Abstract
Atherogenesis is enhanced in arterial segments exposed to disturbed blood flow, indicating the active participation of the hemodynamic environment in lesion formation. Turbulent shear stress selectively regulates responsive genes in the endothelium and increases the damage induced by free radicals. The purpose of the present study was to evaluate the effects of intervention with antioxidants and l-arginine on endothelial NO synthase (eNOS) and oxidation-sensitive gene perturbation induced by disturbed flow in vitro and in vivo. Both human endothelial cells exposed to shear stress and high atherosclerosis-prone areas of hypercholesterolemic low-density lipoprotein receptor knockout (LDLR−/−) mice showed increased activities of redox-transcription factors (ELK-1, p-Jun, and p-CREB) and decreased expression of eNOS. Intervention with antioxidants and l-arginine reduced the activation of redox-transcription factors and increased eNOS expression in cells and in vivo. These results demonstrate that atherogenic effects induced by turbulent shear stress can be prevented by cotreatment with antioxidants and l-arginine. The therapeutic possibility to modulate shear stress-response genes may have important implications for the prevention of atherosclerosis and its clinical manifestations.
Keywords: eNOS‖ELK-1‖p-CREB‖p-Jun‖vascular disease
Vascular endothelial cells (EC) are constantly subjected to the influence of hemodynamic forces including high shear stress imposed by blood flow, which stimulates the release of NO (1). Turbulent shear stress alone, or shear stress associated with other risk factors of atherosclerosis, may activate a variety of signal transduction events that in turn may lead to endothelial dysfunction and enhanced atherogenesis (2). This concept would explain the long-standing observation that early atherosclerosis develops in characteristic topographical patterns primarily to branch points and other areas subjected to oscillatory shear stress (3, 4). Detailed analyses of hemodynamic mechanics in areas highly prone (HP) to atherosclerosis have identified unique patterns of disturbed flow, characterized by regions of flow separation, recirculation, and temporal and spatial gradients of shear stress (4). Moreover, it has been clearly demonstrated that vascular EC under high shear stress increased their release of free radicals and reactive oxygen species (5–10). Recently, using DNA microarray technology, differential expression of a plethora of genes has been shown after high shear stress stimulation in vivo (11, 12).
At low concentrations, like those produced by constitutive endothelial NO synthase (eNOS) in the vasculature in vivo, NO acts as a paracrine-signaling molecule, mediating vasodilation, inhibition of platelet activation, monocyte and leukocyte adhesion, and smooth muscle cell proliferation. It also controls vascular oxidative stress and expression of redox-regulated genes (13). However, its activity is influenced by the vascular wall milieu, and complex pathophysiological interactions among NO, l-arginine, and antioxidants dictate the process of atherogenesis (14). This scenario is complicated further by local effects of systemic risk factors (2, 4).
To determine whether proatherogenic conditions induced by turbulent shear stress can be modulated by antioxidants and/or l-arginine, we first studied cultured human EC subjected to shear stress. We then analyzed HP and low-prone (LP) atherosclerotic aortic areas of low-density lipoprotein (LDL) receptor knockout (LDLR−/−) mice after short (1 week) or long (8 weeks) treatment with l-arginine and/or antioxidants. Administration of l-arginine, the precursor of NO, has been shown to augment endothelium-dependent vasodilation in LDLR−/− mice (15, 16). Similarly, antiatherogenic effects of antioxidants have been demonstrated in hypercholesterolemic mice (17–19). Here, we show that intervention with antioxidants and l-arginine reduces redox-gene activation and increases eNOS expression both in cells and in vivo. These findings also demonstrate that redox-sensitive transcription factors and NO generation by eNOS are affected by shear stress and that the pathogenic effects are reversible.
Materials and Methods
Cell Culture.
Human coronary EC were cultured as described (20, 21). Cells were incubated at 37°C for 4 days in a humidified atmosphere of 95% air and 5% CO2. The incubation medium (delipidated DMEM) was supplemented with 10 ng/ml human epidermal growth factor, penicillin/streptomycin, amphotericin B, and glutamine (20, 21).
Flow Apparatus.
Cells were subjected to shear stress in a cone-and-plate viscometer at low level of 1 dyne (1 dyne = 10 μN) per cm3 (mean shear stress in veins) and at high level of 15 dynes per cm3 (mean shear stress in arteries) 1 day after reaching confluence, as described in detail (22). We selected these forces on the basis of several published studies (7–12). The viscometer consists of a cone with a 0.5° angle rotating on top of a 94 × 16-mm cell culture dish. Flow conditions thus achieved are mainly considered laminar, because the parameter R (r2ωα2/12ν) was smaller than 4 (R1 and R15: 0.006 and 0.03 dynes per cm2, respectively) (22). However, forces generated in vitro can also induce oscillatory or other forms of disturbed shear stress. Forces of 1 dyne per cm2 (0.1 N/m2) or 15 dynes per cm2 (1.5 N/m2) were applied by constant angular velocity in a humidified environment with 5% CO2 at 37°C. Viscosity of the cell medium was 0.007 dyne per s/cm2. For application of arterial shear stress (15 dynes per cm2), 5% dextran (molecular weight: 71,400) was added to the medium to increase the viscosity 2.95-fold to 0.02065 dyne per s/cm2. A control dish accompanied each cell culture dish from the same cell preparation, which was incubated with cell culture medium with or without 5% dextran for 2 h without application of shear stress. Dextran had no influence on the expression of genes studied. EC were also exposed to different flows for 24 h in the absence or presence of 50 μM α-tocopherol and 10 μM ascorbic acid (Sigma) and/or 10 μM l-arginine (Sigma). Additional control cells were incubated and changed to the new culture medium under static conditions.
Mice and Treatments.
Six-month-old male LDLR−/− mice (n = 18, mean weight 42.1 ± 10.3 g) were fed a cholate-free high cholesterol diet (21% milk fat, 1.5% cholesterol, and 19.5% casein; no. 8137, Harlan/Teklad, Madison, WI) for 6 months (19, 23, 24). This diet raised their mean plasma cholesterol levels to about 1,200 mg/dl and plasma triglyceride levels to 400 mg/dl. To assess whether antioxidants and/or l-arginine influence gene regulation, 60 LDLR−/− mice on the same proatherogenic diet were randomly divided into six groups (n = 10 each). One group received the high-fat diet supplemented with antioxidants (1.0% vitamin E added to the chow and 0.05% vitamin C added to the drinking water) (17). The diet of the second and third groups was supplemented only with l-arginine (6% l-arginine added to the drinking water) (15, 16) and a combination of antioxidants and l-arginine (1.0% vitamin E added to the chow and 0.05% vitamin C and 6% l-arginine added to the drinking water), respectively. These three groups were killed after 1 week of treatment and used for gene analysis. The remaining three groups followed the same chronic supplementation for an additional 8 weeks. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996) and the Guidelines of the American Heart Association.
Preparation of Arterial Samples and Western Blot Analysis.
Mice were killed by CO2 asphyxia. Blood was drawn into heparinized tubes from the inferior vena cava (19, 23, 24). The aortic trunk was washed by perfusion with PBS containing 10 μg/ml aprotinin and 0.1 mmol/liter PMSF through the left ventricle. The dissection of the aorta was performed under a stereomicroscope from the iliac bifurcation up to the heart, including the beginning of the carotid and subclavian arteries (19, 23, 24). The adventitia was carefully removed. The aorta was continuously immersed in PBS containing 10 μg/ml aprotinin and 0.1 mmol/liter PMSF from the time of the dissection until the computerized determination of the lesion area was completed (19, 23). Intercellular junctions were stained with 0.25% silver nitrate to visualize endothelium in the HP and LP atherosclerotic aortic regions (located mainly in the proximal aorta), according to Iiyama et al. (25). Although the method used here does not allow the control of blood pressure at the plaque level, EC in the HP regions have variable shapes and random orientation, whereas EC in the LP region are elongated and aligned in the direction of blood flow (25–27). Arterial segments from healthy vessels (HV) served as controls. Tissue sections (5 μm) from the different arterial regions were homogenized in 250 μl of protein extraction buffer [50 mmol/liter Tris (pH 8)/150 mmol/liter NaCl/1% Triton X-100/1% sodium deoxycholate/5 mmol/liter EDTA/1 mmol/liter DTT/10 mmol/liter β-glycerophosphate/10 μg/ml aprotinin/10 μg/ml trypsin inhibitor/2 μg/ml leupeptin/0.1 mmol/liter PMSF] for determination of protein content by Western blot (19, 21, 28). Approximately 15 μg of protein extract was separated by 10% SDS/PAGE (19, 21, 28). The gel was transblotted onto a nitrocellulose membrane; blocked with 10% milk powder in Tris-buffered saline (pH 7.4) with 0.1% Tween 20 (TBST) overnight; incubated with mAbs (1:500 dilution for 1.5 h at room temperature) against Elk-1 (I-20, goat antibody), p-CREB (Sc:7978), and eNOS III [N-20, epitope corresponding to an amino acid sequence mapping at the amino terminus of NOS-III, no cross reactivity with NOS-I or NOS-II, neuronal and inducible forms, respectively; purchased from Santa Cruz Biotechnology (21, 28)]. Ab against the phosphorylated form of Jun (p-Jun) (420110-S) was purchased from Calbiochem. After 5 washes with TBST, the signal was detected by using a chemiluminescence kit (Amersham Pharmacia Biotech ECL kit) (19, 21, 28). Membranes were normalized with a polyclonal Ab against γ-tubulin protein (Sigma). Because qualitative analysis of blots can overestimate the magnitude of results, semiquantitative scan densitometry of blots was done with a Scan LKB (Amersham Pharmacia) (19, 21, 28).
Statistical Analysis.
Results are expressed as the mean ± SD. The difference among groups was evaluated by a one- or two-factor ANOVA by two independent investigators in a blinded fashion. Significance was accepted at P < 0.05.
Results
Shear Stress in Cultured EC.
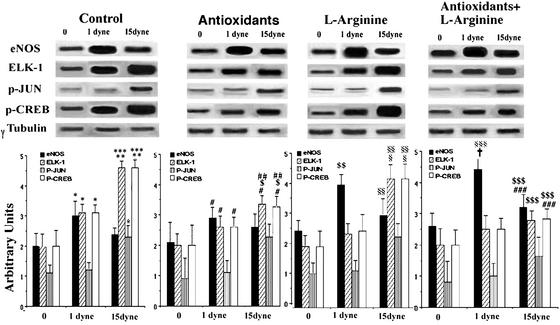
A strictly controlled densitometric analysis showed that basal eNOS protein was increased by ≈50% at 1 dyne per cm2 shear stress (P < 0.01) but increased less at 15 dynes per cm2 (20 ± 5%, P = NS) (Fig. 1). Thus, in our experimental conditions, a high rate of shear stress increased eNOS expression only modestly. After l-arginine administration, eNOS protein showed a significant further increase at 1 dyne per cm2 (by ≈60%, P < 0.01 vs. control) and by 25 ± 7% at 15 dynes per cm2 (P = NS). Similarly, administration of antioxidants induced a significant increase of eNOS protein at 1 dyne per cm2 (40%; P < 0.05 vs. control), which did not achieve statistical significance at 15 dynes per cm2 (≈15%; P = NS). At 15 dynes per cm2, cotreatment with antioxidants and l-arginine resulted in a synergistic action on eNOS activity (P < 0.001) that was significantly greater than antioxidants or l-arginine alone (+25 ± 3%; P < 0.05; 1 dyne per cm2) and control (Fig. 1).
Figure 1.
Effect of shear stress (0, 1, or 15 dynes per cm2) on eNOS, Elk-1, p-Jun, and p-CREB protein levels measured by Western blot in cultured EC. Control EC or EC treated with l-arginine and/or antioxidants were exposed to laminar shear stress for 24 h. (Lower) Corresponding densitometric analysis of eNOS, Elk-1, p-Jun, and p-CREB. Data are representative of the mean ± SD of four different experiments. *, P < 0.01 vs. control; **, P < 0.001 vs. control; ***, P < 0.01 vs. 1 dyne per cm2 control; #, P < 0.05 vs. antioxidants at 0 dynes per cm2; ##, P < 0.01 vs. 1 dyne per cm2 antioxidants; $, P < 0.01 vs. 15 dynes per cm2 control; $$, P < 0.01 vs. 0 dynes per cm2 l-arginine; §, P < 0.01 vs. 0 dynes per cm2 l-arginine; §§, P < 0.001 vs. 1 dyne per cm2 l-arginine; §§§, P < 0.001 vs. 0 dynes per cm2 antioxidants + l-arginine; $$$, P < 0.01 vs. 0 dynes per cm2 antioxidants + l-arginine; ###, P < 0.01 vs. 15 dynes per cm2 control; †, P < 0.05 vs. 1 dyne per cm2 antioxidants.
Shear stress also modulates linearly oxidation-sensitive Elk-1 and p-CREB expression. Indeed, Elk-1 was increased by 60% at 1 dyne per cm2 (P < 0.01 vs. control) and by 125% at 15 dynes per cm2 (P < 0.01 vs. 1 dyne per cm2); comparable results were obtained for p-CREB (Fig. 1). Antioxidants alone or in combination with l-arginine reduced Elk-1 and p-CREB protein levels by 25–45% at 15 dynes per cm2 compared with the untreated control at the same flow (P < 0.01). l-Arginine alone resulted in a nonsignificant reduction of Elk-1 and p-CREB expression (Fig. 1). p-Jun accumulation, an index of JNK activity (5), was increased by 110% at 15 dynes per cm2, whereas no significant effect was observed at 1 dyne per cm2 (Fig. 1). Antioxidant treatment alone or cotreatment with antioxidants and l-arginine slightly reduced p-Jun expression (Fig. 1). l-arginine alone did not exert any effect on p-Jun expression. Thus, these therapeutic interventions may induce some protective effects in cultured EC exposed to shear stress.
Effects of Turbulent Shear Stress in Vivo: Effects of Short-Term 1-Week Treatment with Antioxidants and/or l-Arginine.
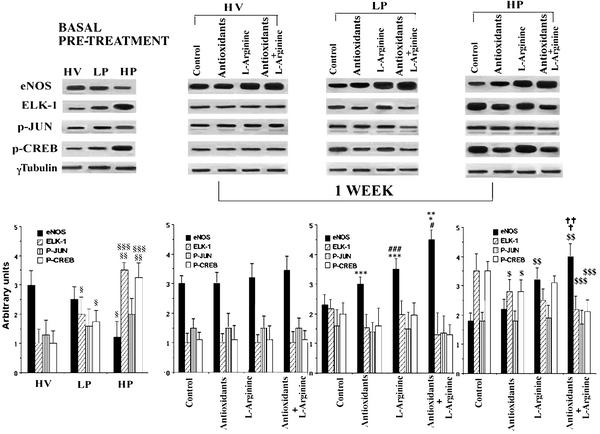
As expected, after 6 months of high-fat diet the control LDLR−/− mice had intermediate lesions in LP areas with an average cross-sectional area of 11,080 ± 1,010 μm2 and large advanced lesions in HP areas (121,558 ± 18,528 μm2). Lesion sizes in both HP and LP areas in mice receiving an additional 1-week treatment with antioxidants and/or l-arginine did not differ significantly from those of untreated groups (not shown). Densitometric analysis of Western blots showed that compared with HV, basal eNOS activity before treatments was decreased by ≈16% (P = NS) and 40% (P < 0.01) in LP and HP areas, respectively (Fig. 2). After 1 week with antioxidants alone, the eNOS activity increased modestly both in LP and HP areas (Fig. 2). Furthermore, eNOS activity in LP and HP areas showed an even greater increase by l-arginine (P < 0.01 vs. control, P < 0.01 vs. antioxidants). Cotreatment with antioxidants and l-arginine indicated a synergistic effect (P < 0.01 vs. control, P < 0.01 vs. antioxidants, and P < 0.01 vs. l-arginine) (Fig. 2).
Figure 2.
Effects of 1-week intervention on protein levels in the aortas of LDLR−/− mice. (Upper) Western blot analysis of total protein extracts from HV, LP, and HP areas. (Lower) Corresponding densitometric analysis. §, P < 0.01 vs. HV; §§, P < 0.001 vs. HV; §§§, P < 0.05 vs. LP; ***, P < 0.05 vs. control LP; ###, P < 0.01 vs. antioxidants LP; *, P < 0.001 vs. control LP; **, P < 0.001 vs. antioxidants LP; #, P < 0.01 vs. l-arginine LP; $, P < 0.05 vs. control HP; $$, P < 0.001 vs. control; ##, P < 0.001 vs. control HP; $$$, P < 0.05 vs. HP control; †, P < 0.001 vs. antioxidants basal treatment; ††, P < 0.01 vs. l-arginine basal treatment.
Elk-1 and p-CREB basal levels in LP and HP areas were increased by 6 months of hypercholesterolemia (Fig. 2). Elk-1 was increased by ≈70% in LP (P < 0.01 vs. HV) and by 225% in HP (P < 0.001 vs. HV). Similarly, p-CREB was increased by about 60% in LP (P < 0.01 vs. HV) and 190% in HP (P < 0.001 vs. HV). After 1-week treatment with antioxidants alone, both Elk-1 and p-CREB were significantly decreased by 20% in LP areas and by 25% in HP areas (P = NS vs. control LP and P < 0.05 vs. control HP areas) (Fig. 2). The combination of antioxidants and l-arginine, but not l-arginine alone, induced a further 25% reduction of both Elk-1 and p-CREB activities in LP and HP areas when compared with antioxidants alone (P < 0.05) (Fig. 2). Different treatments did not significantly modify the p-Jun protein level, whose basal level was increased especially in the LP areas (Fig. 2)
Effects of Chronic 8-Week Treatment with Antioxidants and/or l-Arginine.
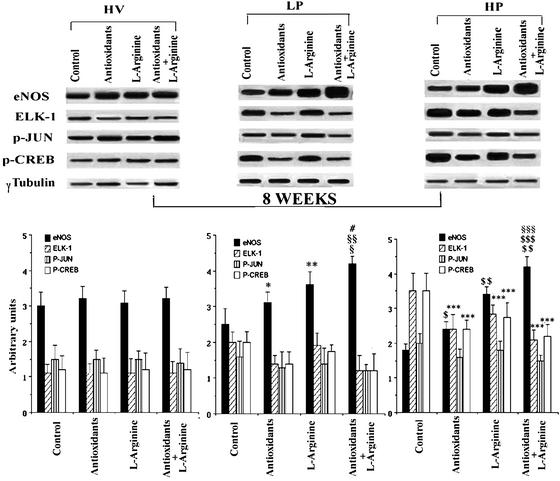
To investigate whether prolonged treatment with antioxidants and/or l-arginine further prevented gene disregulation induced by disturbed shear stress, we analyzed total protein extracts from HV, LP, and HP areas of LDLR−/− mice after 8 weeks of intervention. Consistent with previous studies (14–19), LDLR−/− mice receiving antioxidants and/or l-arginine had reduced atherosclerotic lesions in LP areas. The average area was 6,245 ± 870 μm2 in the antioxidant + l-arginine group; 7,678 ± 1,034 μm2 in the antioxidant group; and 9,005 ± 1,188 μm2 in the l-arginine group (all P < 0.01 vs. the untreated control group that showed an average lesion area of 13,658 ± 1,256 μm2). The same was true in HP areas (88,776 ± 11,239 μm2 in the antioxidant + l-arginine group; 93,456 ± 13,992 μm2 in the antioxidant group; and 95,663 ± 12,555 μm2 in the l-arginine group; all P < 0.01 vs. untreated control group that showed an average lesion area of 139,658 ± 20,855 μm2). When mice were fed a diet enriched with antioxidants alone, the eNOS activity increased significantly in LP and HP areas (P < 0.05, Fig. 3). As expected, eNOS activity also increased significantly in LP and HP areas by using diet enriched with l-arginine (P < 0.01 vs. control and P < 0.01 vs. antioxidants) and was synergistically enhanced by cotreatment with antioxidants and l-arginine (P < 0.01 vs. control, P < 0.01 vs. antioxidants, and P < 0.01 vs. l-arginine).
Figure 3.
Western blot analysis of proteins extracted from LDLR−/− mice aortas after 8 weeks of chronic intervention. (Lower) Corresponding densitometric analysis. *, P < 0.05 vs. control LP; **, P < 0.01 vs. control LP; #, P < 0.001 vs. control LP; §, P < 0.05 vs. l-arginine LP; §§, P < 0.01 vs. antioxidants LP; $, P < 0.05 control HP; $$, P < 0.001 vs. control HP; ***, P < 0.01 vs. control HP; §§§, P < 0.01 vs. antioxidants HP; $$$, P < 0.01 vs. l-arginine HP.
After 8 weeks of treatment with antioxidants alone, both Elk-1 and p-CREB protein levels were slightly decreased in LP areas (approximately −30%, P = NS vs. control LP) and significantly decreased in HP areas (−35 ± 8%, P < 0.05 vs. control HP areas). Combination with antioxidants and l-arginine, but not l-arginine alone, induced a further mean reduction by 38% of both Elk-1 and p-CREB activities in LP and HP areas when compared with antioxidants alone (P < 0.05) (Fig. 3). On the other hand, different treatments did not significantly modify the p-Jun protein level (Fig. 3). Thus, therapeutic effects seen after 1 week were maintained during chronic intervention, although in HP areas an 8-week regimen of antioxidants and l-arginine alone seemed to be more effective in increasing eNOS and decreasing redox gene expression.
Discussion
Our results demonstrate that disturbed shear stress modulates eNOS activity and redox-sensitive transcription factors both in vitro and in vivo and show that prolonged therapeutic intervention with antioxidants and l-arginine can normalize this proatherogenic disequilibrium.
Several studies have established that shear stress can influence transcriptional events in cultured arterial cells (1, 2, 6, 8–12, 29–36). A large body of published data conducted under several experimental settings showed that eNOS expression was steadily increased by shear stress in the range of 1–25 dynes per cm2 (2, 8–12, 29–36). Discrepancy among these studies can be due to different cell types (bovine or human cells), evaluation of eNOS expression mainly by mRNA and not by protein measurements, the overestimation of qualitative analysis of the results in comparison to the use of semiquantitative densitometric analysis, a huge number of devices used for generating forces in vitro, different times of in vitro exposure to shear stress (from 3 to 48 h), and several other experimental peculiarities. Moreover, persistent transduction effects are regulated by a multitude of coregulatory and corepressor mechanisms at the level of transcription (6). Therefore, although all of this information is beyond the scope of the present study, further investigations should elucidate whether shear-stress-dependent changes in e-NOS protein levels can induce transcriptional changes, mRNA stability, and/or coregulatory mechanisms.
Disturbed flow can influence several signal transduction pathways that lead to alteration in expression levels of numerous redox-sensitive genes (2, 5, 6). It has also been demonstrated that turbulent flow increases the oxidative stress induced by EC (6–8, 10, 29). Here, we investigated ELK-1 and p-CREB (redox factors that play a critical role in the growth regulation) (5, 6) and found that expression of these factors was activated by high shear stress. We show that those factors are influenced by turbulent shear stress in hypercholesterolemic mice and that intervention with antioxidants and l-arginine blunted the deleterious effects of disturbed flow, inducing an increase of eNOS activity and preventing the Elk-1 and p-CREB accumulation. Under our experimental conditions, JNK activity was only modestly affected.
In general, areas of flow separation and oscillatory shear stress are the main areas that developed atherosclerotic lesions (2, 6). We observed that eNOS, p-CREB, and Elk-1 were “primed” to respond to systemic activation stimuli in HP vs. LP areas that represent areas with the most and least atherosclerosis in mice and pigs (25–27). We demonstrated that these redox proteins were up-regulated in endothelium of HP atherosclerotic areas in LDLR−/− mice, whereas eNOS activity was relatively decreased. Indeed, if we consider eNOS as an atheroprotective protein (14) and Elk-1, p-Jun, and p-CREB as pro-atherogenic redox-sensitive elements (5, 6), in HP regions the equilibrium between these proteins is shifted in favor of atherogenesis. With therapeutic intervention, we demonstrate that it is possible to attenuate this proatherogenic shift. These results are consistent with the observation that long-term oral administration of l-arginine is associated with a significant improvement in NO-dependent vasodilation in LDLR−/− mice (15, 16). Moreover, l-arginine enhances aerobic exercise capacity as well as NO production in apolipoprotein E knockout mice (37). Other NO-dependent vascular functions are also modulated by chronic supplementation with l-arginine. Indeed, endothelial leukocyte adhesion (15) and platelet aggregation (38, 39) are reduced, and vascular smooth muscle cell proliferation is attenuated (40). Consistent with this finding, an impressive number of clinical studies have reported that l-arginine administration reduces symptoms of coronary heart disease in patients (41–44).
Atherogenic lipids, particularly oxidized LDL (oxLDL), are responsible for a wide range of cellular dysfunctions within the vessel wall (reviewed in refs. 5 and 14). Oxidative modification of LDL plays a pivotal role in human early atherogenesis (45, 46). Concerning the regulation of vascular tone, oxLDL may disturb cellular relaxation functions or act directly against NO (14). oxLDL can down-regulate or uncouple NOS (14, 47), and may induce a decreased endothelial uptake of l-arginine (14). The local depletion of the l-arginine substrate may derange the eNOS, leading to overproduction of reactive oxygen species (ROS) (14). Furthermore, the possible pathogenic role of the competitive inhibitor asymmetric N(G), N(G)-dimethylarginine (ADMA) (14) in high shear stress areas needs further study. In general, a multitude of oxidation-sensitive apoptotic signaling effects can interact with NO in the arterial wall (14). Thus, the balance between NO bioactivity and oxidative stress may play an important role in atherogenesis, and the “l-arginine” and the “oxidation” hypotheses may actually be complementary. The results of the present study support this synergism between antioxidants and l-arginine in reversing the proatherogenic profile induced by high shear stress. Antioxidants present in the vascular wall decrease cellular production and release of ROS, inhibit endothelial activation, and improve the biologic activity of NO through a cell- or tissue-specific antioxidant action (14). However, whereas l-arginine only increased NO formation and reduced superoxide release, antioxidants antagonized many oxidation-sensitive mechanisms in atherogenesis (5, 6). This finding may explain the complementary nature of their interaction, as observed in the current study. The role of antioxidants in human atherosclerosis remains unresolved. Some trials showed clinical benefits (on a composite end-point of nonfatal infarction, stroke, or death from cardiovascular causes) (48, 49), whereas others did not (50–53). LDL oxidation and redox-sensitive mechanisms in the arterial wall (5, 6) already occur in very early lesions in human fetuses and children (45, 46). Antioxidant intervention may affect long-term lesion progression but not necessarily modulate the properties of preexisting advanced atherosclerotic lesions (i.e., coronary heart disease, cerebrovascular disease, and peripheral arterial disease) or reduce the clinical manifestations of plaque rupture (5, 14, 53). Thus, to test whether antioxidants inhibit atherosclerosis it is necessary to investigate the progression of early atherosclerotic lesions in young adults. In addition, such intervention may prevent proatherogenic programming events during fetal development (54).
One of the most important findings from our study is the agreement of in vitro and in vivo findings, and that eNOS protein and several transcription factors downstream of the extracellular signal-regulated kinase (ERK)1/2 pathway, such as Elk-1 and p-CREB, are primed to respond in vivo to shear stress. The involvement of shear-stress-induced p-CREB overexpression was also confirmed in another recent study done in osteoblastic cells (55). We have shown that p-CREB is involved in oxidative stress and apoptosis in human EC (21). Thus, p-CREB-dependent signaling could be a crucial pathway in the development and maintenance of both endothelial function and bone integrity. Moreover, shear-tress inhibits tumor necrosis factor-α activation of JNK but not ERK1/2 or p38 in human umbilical vein EC (56), suggesting a possible inhibitory crosstalk among mitogen-activated protein kinase (MAPK) family members. However, another study revealed that shear stress signal transduction involves MAPK, c-fos, and connexin 43 (31). Finally, serine-threonine kinase, IKK beta, is necessary for the cytokine-induced inflammatory phenotype of human endothelium and endothelial recruitment of human monocytes under laminar flow (57). Further studies are needed to clarify the pathogenic role of multiple kinase-dependent pathways modulated by shear stress.
In summary, synergistic therapeutic intervention with antioxidants and l-arginine can induce a sustained correction of the disturbed shear-stress-induced pro-atherogenic profile. This therapeutic option of modulating early-response genes may have important implications for the pathogenesis and prevention of atherogenesis and its clinical sequelae.
Acknowledgments
This study was supported by National Institutes of Health Grants HL-56989, HL-63282, HL-58433, and HL-66999, and by the Mayo Foundation.
Abbreviations
- eNOS
endothelial NO synthase
- LDL
low-density lipoprotein
- LDLR
LDL receptor
- EC
endothelial cells
- HP
highly prone
- LP
low prone
- HV
healthy vessels
Footnotes
See commentary on page 768.
References
- 1.Buga G M, Gold M E, Fukuto J M, Ignarro L J. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone M A., Jr Am J Pathol. 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman M H, Hutchins G M, Bergerson C B, Deters O J, Mark F F. Atherosclerosis. 1981;39:425–436. doi: 10.1016/0021-9150(81)90027-7. [DOI] [PubMed] [Google Scholar]
- 4.Glagov S, Zarins C, Giddens D P G, Ku D N. Arch Pathol Lab Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 5.Napoli C, de Nigris F, Palinski W. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 6.de Nigris F, Lerman L O, Condorelli M, Lerman A, Napoli C. Antioxid Redox Signal. 2001;3:1119–1130. doi: 10.1089/152308601317203620. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh H J, Cheng C C, Wu S T, Chiu J J, Wung B S, Wang D L. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Chiu J J, Wung B S, Shyy J Y, Hsieh H J, Wang D L. Arterioscler Thromb Vasc Biol. 1997;17:3570–3577. doi: 10.1161/01.atv.17.12.3570. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita S, Inoue N, Ueyama T, Kawashima S, Yokoyama M. Biochem Biophys Res Commun. 2000;273:66–71. doi: 10.1006/bbrc.2000.2898. [DOI] [PubMed] [Google Scholar]
- 10.Silacci P, Desgeorges A, Mazzolai L, Chambaz I, Hayoz D. Hypertension. 2001;38:1162–1166. doi: 10.1161/hy1101.095993. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Gardena G, Comander J, Anderson K R, Blackman B R, Gimbrone M A. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick S M, Eskin S G, McIntire L V, Teng C L, Lu C M, Russell C G, Chittur K K. Proc Natl Acad Sci USA. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ignarro L J, Cirino G, Casini A, Napoli C. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Napoli C, Ignarro L J. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 15.Bonthu S, Heistad D D, Chappell D A, Lamping K G, Faraci F M. Arterioscler Thromb Vasc Biol. 1997;17:2333–23440. doi: 10.1161/01.atv.17.11.2333. [DOI] [PubMed] [Google Scholar]
- 16.Aji W, Ravalli S, Szabolcs M, Jiang X C, Sciacca R R, Michler R E, Cannon P J. Circulation. 1997;95:430–437. doi: 10.1161/01.cir.95.2.430. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S, Shortal B P, Witztum J L, Palinski W. Arterioscler Thromb Vasc Biol. 2000;20:689–697. doi: 10.1161/01.atv.20.3.689. [DOI] [PubMed] [Google Scholar]
- 18.Palinski W, Napoli C, Reaven P D. In: Contemporary Cardiology: Vascular Disease and Injury–Preclinical Research, Harvard Series. Simon D I, Rogers C, editors. Totowa, NJ: Humana; 2000. pp. 149–174. [Google Scholar]
- 19.Napoli C, de Nigris F, Welch J S, Calara F B, Stuart R, Glass C K, Palinski W. Circulation. 2002;105:1360–1367. doi: 10.1161/hc1102.106792. [DOI] [PubMed] [Google Scholar]
- 20.de Nigris F, Youssef T, Ciafré S, Franconi F, Anania V, Condorelli G, Palinski W, Napoli C. Circulation. 2000;102:2111–2117. doi: 10.1161/01.cir.102.17.2111. [DOI] [PubMed] [Google Scholar]
- 21.Napoli C, Quehenberger O, de Nigris F, Abete P, Glass C K, Palinski W. FASEB J. 2000;14:1996–2007. doi: 10.1096/fj.99-0986com. [DOI] [PubMed] [Google Scholar]
- 22.Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. J Physiol. 2000;525:761–770. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napoli C, Cirino G, Del Soldato P, Sorrentino R, Sica V, Condorelli M, Pinto A, Ignarro L J. Proc Natl Acad Sci USA. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calara F, Silvestre M, Casanada F, Napoli C, Palinski W. J Pathol. 2001;195:257–263. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]
- 25.Iiyama K, Hajira L, Iiyama M, Li H, DiChiara M, Medoff B D, Cibulsky M I. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 26.de Nigris F, Lerman L O, Rodriguez-Porcel M, Demontis M P, Lerman A, Napoli C. Biochem Biophys Res Commun. 2001;281:945–950. doi: 10.1006/bbrc.2001.4431. [DOI] [PubMed] [Google Scholar]
- 27.Hajra L, Evans A I, Chen M, Hyduk S J, Collins T, Cybulsky M I. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Nigris F, Franconi F, Maida I, Palumbo G, Anania V, Napoli C. Biochem Pharmacol. 2000;59:1477–1487. doi: 10.1016/s0006-2952(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 29.Topper J N, Cai J, Falb D, Gimbrone M A., Jr Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y S, Shyy Y J, Li S, Lee J, Su B, Karin M, Chien S. Mol Cell Biol. 1996;16:5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao X, Clark C B, Frangos J A. Am J Physiol. 2000;278:H1598–H1605. doi: 10.1152/ajpheart.2000.278.5.H1598. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu M, Ohara Y, Navas J P, Nishida K, Murphy T J, Alexander R W, Nerem R M, Harrison D G. Am J Physiol. 1995;269:C1371–C1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 33.Silacci P, Formentin K, Bouzourene K, Daniel F, Brunner H R, Hayoz D. Nitric Oxide. 2000;4:47–56. doi: 10.1006/niox.2000.0271. [DOI] [PubMed] [Google Scholar]
- 34.Drummond G R, Cai H, Davis M E, Ramasamy S, Harrison D G. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 35.Davis M E, Cai H, Drummond G R, Harrison D G. Circ Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 36.Boo Y C, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 37.Maxwell A J, Ho H V, Le C Q, Lin P S, Bernstein D, Cooke J P. J Appl Physiol. 2001;90:933–938. doi: 10.1152/jappl.2001.90.3.933. [DOI] [PubMed] [Google Scholar]
- 38.Boger R H, Bode-Boger S M, Phivthong-Ngam L, Brandes R P, Schwedhelm E, Mugge A, Bohme M, Tsikas D, Frolich J C. Atherosclerosis. 1998;141:31–43. doi: 10.1016/s0021-9150(98)00145-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsao P S, Theilmeier G, Singer A H, Leung L L, Cooke J P. Arterioscler Thromb. 1994;14:1529–1533. doi: 10.1161/01.atv.14.10.1529. [DOI] [PubMed] [Google Scholar]
- 40.Bode-Boger S M, Boger R H, Kienke S, Bohme M, Phivthong-Ngam L, Tsikas D, Frolich J C. Cardiovasc Res. 1998;37:756–764. doi: 10.1016/s0008-6363(97)00295-2. [DOI] [PubMed] [Google Scholar]
- 41.Cooke J P, Oka R K. Curr Atheroscler Rep. 2001;3:252–259. doi: 10.1007/s11883-001-0068-x. [DOI] [PubMed] [Google Scholar]
- 42.Tousoulis D, Davies G J, Tentolouris C, Crake T, Katsimaglis G, Stefanadis C, Toutouzas P. Am J Cardiol. 1998;82:1110–1113. doi: 10.1016/s0002-9149(98)00563-3. [DOI] [PubMed] [Google Scholar]
- 43.Fujita H, Yamabe H, Yokoyama M. J Nucl Cardiol. 2000;7:97–102. doi: 10.1016/s1071-3581(00)90028-x. [DOI] [PubMed] [Google Scholar]
- 44.Blum A, Hathaway L, Mincemoyer R, Schenke W H, Kirby M, Csako G, Waclawiw M A, Panza J A, Cannon R O., III Circulation. 2000;101:2160–2164. doi: 10.1161/01.cir.101.18.2160. [DOI] [PubMed] [Google Scholar]
- 45.Napoli C, D'Armiento F P, Witztum J L, Mancini F P, Postiglione A, Palumbo G, Palinski W. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napoli C, Glass C K, Witztum J L, Deutch R, D'Armiento F P, Palinski W. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 47.Pritchard K A, Jr, Groszek L, Smalley D M, Sessa W C, Wu M, Villalon P, Wolin M S, Stemerman M B. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 48.Stephens N G, Parsons A, Schofield P M, Kelly F, Cheeseman K, Mitchinson M J. Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 49.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green M S. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 50.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico–Prevenzione Investigators. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 51.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 52.Pryor W A. Free Radical Biol Med. 2000;28:141–164. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg D, Witztum J L. Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 54.Napoli C, Palinski W. Eur Heart J. 2001;22:4–9. doi: 10.1053/euhj.2000.2147. [DOI] [PubMed] [Google Scholar]
- 55.Ogasawara A, Arakawa T, Kaneda T, Takuma T, Sato T, Kaneko H, Kumegawa M, Hakeda Y. J Biol Chem. 2001;276:7048–7054. doi: 10.1074/jbc.M008070200. [DOI] [PubMed] [Google Scholar]
- 56.Surapistchat J, Hoefen R J, Pi X, Toshizumi M, Yan C, Berk B. Proc Natl Acad Sci USA. 2001;98:6476–6481. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meiler S E, Hung R R, Gerszten R E, Gianetti J, Li L, Matsui T, Gimbrone M A, Jr, Rosenzweig A. J Mol Cell Cardiol. 2002;34:349–359. doi: 10.1006/jmcc.2001.1519. [DOI] [PubMed] [Google Scholar]