Abstract
Estimates of hemodynamic amplitude, delay, and width were combined to investigate system dynamics involved in lexical decision making. Subjects performed a lexical decision task using word and nonword stimuli rotated 0°, 60°, or 120°. Averaged hemodynamic responses to repeated stimulation were fit to a Gamma-variate function convolved with a heavyside function of varying onset and duration to estimate each voxel's activation delay and width. Consistent with prolonged reaction times for the rotated stimuli and nonwords, the motor cortex showed delayed hemodynamic onset for both conditions. Language areas such as the lingual gyrus, middle temporal gyrus, fusiform gyrus, and precuneus all showed delayed hemodynamic onsets to rotated stimuli but not to nonword stimuli. The inferior frontal gyrus showed both increased onset latency for rotated stimuli and a wider hemodynamic response to nonwords, consistent with prolonged processing in this area during the lexical decision task. Phonological processing areas such as superior temporal and angular gyrus showed no delay or width difference for rotated stimuli. These results suggest that phonological routes but not semantic routes to the lexicon can proceed regardless of stimulus orientation. This study demonstrates the utility of estimating hemodynamic delay and width in addition to amplitude allowing for more quantitative measures of brain function such as mental chronometry.
Among the major utility-enhancing advances in functional MRI (fMRI) was the development of event-related designs and analyses procedures (1–7). The advent of event-related designs led the way to several notable methods for extracting information from the hemodynamic response. These include sorting hemodynamic responses with regard to behavioral performance (8–10), mental chronometry using hemodynamic delay estimates (7) to infer neural information flow (11–13), and estimates of hemodynamic width to infer areas with increased processing demand (14–19). An ability to measure ever more subtle hemodynamic response modulations to specific stimulus modulations will help us better understand the complex relationship between neuronal activity and fMRI responses, thereby allowing investigators to ask more sophisticated neuroscience questions. Simultaneous measures of hemodynamic delay and width would aide in resolving mental chronometry and relative processing time, resulting in more detailed investigation of cognitive neuroscience models that include relative timing information.
Prior work using hemodynamic response amplitude and delays to infer cognitive processes specific to a neural circuit (14–20) have run into two main difficulties. First, estimates of amplitude and delay can be dramatically biased by changes in response width. For example, a least squares fit of a response model to a wider empirical response will artificially lengthen the delay estimate, thereby confounding interpretation. Here, we implement a direct method that produces delay and width estimates that are uncorrelated, eliminating bias effects between the two estimates. The second experimental challenge arises when attempts are made to compare temporal characteristics of the hemodynamic response across brain regions. The ability to compare hemodynamic measures throughout an entire neural system would enable investigators to decipher what system component participates in which processing stage. The major obstacle in comparing the hemodynamic response across systems, and even within systems, is that different brain regions exhibit biologically determined differences (spatial bias) in hemodynamic response properties that are unrelated to the underlying neural function. Delay and/or width estimate vary within and across regions by up to ±2 s (11, 14, 21–24). To date, studies comparing delay and width estimates have avoided such pitfalls by focusing on changes correlated with task variability within a single task type and averaging delays across a predefined set of regions, or simply mapped delay estimates from separate tasks onto functionally derived regions of interest (ROI; refs. 13 and 18). Because these methods fail to compensate for the nonneuronal hemodynamic variability or undetermined spatial bias, inferences about mental chronometry between voxels in ROI A and ROI B can be misleading. One way to address this issue is by examining differences among levels along a continuum of task modulations relative to a comparator task with the stipulation that task-related modulation in the hemodynamic response is independent from hemodynamic spatial bias. In this experimental design, the response delays of the comparator task are subtracted from delays of the target task modulations, thereby eliminating the spatially bias in response delays within and between ROIs.
Neuroimaging has served as a tool for attaching neuroanatomical correlates to cognitive models of human brain processing that previously relied on reaction time data and lesion studies. Advances in fMRI methodology should enable investigators to correlate neuroanatomical markers with cognitive processes and test cognitive neuroscience models of brain function. For example, Koriat and Norman (25, 26) showed that reaction times in a lexical decision task using rotated stimuli are proportional to the degree of rotation above 60°. This suggests that a perceptual perspective change occurs before language processing. From a neuroscience perspective, one might predict that different functional areas will respond with different latency delays and increased duration relative to the amount of stimulus rotation. In particular, language-processing areas should show delayed responses to stimuli rotated >60° but no magnitude or width changes, whereas areas involved in stimulus rotation may show increased response duration relative to the degree of rotation.
Connectionist theories of single-word processing propose that the same neural systems process both words and nonwords (27–30). These models predict that the phonological component of visual word recognition is prolonged when either pronounceable nonwords or low frequency words are presented, whereas common words can take a more direct route to the lexicon via semantic processing. Therefore, differences in brain activation between presentation of words and nonwords would be related to differential processing demands within the same neural system. Previous neuroimaging studies comparing words and nonwords have shown magnitude differences in a multitude of brain regions that seem to support a dual route model (30) of lexical access (31–33) or may simply reflect prolonged processing in these regions or components in the postlexical decision process (34). Although reaction time differences in lexical decision tasks are small compared with the spatial variability of the blood oxygenation level-dependent response, our method of voxel-wise task comparisons of delay and width should enable us to measure hemodynamic property changes of this scale. The temporal variance of the hemodynamic response (11, 35) suggests that estimates on the order of 100 ms can be robustly obtained by averaging runs collected during a typical imaging session.
The purpose of this study is to demonstrate the utility of creating voxel-wise task-comparison measures from estimates of hemodynamic delay and width for understanding the neural correlates of specific task demands and for dissecting the cognitive processing stream. Through manipulation of task performance and combined estimation of delay and width, we should decrease the bias introduced when estimating them separately and allow for a dissociation of areas involved in prolonged cognitive processing from areas further down the processing stream. We apply this strategy to test whether language processing is automatic or is delayed when the stimuli are rotated more than 60° from horizontal. Following the findings of Koriat and Norman (25), we predict a larger hemodynamic delay to be present in all language areas not involved in perceptual perspective changing when stimuli are presented at 120° of rotation. Additionally, we predict that voxels in the left inferior frontal gyrus, proposed to be involved in phonological, semantic, and postlexical processing (31, 34, 36–46) components of the lexical decision task, will show a wider hemodynamic response when nonwords are presented compared with real words and that this will be true regardless of degree of stimulus rotation.
Materials and Methods
Participants.
Twelve (eight male) right-handed, fluent English-speaking adults (23–32 years of age) were each scanned for ≈90 min. Data from two subjects were discarded before analysis as a result of head movement or scanner stability problems. All subjects signed informed consents in compliance with the Institutional Review Board at the National Institutes of Health. All subjects were monetarily compensated for their participation.
Tasks and Stimuli.
Subjects performed a lexical decision task composed of words and pronounceable nonwords rotated at 0°, 60°, or 120° from horizontal. Forty novel stimuli from each of the six possible conditions (stimulus type × rotation) were presented pseudorandomly throughout the four scanning series. Each stimulus was presented for 750 ms followed by a fixation crosshair. The average interstimulus interval was 3,000 ms but varied between 2,000 and 4,000 ms. All subjects responded to the task with a response box held in their left hands and were instructed to depress the left-most key for words and the adjacent right key for nonwords. Before being placed in the scanner, subjects were trained on the lexical decision task using an additional 10 words and nonwords. Subjects were instructed to decide if the stimulus was a word or nonword regardless of stimulus orientation and received explicit instructions to respond as quickly as possible without sacrificing accuracy for speed.
Words used during scanning consisted of 120 six-letter nouns with a Kucera–Francis written frequency ranging from 10 to 100. Random permutations of the six letters were used to create the 120 pronounceable nonwords. In a majority of randomizations, vowels were either shifted or inserted as a replacement for a constant to ensure pronouncability. All stimuli were presented at a visual angle of ≈4° in black Helvetica font on a white background.
Image Acquisition and Data Analysis.
Imaging was performed on a 3 Tesla General Electric Signa scanner. A brain-specific quadrature head coil was used for these studies (Medical Advances, Milwaukee, WI). Functional imaging of the entire brain was conducted using a gradient-echo echoplanar sequence (TE = 30 ms, TR = 1,000 ms, FOV = 24 cm, matrix = 64 × 64, slice thickness = 6 mm, and gap of 3 mm). A series of 384 consecutive image volumes were acquired during each of four functional runs. An additional set of high resolution, T1-weighted, spoiled gradient-echo anatomic reference images (TE = 5.3 ms, TR = 12 ms, FOV = 24 cm, matrix = 256 × 192, slice thickness = 1.2 mm, and 17° flip angle) were obtained for localization purposes.
All image processing and statistical analyses were performed with the AFNI software package (47, 48) except for delay and width estimates that were performed with a customized analyses script written in MATLAB (49). Subjects' four echo-planar time series were each motion corrected (50), concatenated, and all images were then reregistered to the fifth volume in the first run. The hemodynamic response function (HRF) and multivariate statistics corresponding to each of the six conditions were obtained by deconvolving the input for each from the concatenated time series using a least squares procedure with a finite impulse response model. A priori general linear model tests of means were conducted among all levels of rotation and also between words and nonwords.
The full model F statistic from the deconvolution procedure was used to restrict the hemodynamic characterization analyses to those voxels that were significantly activated at P < 0.05 (uncorrected). For each subject, HRFs of activated voxels were upsampled by a factor of 10 and modeled as a convolution of a gamma variate function with a heavyside function of variable delay and width. To account for subject-to-subject variability in the average HRF, separate estimates of the gamma variate function parameters were obtained for each subject by fitting the function (51, 52) to the average HRF. Delay and width of the HRF of each activated voxel were obtained using the Nelder–Mead simplex method to fit the HRF model to the empirical HRF estimate. To improve the convergence of the algorithm to a proper solution, we computed the fit using a set of initial values for delay and width ranging in 0.5-s increments between 0 and 4. The parameter combination resulting in the best fit was then used as a center for another set of delay and width values covering 1 s in finer increments of 0.2-s. The result of the second pass provided the final estimate of delay and width for a particular voxel's HRF. From simulated data obtained by generating HRFs from input functions of random delays and widths, we found that estimates of delay and width were uncorrelated (r = 0.07). This indicated that the delay estimates were not biased by response width and vice versa. However, although width and amplitude estimates of the heavyside function are linearly independent, the hemodynamic response function introduces correlation between them. To aid in explanatory power, we provide averaged estimates of the HRF to determine whether differences are caused by differences in peak amplitude or width and functional maps that show a spatial dissociation of the two effects.
It has been shown that standing differences in response delay (11, 21) and possibly widths are present across voxels. To avoid having such differences confound task-related delay and width differences, we calculated, on a voxel-by-voxel basis, the difference in response delay and width between words vs. nonwords, 120° rotation vs. no rotation, and 60° rotation vs. no rotation contrasts. These maps were then put into standard stereotaxic space and averaged across subjects using a 2-mm rms Gaussian blur. Average delay and width difference maps depict voxels with a delay difference greater than half the smallest average reaction time difference (reaction time difference between nonrotated stimuli and 60° rotated stimuli, 98 ms). HRFs were converted to percent change relative to the mean intensity value from the entire time series less the first five images of each run.
ROI Selection.
Regions of interest were selected covering the left inferior frontal gyrus, right precentral gyrus, and left middle temporal gyrus. ROIs used for within-region comparison were created by selecting voxels with overlap between the group full model F statistic (resampled for each individual) and thresholded difference maps created. Data on delay, width, and averaged time series were collected from the intersecting voxels. To determine the statistical significance of delay and width differences in these regions, separate random effects repeated measures analyses of variance were conducted for each region.
Results
Behavioral Data.
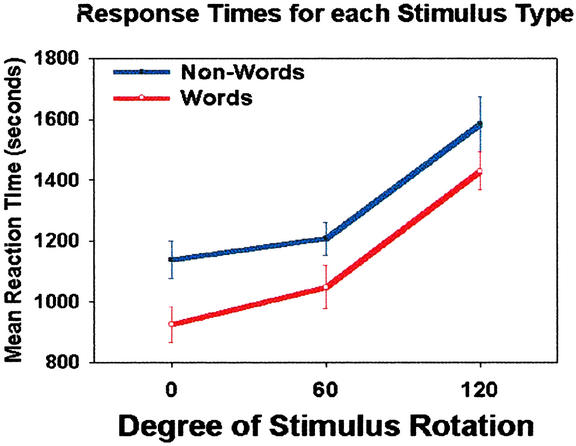
Subjects' overall 92.5% correct performance on the lexical decision task did not differ among levels of rotation (F < 1.0). As depicted in Fig. 1, stimulus rotation of 120° but not 60° resulted in longer reaction times than nonrotated words and nonwords, F (2,42) = 27.7, P < 0.01. Across all levels of stimulus rotation, nonwords showed a significantly longer response time than words, F (1, 42) = 10.0, P < 0.01. Although the reaction time difference between words and nonwords seems to decrease across levels of stimulus rotation, no significant interaction was found in this behavioral measure (F < 1.0).
Figure 1.
Mean reaction time for each condition. Error bars depict the SEM.
fMRI Data.
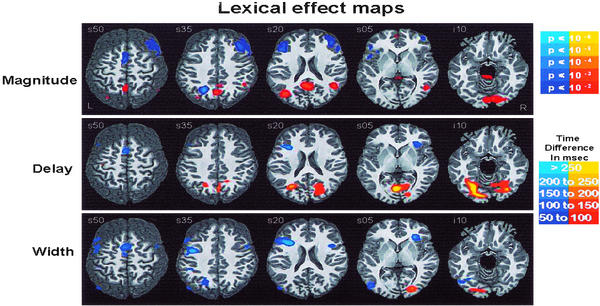
Fig. 2 depicts averaged amplitude, delay, and width difference group maps for the Lexical main effect (word vs. nonword), and Fig. 3 depicts the same for the rotation main effect (no rotation vs. either 60° or 120° rotation). Consistent with previous fMRI studies of lexical processing, subjects showed magnitude differences between words and nonwords in language areas attributed to orthographic, phonological, semantic, and postlexical processing, including fusiform gyrus, lingual gyrus, middle temporal gyrus, precuneus, superior temporal gyrus, and middle and inferior frontal gyrus.
Figure 2.
Warm colors (red/yellow) are areas where words are more than nonwords. Cool colors (blues) are areas where nonwords are more than words. The left hemisphere is toward the left margin.
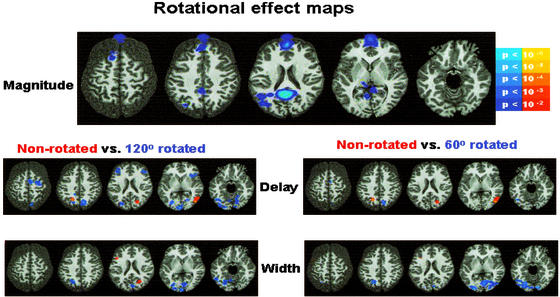
Figure 3.
Warm colors (red/yellow) are areas where nonrotated stimuli are more than rotated. Cool colors (blues) are areas where rotated stimuli are more than nonrotated. The left hemisphere is toward the left margin.
Mean delays were calculated for each condition in all regions surviving the full model F statistic threshold. Random effects ANOVA using level of rotation and word type as factors were conducted for each region. Regions showing a statistically significant (P < 0.05) or trend (P < 0.075) toward hemodynamic onset delay to stimuli rotated 120° included a motor response area (precentral gyrus) and a language/decision network that includes bilateral inferior frontal gyrus, middle temporal gyrus, and precuneus and lingual gyrus. Although no significant reaction time difference was evident between stimuli rotated 60° and not rotated, significant hemodynamic delays existed between the rotated stimuli bilaterally in the precuneus, right posterior temporal regions. Because these regions are also found in the 120° rotated stimuli comparison, it suggests that they are involved in rotation processing or perspective processing that is not involved in slowing language processing.
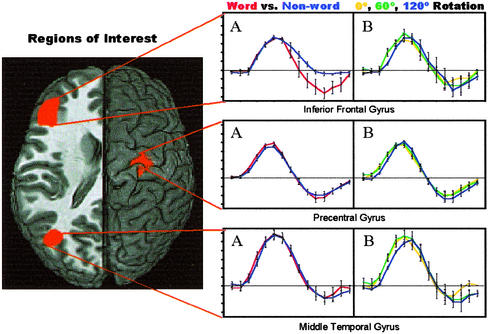
Bilateral inferior frontal gyrus showed both a delayed onset to rotated stimuli [F (2, 41) = 8.31; P < 0.001] and a width difference between HRFs for words and nonwords [F (1, 41) = 5.76; P < 0.05], suggesting that processing was prolonged in this region during the lexical decision component of the task. Averaged time series obtained in the left inferior frontal gyrus are presented as support for both the delay and width estimates. Similarly, width differences between estimated HRFs for words and nonwords are clearly shown from our estimate of the width and the averaged HRF shown in Fig. 4. There were three functionally separate regions within the middle temporal gyrus. The superior regions of the left middle temporal gyrus showed widening of the hemodynamic response to nonwords [F (1, 41) = 4.73; P < 0.05]. In contrast, a more ventral region showed no differential widths (F < 1.0) between stimuli but rather showed a delayed onset time for stimuli rotated at 120°. The anterior left middle temporal gyrus showed delayed responses to words relative to nonwords but had no differential effects of stimulus rotation. Differential responses in the middle temporal gyrus suggest that different areas have different functional roles in accessing the mental lexicon. Averaged HRFs grouped to show both the lexical and rotational effects are presented for the inferior middle temporal gyrus. These averaged HRFs are representative of a more anterior middle temporal gyrus language area that showed a delayed onset for rotated stimuli but no width difference for words vs. nonwords (F < 1.0). Like the middle temporal gyrus, the fusiform gyrus showed significantly longer word onset delays relative to nonword delays. This finding suggests that these areas participate earlier in the processing of nonwords than words, possibly due to postlexical automatic semantic processing of word stimuli.
Figure 4.
Graphs depicting the estimated hemodynamic impulse response functions for both the lexical (words vs. nonwords) and rotation effects (degree of rotation). The x axis depicts time in seconds, and the y axis depicts percent signal change. Error bars represent the SEM, and the left hemisphere is toward the left margin.
Right precentral gyrus shows a delayed hemodynamic onset for stimuli rotated 120°. Although the averaged delay difference is similar to the reaction time difference, no significant correlation exists between estimated hemodynamic delay and subject reaction time. The correlation between estimated hemodynamic onset delay and reaction time was also nonsignificant in the inferior frontal gyrus.
Discussion
The present study was conducted to determine whether a voxel-wise characterization of the hemodynamic response beyond standard measures of amplitude would aide in attaching neuroanatomical markers to cognitive processes involved in single word processing. Combinatory use of the magnitude, delay, and width maps demonstrates that rotation produced delayed access to the mental lexicon only via a semantic/orthographic route including the lingual, fusiform, and middle temporal gyri ventrally and the precuneus dorsally. These maps also show that the inferior and middle frontal gyri do not simply show greater peak levels of activation for nonwords compared with words but that the difference is the result of an increase in the HRF width. The present data show that magnitude differences between words and nonwords were mostly due to varying widths rather than one condition not showing an increase from fixation. As a whole, the results demonstrate that increasing the amount of information derived from the blood oxygenation level-dependent signal can yield novel details about the neural substrates of cognitive tasks.
Consistent with previous cognitive neuroscience and brain imaging studies (18, 38–40), the present study showed increased amplitudes for nonwords relative to words in the inferior frontal gyrus (IFG) during a lexical task. As in Henson et al. (18), this region (centered in the inferior frontal sulcus) showed slight delay differences. The present study further shows large hemodynamic width differences between words and nonwords in the IFG, as evident in the time series data. This width difference may reflect a persistence of activation for nonwords due to postlexical decision processing. Balota and Chumbley (34) proposed a two-stage model of lexical decision processing. In the first stage, an initial response threshold is set by word frequency and phonologic plausibility. During this stage, high frequency words and unpronounceable nonwords are either accepted or rejected rapidly. The remaining words including pronounceable nonwords and lower frequency words are then reassessed for lexical validity. Persistent activation during nonword processing is consistent with a reassessment of both phonologic and semantic plausibility of that nonword. Therefore, the role of the IFG may involve processing the increased semantic and phonological demands for decisions regarding nonwords and suggests that the inferior frontal gyrus may be the “bottleneck” in the processing stream that results in an increase reaction time during the lexical decision task. Portions of the IFG have been implicated in semantic processing (32, 41) like many of the other regions that showed a delayed response when stimuli were rotated (see below). The design of the current study does not allow us to determine whether the persistence of activation in the IFG is relevant to phonological, semantic, postlexical decision processes or some combination of these. However, the IFG response is more similar to phonological processing areas as it did not show a delayed onset to rotated stimuli, as did many semantic areas.
Although we predicted that a more extensive network of regions associated with language processing would show delays for stimuli rotated 120°, rotational delays occurred mostly in areas involved in semantic processing and retrieval. These areas included the bilateral precuneus, ventral temporal-occipital pathway, and middle temporal gyrus. It is possible that if access to the mental lexicon can be gained via two separate routes, then these data suggest that the phonological route is activated automatically and that the semantic route depends upon a more thorough perceptual analysis to be completed and thus is delayed until the perspective of the subject is rotated 120°. The value of including measures of delay and width can also be seen in posterior temporal-occipital regions. Consistent with previous studies (18), left posterior regions showed longer delays for words relative to nonwords. However, in the present study, we found no difference in magnitude or width for the lexical comparison and differences only in the right ventral temporal areas for the rotational manipulation. This could result from further automatic semantic or associative processing being done after the lexical component of the task is completed.
The current study demonstrates that hemodynamic delays related to reaction time differences of ≈100 ms can be detected using TR as long as 1 s. The observed reaction time differences are, in some cases, an order of magnitude different from most that are reported in the cognitive literature (e.g., repetition priming effects). Nevertheless, with a greater number of trials, improved field stability and corrections for physiological noise, the variability of delay and width estimates will be significantly improved. Although the addition of delay and width estimates improves the utility of fMRI, their correlation with reaction time measures was marginal (P < 0.08 in the precentral gyrus). A similar nonlinear relationship between delays and reaction time were found by Henson et al. (18).
The design and analysis technique presented in the present study yield unique insight into the cognitive systems involved in single word processing and in particular lexical decision. Amplitude measures of differences between words and nonwords have shown that the IFG is more active for nonwords; however, magnitude can differ in at least three ways. First, one stimulus may activate an additional region, whereas the other does not. Second, one stimulus can cause a higher degree of neuronal activity in the same region, therefore causing an elevation in the MR signal. Third, one stimulus can have more persistent activation leading to a greater magnitude and wider BOLD response. Stimulus estimates of delay provide information regarding the stream that processing may take, and width estimates provide a sketch of areas that have prolonged processing demands put upon it. However, variability in brain vasculature between regions and even between voxels demands that each of these measures be standardized or calibrated. This study shows that appropriate task comparisons can produce difference maps that standardize the delay, and width measures between voxels allow for more quantitative mental chronometric maps, adding a new dimension to the utility of fMRI.
Acknowledgments
We thank Dr. R. W. Cox for providing advice with computational and statistical matters, Elisa Kapler for assistance with scanning and data processing, and Rasmus Birn for his contributions in experimental design. This study was supported by the National Institute of Mental Health-Intramural Research Program.
Abbreviations
- fMRI
functional MRI
- HRF
hemodynamic response function
- ROI
region of interest
- IFG
inferior frontal gyrus
References
- 1.Buckner R L, Bandettini P A, O'Craven K M, Savoy R L, Petersen S E, Raichle M E, Rosen B R. Proc Natl Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner R L. Hum Brain Mapp. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birn R M, Cox R W, Bandettini P A. NeuroImage. 2002;15:252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- 4.Friston K J, Zarahn E, Josephs O, Henson R N, Dale A M. NeuroImage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 5.Dale A M. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen B R, Buckner R L, Dale A M. Proc Natl Acad Sci USA. 1998;95:773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friston K J, Fletcher P, Josephs O, Holmes A, Rugg M D, Turner R. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 8.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neuron. 2002;35:975. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 9.Wagner A D, Schacter D L, Rotte M, Koutstaal W, Maril A, Dale A M, Rosen B R, Buckner R L. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 10.Brewer J B, Zhao Z, Desmond J E, Glover G H, Gabrieli J D. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 11.Bandettini P. In: Functional MRI. Moonen C T W, Bandettini P, editors. Berlin: Springer; 1999. pp. 205–220. [Google Scholar]
- 12.Menon R S, Kim S G. Trends Cogn Sci. 1999;3:207–216. doi: 10.1016/s1364-6613(99)01329-7. [DOI] [PubMed] [Google Scholar]
- 13.Formisano E, Linden D E, Di Salle F, Trojano L, Esposito F, Sack A T, Grossi D, Zanella F E, Goebel R. Neuron. 2002;35:185–194. doi: 10.1016/s0896-6273(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 14.Saad Z S, Ropella K M, Cox R W, DeYoe E A. Hum Brain Mapp. 2001;13:74–93. doi: 10.1002/hbm.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter W, Ugurbil K, Georgopoulos A P, Kim S G. NeuroReport. 1997;8:3697–3702. doi: 10.1097/00001756-199712010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Richter W, Anderson P M, Georgopoulos A P, Kim S G. NeuroReport. 1997;8:1257–1261. doi: 10.1097/00001756-199703240-00040. [DOI] [PubMed] [Google Scholar]
- 17.Dukelow S P, Vilis T, Hassard F A, Gati J S, Menon R S, Goodale M A. J Neurophysiol. 1999;81:388–393. doi: 10.1152/jn.1999.81.1.388. [DOI] [PubMed] [Google Scholar]
- 18.Henson R N, Price C J, Rugg M D, Turner R, Friston K J, Maratos E J. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- 19.Menon R S, Luknowsky D C, Gati J S. Proc Natl Acad Sci USA. 1998;95:10902–10907. doi: 10.1073/pnas.95.18.10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weilke F, Spiegel S, Boecker H, von Einsiedel H G, Conrad B, Schwaiger M, Erhard P. J Neurophysiol. 2001;85:1858–1863. doi: 10.1152/jn.2001.85.5.1858. [DOI] [PubMed] [Google Scholar]
- 21.Huettel S A, McCarthy G. NeuroImage. 2001;14:967–976. doi: 10.1006/nimg.2001.0900. [DOI] [PubMed] [Google Scholar]
- 22.Miezin F M, Maccotta L, Ollinger J M, Petersen S E, Buckner R L. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 23.Lee A T, Glover G H, Meyer C H. Magn Reson Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- 24.Moonen C T W, Bandettini P A. Functional MRI. Berlin: Springer; 1999. p. xii. and 575. [Google Scholar]
- 25.Koriat A, Norman J. J Exp Psychol Hum Percept Perform. 1985;11:490–508. doi: 10.1037//0096-1523.11.4.490. [DOI] [PubMed] [Google Scholar]
- 26.Koriat A, Norman J. J Exp Psychol Learn Mem Cognit. 1984;10:421–434. doi: 10.1037//0278-7393.10.3.421. [DOI] [PubMed] [Google Scholar]
- 27.Seidenberg M S, McClelland J L. Psychol Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- 28.Seidenberg M S, Plaut D C, Petersen A S, McClelland J L, McRae K. J Exp Psychol Hum Percept Perform. 1994;20:1177–1196. doi: 10.1037//0096-1523.20.6.1177. [DOI] [PubMed] [Google Scholar]
- 29.Plaut D C, McClelland J L, Seidenberg M S, Patterson K. Psychol Rev. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Coltheart M, Curtis B, Atkins P, Haller M. Psychol Rev. 1993;100:589–608. [Google Scholar]
- 31.Price C J, Wise R J, Frackowiak R S. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- 32.Rumsey J M, Horwitz B, Donohue B C, Nace K, Maisog J M, Andreason P. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- 33.Mummery C J, Shallice T, Price C J. NeuroImage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- 34.Balota D A, Chumbley J I. J Exp Psychol Hum Percept Perform. 1984;10:340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- 35. Saad, Z. S., DeYoe, E. A. & Ropella, K. M. (2003) NeuroImage, in press. [DOI] [PubMed]
- 36.Roskies A L, Fiez J A, Balota D A, Raichle M E, Petersen S E. J Cognit Neurosci. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- 37.Fiez J A. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- 38.Bokde A L, Tagamets M A, Friedman R B, Horwitz B. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 39.Fiebach C J, Friederici A D, Muller K, von Cramon D Y. J Cognit Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- 40.Rossell S L, Bullmore E T, Williams S C, David A S. Neuropsychologia. 2001;39:1167–1176. doi: 10.1016/s0028-3932(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 41.Price C J. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price C C, Davis K L, Kaplan E, Libon D J, Price C J. Neuropsychologia. 2002;40:435–445. doi: 10.1016/s0028-3932(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 43.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 44.Herbster A N, Mintun M A, Nebes R D, Becker J T. Hum Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 45.Poldrack R A, Wagner A D, Prull M W, Desmond J E, Glover G H, Gabrieli J D. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 46.Chumbley J I, Balota D A. Mem Cognit. 1984;12:590–606. doi: 10.3758/bf03213348. [DOI] [PubMed] [Google Scholar]
- 47.Cox R W. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Cox R W, Hyde J S. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.matlab v. Signal Processing Toolbox User's Guide. Natick, MA: Mathworks; 1994. [Google Scholar]
- 50.Cox R W, Jesmanowicz A. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 51.Cohen M S. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 52.Boynton G M, Engel S A, Glover G H, Heeger D J. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]