Abstract
Endothelial cells in most vascular beds release a factor that hyperpolarizes the underlying smooth muscle, produces vasodilatation, and plays a fundamental role in the regulation of local blood flow and systemic blood pressure. The identity of this endothelium-derived hyperpolarizing factor (EDHF), which is neither NO nor prostacyclin, remains obscure. Herein, we demonstrate that in mesenteric resistance arteries, release of C-type natriuretic peptide (CNP) accounts for the biological activity of EDHF. Both produce identical smooth muscle hyperpolarizations that are attenuated in the presence of high [K+], the Gi G protein (Gi) inhibitor pertussis toxin, the G protein-gated inwardly rectifying K+ channel inhibitor tertiapin, and a combination of Ba2+ (inwardly rectifying K+ channel blocker) plus ouabain (Na+/K+-ATPase inhibitor). Responses to EDHF and CNP are unaffected by the natriuretic peptide receptor (NPR)-A/B antagonist HS-142-1, but mimicked by the selective NPR-C agonist, cANF4–23. EDHF-dependent relaxation is concomitant with liberation of endothelial CNP; in the presence of the myoendothelial gap-junction inhibitor 18α-glycyrrhetinic acid or after endothelial denudation, CNP release and EDHF responses are profoundly suppressed. These data demonstrate that acetylcholine-evoked release of endothelial CNP activates NPR-C on vascular smooth muscle that via a Gi coupling promotes Ba2+/ouabain-sensitive hyperpolarization. Thus, we have revealed the identity of EDHF and established a pivotal role for endothelial-derived CNP in the regulation of vascular tone and blood flow.
The endothelium releases a number of vasorelaxant factors, including NO and prostacyclin (PGI2), which play a fundamental role in the regulation of vascular tone, local blood flow, and systemic blood pressure. In many arteries, however, particularly those in the resistance vasculature, a significant endothelium-dependent relaxation remains despite blockade of NO and PGI2 synthesis. The mediator responsible for this activity has been termed endothelium-derived hyperpolarizing factor (EDHF) (1–4), because this component of endothelium-dependent relaxation is associated with smooth muscle hyperpolarization. Despite considerable attention, the identity of this factor(s) remains obscure, with potential candidates as diverse as cytochrome P450 (2C8/34) products [e.g., 11,12-epoxyeicosatrienoic acid (11,12-EET); ref. 1], anandamide (5), and K+ ions (4). Investigation of the physiological importance of EDHF has been hampered not only by controversy over its identity, but also by the lack of selective inhibitors of its activity. Only recently has it been accepted that “the hallmark of an EDHF-mediated response” (6) is the blockade of its release by a combination of the calcium-dependent K+ channel blockers charybdotoxin (CTx) plus apamin (7). In addition, there is considerable support for the concept that EDHF-dependent smooth muscle hyperpolarization is prevented by barium [Ba2+; inwardly rectifying K+ (KIR) channel inhibitor] plus ouabain (Na+-K+ ATPase inhibitor; refs. 4, 8, and 9). Because the importance of EDHF as an endothelial-derived vasorelaxant appears to be inversely related to vessel diameter, with the greatest activity being found in the resistance vasculature (10), EDHF is likely to be fundamental to the regulation of local blood flow and blood pressure. Moreover, altered EDHF responses may contribute to, or compensate for, the endothelial dysfunction associated with the pathogenesis of a number of cardiovascular diseases, such as atherosclerosis, hypertension, and sepsis. Therefore, identification of EDHF, and selective activators or inhibitors of its biological activity, will have a significant impact on our understanding of cardiovascular (patho)physiology and may provide the rationale for the design of novel therapeutics.
C-type natriuretic peptide (CNP) belongs to a family of vasoactive peptides [including atrial natriuretic peptide (ANP); brain natriuretic peptide (BNP), and urodilatin] that have vasodilator and diuretic properties and play an important role in cardiovascular homeostasis (11–13). This 22-aa peptide is widely distributed in the cardiovascular system, being found in particularly high concentrations in vascular endothelial cells (11). CNP is a potent relaxant of vascular smooth muscle, particularly in the coronary circulation (14, 15), and also inhibits smooth muscle cell proliferation (16) and aldosterone production (17, 18). The cardiovascular actions of CNP are mediated via activation of the NPR subtypes, NPR-B (for which it is the preferential ligand) and NPR-C (19), the latter accounting for ≈95% of NPRs expressed in vivo (20). Moreover, recent evidence suggests that the mechanism by which CNP mediates vasorelaxation (including human vessels) is via an NPR-mediated hyperpolarization of vascular smooth muscle, because responses are sensitive to blockade by tetraethylammonium (21).
Therefore, because CNP is stored by vascular endothelial cells, NPR-B and NPR-C are found in abundance on the underlying smooth muscle, and CNP-mediated vasorelaxation involves hyperpolarization, we investigated the hypothesis that CNP is EDHF in mesenteric resistance arteries.
Materials and Methods
All experiments were conducted according to the Animals (Scientific Procedures) Act 1986, United Kingdom.
Materials.
All drugs used were obtained from Sigma except apamin (Calbiochem), cANF4–23 (Peninsula Labs, San Carlos, CA), CNP (Calbiochem), CTx (Calbiochem), HS-142-1 (kind gift of Matsuda, Kyowa Hakko Kogyo, Tokyo), tertiapin (Alomone Labs, Jerusalem), and U-46619 (Biomol, Plymouth Meeting, PA). U-46619 was dissolved in ethanol and then diluted in saline, CNP and cANF4–23 were dissolved in 5% acetic acid and diluted in saline, Indomethacin was dissolved in 5% NaHCO3 and diluted in saline, and all other drugs were prepared using saline (0.9%).
Isolated Mesenteric Artery.
Male rats (Sprague–Dawley; ≈250 g) were culled by stunning and cervical dislocation and the mesentery removed and placed in cold Krebs solution of composition (mM) 119 NaCl/4.7 KCl/2.5 CaCl2/1.2 MgSO4/25 NaHCO3/1.2 KH2PO4/5.5 glucose. Third-order arteries were cleaned of surrounding fat and mounted in a tension myograph containing Krebs solution maintained at 37°C and gassed with 5% CO2 in O2.
After a 60-min wash period, mesenteric arteries were normalized using standard procedures (22). Subsequently, the resistance arteries were repeatedly contracted with the thromboxane-A2 mimetic U46619 (1 μM) until responses were reproducible. Relaxation of U46619 (1 μM)-precontracted vessels to acetylcholine (Ach) (10 μM) was used to determine endothelial integrity (vessels that relaxed by at least 50% were deemed endothelium-intact). Arteries were incubated with NG-nitro-l-arginine methyl-ester (l-NAME) (300 μM) and indomethacin (5 μM) for 30 min before being contracted with an approximate EC50 concentration of U-46619 (10–100 nM) and cumulative concentration–response curves constructed to ACh (0.001–10 μM), CNP (0.001–1 μM), cANF4–23 (0.001–1 μM), or spermine-NONOate (SPER-NO) (0.01–30 μM).
Electrophysiology.
Small mesenteric arteries were mounted in a tension myograph, normalized, and equilibrated using U46619 as described above. Vessels were incubated with l-NAME (300 μM) and indomethacin (5 μM) for 30 min before being contracted with an approximate EC50 concentration of U-46619 (10–300 nM). On attainment of a stable plateau, a single concentration of ACh (10 μM) or CNP (1 μM) was added directly to the bath. Membrane potential was measured continuously throughout the course of the experiment by using aluminium silicate microelectrodes (1 mm in diameter, World Precision Instruments, Sarasota, FL) that had resistances between 50 and 90 MΩ when filled with 3 M KCl. Membrane potential (mV) was measured using an oscilloscope (Gould-Nicolet Technologies, Essex, U.K.) connected to an amplifier (Intra 767 electrometer, World Precision Instruments) and recorded on a chart recorder (BBC Goerz Metrawatt, Wiener Neudorf, Austria). Electrode entry into a vascular smooth muscle cell was determined by an abrupt drop in voltage, followed by a sharp return to baseline on exit, with a minimal change (no more than 10%) in resistance (23).
CNP Bioassay.
Rats were heparinized and killed by cervical dislocation. An abdominal midline incision was made and the superior mesenteric artery isolated and cannulated, before being perfused with 10 ml of warmed Krebs solution to eliminate blood. After cannulation of the superior mesenteric artery, the entire mesenteric bed was excised and placed in a 37°C water-jacketed organ bath, perfused at a constant rate of 4 ml/min with 37°C Krebs solution containing l-NAME (300 μM) and indomethacin (5 μM), and oxygenated with 5% CO2 in O2 by using a roller pump (Miniplus 2, Gilson). The preparation was covered with a piece of parafilm to prevent drying and maintain temperature. Perfusion pressure [basal = 20.9 ± 3.5 mmHg (1 mmHg = 133 Pa); n = 16] was measured by attaching the cannula to an in-line transducer (P23XL, Becton Dickinson) and was continuously recorded on a PC using chart software (AD Instruments, Castle Hill, Australia). Because flow rate is kept constant throughout the experiment, changes in pressure reflect alterations in vascular resistance. The preparation was equilibrated for 45 min before being exposed to 1 μM U46619; after washout for 45 min, U46619 (1 μM) was readministered and endothelial integrity tested using ACh (10 μM). Preparations that did not relax by at least 50% were excluded. Endothelial denudation was achieved by perfusing with 0.1% Triton X-100 for 30 s. Subsequently, vascular smooth muscle and endothelial integrity were tested by addition of U46619 (1 μM) and ACh (1 μM), respectively (endothelial denudation was deemed to have been achieved if responses to ACh were <10% of controls).
A single concentration of CNP (1 μM) or ACh (10 μM; both ≈EC95) was perfused into individual tissue preparations in the absence and presence of endothelial denudation or 18α-glycyrrhetinic acid (18α-GA) (100 μM) and the relaxant responses observed. During the course of the relaxant response to each compound the effluent from the tissue was collected (≈10 ml) into a vial containing the protease inhibitor aprotinin (0.6 units/ml) and stored immediately at −20°C.
To assay for CNP, 3-ml C18AR columns (DRG Diagnostics, Marburg, Germany) were equilibrated with 1.5 ml of buffer A (1% trifluoroacetic acid; HPLC grade), each sample applied to a separate column and allowed to flow through by gravity. Columns were washed twice with 1.5 ml of buffer A and the CNP eluted twice with 1 ml of buffer B (60% acetonitrile in 1% trifluoroacetic acid). The eluate was then evaporated to dryness at 37°C under N2 and the residue dissolved in 220 μl of RIA buffer and CNP concentrations, according to the manufacturer's instructions [RIA not cross-reactive with atrial natriuretic peptide (ANP) or brain natriuretic peptide (BNP); DRG Diagnostics].
Calculations and Statistics.
Relaxations are expressed as percentage reversal of induced tone. All data are shown as mean ± SEM. Tests of significance between curves were conducted using two-way ANOVA for multiple comparisons or Student's t test for differences between two data groups, where P < 0.05 was considered significant. The n values quoted similarly indicate the number of experiments and animals used.
Results
Tension myography was used to investigate the vasoreactivity of mesenteric arteries (≈200 μm in diameter) that exhibit archetypal EDHF responses (4, 24–28). A direct comparison was made between the vasorelaxant activity of EDHF, elicited by the administration of ACh, and CNP. All experiments were conducted in the presence of the NO synthase inhibitor, l-NAME (300 μM), and cyclooxygenase inhibitor, indomethacin (5 μM), to abolish responses to NO and PGI2 and, therefore, reveal EDHF-dependent relaxations.
Effect of Inhibitors of EDHF on CNP-Induced Vasorelaxation.
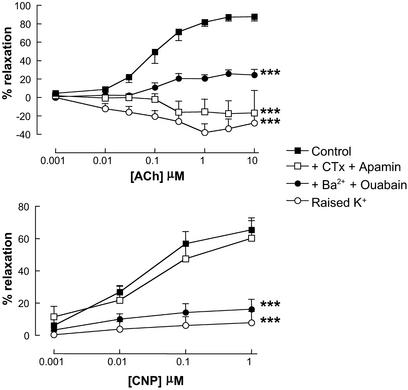
ACh (0.001–10 μM)-induced relaxations were inhibited by a combination of CTx (100 nM) plus apamin (100 nM), Ba2+ (30 μM) plus ouabain (1 mM), high [K+] (30 mM; all Fig. 1), and endothelial denudation (data not shown). CNP (0.001–1 μM)-dependent relaxations were also attenuated by Ba2+ plus ouabain and high [K+] but were unaffected in the presence of CTx plus apamin (all Fig. 1) or by endothelial denudation (data not shown).
Figure 1.
Responses to EDHF and CNP are similarly attenuated by barium plus ouabain and high [K+]. Relaxation of rat isolated mesenteric artery by ACh (0.001–10 μM; Upper) and CNP (0.001–1 μM; Lower) in the absence or presence of high [K+] (30 mM), CTx (100 nM) plus apamin (100 nM), or Ba2+ (30 μM) plus ouabain (1 mM). Data are presented as mean ± SEM; n ≥ 5. ***, P < 0.05; significantly different from control.
Involvement of NPR-C and G Protein-Gated Inwardly Rectifying K+ Channels (GIRKs) in the Mechanism of Action of EDHF and CNP.
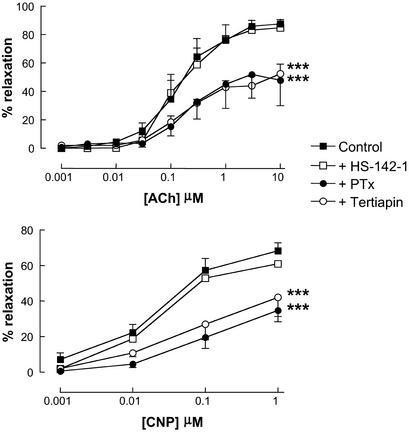
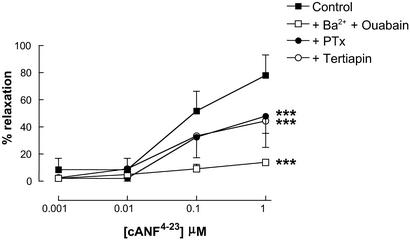
To determine the NPR subtype activated by CNP (and potentially EDHF) to mediate smooth muscle relaxation, the selective NPR-A/B antagonist HS-142-1 was used. Responses to both CNP and EDHF were unaffected by HS-142-1 (10 μM; Fig. 2), at concentrations that significantly attenuated relaxations to ANP (1–100 nM; data not shown). This suggests that the mechanism used by CNP (and possibly EDHF) to elicit smooth muscle relaxation is the non-guanylate cyclase-linked NPR-C. To provide further evidence that NPR-C is involved in the Ba2+/ouabain-sensitive relaxation elicited by CNP (and EDHF), the selective NPR-C agonist cANF4–23 was used. cANF4–23 (0.001–1 μM) produced concentration-dependent relaxations of the mesenteric arteries that were equipotent with responses to CNP and inhibited by Ba2+ (30 μM) plus ouabain (1 mM; Fig. 3).
Figure 2.
Responses to EDHF and CNP are similarly attenuated by pertussis toxin (PTx) and tertiapin. Shown is relaxation of rat isolated mesenteric artery by ACh (0.001–10 μM; Upper) and CNP (0.001–1 μM; Lower) in the absence or presence of HS-142-1 (30 μM), PTx (400 ng/ml), or tertiapin (10 μM). Data are presented as mean ± SEM; n ≥ 5. ***, P < 0.05; significantly different from control.
Figure 3.
Responses to cANF4–23 are attenuated by barium plus ouabain, PTx, and tertiapin. Shown is relaxation of rat isolated mesenteric artery by cANF4–23 (0.001–1 μM) in the absence or presence of Ba2+ (30 μM) plus ouabain (1 mM), PTx (400 ng/ml), or tertiapin (10 μM). Data are presented as mean ± SEM; n ≥ 5. ***, P < 0.05; significantly different from control.
Because the NPR-C has been postulated to act as an effector pathway involving activation of Gi G protein (Gi) (29, 30), we conducted similar experiments in the presence of the Gi inhibitor PTx (400 ng/ml). Responses to EDHF, CNP, and cANF4–23 were similarly inhibited in the presence of PTx (Figs. 2 and 3), supporting our hypothesis that Gi-coupled NPR-C activation is involved in EDHF/CNP-mediated smooth muscle relaxation.
Attenuation of EDHF and CNP responses by PTx intimates that these relaxations are brought about by NPR-C activation and Gi coupling to a smooth muscle K+ channel. To explore this thesis, we used the selective G protein-gated inwardly rectifying K+ channel (GIRK) inhibitor tertiapin (31). Relaxations induced by EDHF, CNP, and cANF4–23 were significantly attenuated in the presence of tertiapin (10 μM; Figs. 2 and 3).
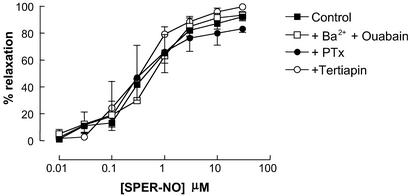
In control experiments, relaxant responses to spermine-NONOate (SPER-NO) (0.01–30 μM; releases NO spontaneously in aqueous solution) were unaffected by Ba2+/ouabain, PTx, or tertiapin (Fig. 4).
Figure 4.
Responses to SPER-NO are unaffected by barium plus ouabain, PTx, and tertiapin. Relaxation of rat isolated mesenteric artery by SPER-NO (0.01–30 μM) in the absence or presence of Ba2+ (30 μM) plus ouabain (1 mM), PTx (400 ng/ml), or tertiapin (10 μM). Data are presented as mean ± SEM; n ≥ 5.
Electrophysiological Comparison of Responses to EDHF and CNP.
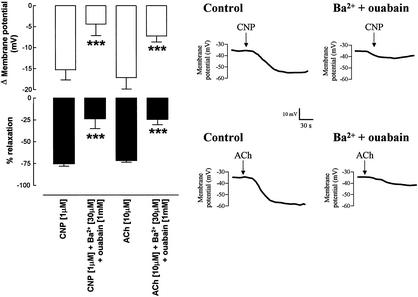
To investigate whether the hyperpolarizations evoked by CNP and EDHF were synonymous, microelectrode membrane potential measurements were made in situ in intact rat mesenteric vessels such that membrane potential and tension could be measured simultaneously. Resting membrane potential in these arteries was −54.5 ± 1.2 mV (n = 7) and increased to −35.5 ± 1.6 mV (n = 7) after addition of the vasoconstrictor U46619 (approximate EC50 = 10–100 nM). Addition of CNP (1 μM) caused a marked hyperpolarization of the tissue (Fig. 5). However, in the presence of Ba2+ (30 μM) plus ouabain (1 mM), both the relaxation and hyperpolarization elicited by CNP were significantly attenuated (Fig. 5). An identical pattern of activity was observed for EDHF-dependent responses elicited by ACh (10 μM; Fig. 5).
Figure 5.
EDHF and CNP produce indistinguishable hyperpolarization of mesenteric vascular smooth muscle. (Left) Membrane potential measurement and relaxation of rat mesenteric vascular smooth muscle elicited by CNP (1 μM) and ACh (10 μM) in the absence and presence of Ba2+ (30 μM) plus ouabain (1 mM). Data are represented as mean ± SEM; n ≥ 5. ***, P < 0.05; significantly different from corresponding control. (Right) Representative membrane potential recordings in rat mesenteric vascular smooth muscle in response to CNP (1 μM) and ACh (10 μM) in the absence and presence of Ba2+ (30 μM) plus ouabain (1 mM). Recordings are representative of at least three separate measurements.
Bioassay of EDHF/CNP Release from the Mesenteric Vascular Bed.
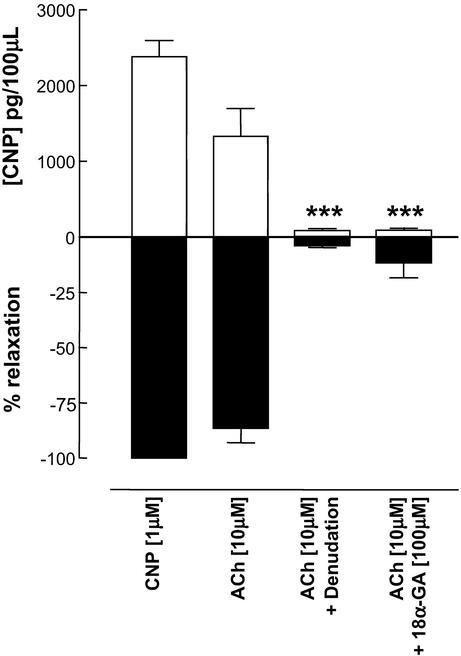
To ascertain whether ACh was able to evoke the release of CNP from vascular endothelial cells, perfused rat mesenteric preparations were set up in vitro in the presence of NO synthase and cyclooxygenase inhibitors (l-NAME, 300 μM, and indomethacin, 5 μM). In U-44619-preconstricted preparations (approximate EC50 = 10–100 nM) a maximal concentration of ACh (10 μM) was administered and the effluent from the tissue collected during the time course of the relaxant response and assayed for CNP. This process was repeated in denuded tissues and in the presence of the myoendothelial gap junction inhibitor 18α-GA (100 μM; refs. 27, 32, and 33). ACh elicited EDHF-dependent relaxations that were associated with the release of CNP. In the absence of endothelium or in the presence of 18α-GA, the release of CNP was abolished and the EDHF-dependent relaxation attenuated (Fig. 6). A maximal concentration of CNP (1 μM) was also administered to certain tissues as a positive control (Fig. 6).
Figure 6.
EDHF-mediated relaxations depend on the release of CNP. Bioassay of CNP released from perfused rat mesenteric preparations and associated decreases in vascular perfusion pressure in response to authentic CNP (1 μM) and EDHF (ACh; 10 μM) under control conditions, after endothelial denudation, or in the presence of 18α-GA (100 μM). Data are presented as mean ± SEM; n ≥ 4. ***, P < 0.05, significantly different from control (ACh; 10 μM).
Discussion
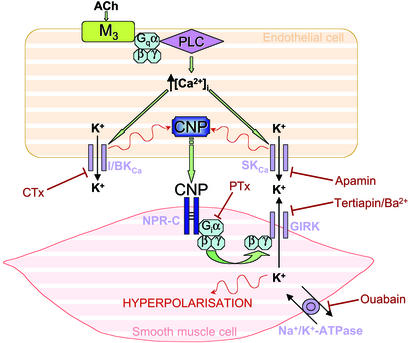
The contribution of EDHF to endothelium-dependent relaxation increases as the vessel size decreases, suggesting that this unidentified factor is likely to play a pivotal role in the regulation of local blood flow within the resistance vasculature, in tissue perfusion, and in systemic blood pressure. Moreover, in vascular disorders characterized by endothelial dysfunction it is unclear whether altered EDHF activity may contribute to pathogenesis or, indeed, represent an important compensatory mechanism to offset the loss of other endothelial-derived vasorelaxant factors such as NO and PGI2. Thus, the need to reveal the identity of EDHF and selective modulators of its biological activity is paramount. In the present study, we have demonstrated that in the mesenteric vasculature the release of CNP accounts for the biological activity of EDHF; thus, we have revealed the identity of EDHF and assigned a pivotal role to endothelial-derived CNP in the regulation of vascular tone and blood flow. Moreover, we have identified a mechanism by which EDHF/CNP mediates smooth muscle hyperpolarization and relaxation; that is, via the activation of NPR-C and the opening of a GIRK (Fig. 7).
Figure 7.
Schematic representation of CNP/EDHF-mediated vascular smooth muscle hyperpolarization. Endothelial release of CNP results in activation of NPR-C on the vascular smooth muscle that, via a Gi coupling, leads to activation of a novel GIRK and hyperpolarization. I/BKCa, intermediate/large-conductance Ca2+-activated K+ channel; PLC, phospholipase C; SKCa, small-conductance Ca2+-activated K+ channel.
In mesenteric arteries, EDHF-mediated responses were blocked by endothelial denudation and in the presence of CTx plus apamin and Ba2+ plus ouabain. Likewise, responses to CNP were attenuated in the presence of Ba2+ plus ouabain but unaltered by CTx plus apamin or endothelial denudation. These observations are in accord with the findings of Edwards et al. (4) and others (8, 25), who proposed that EDHF release from endothelial cells depends on the activity of Ca2+-activated K+ channels (inhibited by CTx and apamin) and vascular smooth muscle hyperpolarization elicited by EDHF depends on inwardly rectifying K+ (KIR) channels and Na+/K+-ATPase (blocked by Ba2+ and ouabain, respectively). The observation that responses to exogenous CNP were only blocked by the latter combination (because exogenous CNP circumvents the requirement for endothelial release) hinted that the mechanism(s) underlying vascular smooth muscle relaxation by EDHF and CNP was similar.
The biological actions of CNP are mediated via activation of specific NPRs, namely the NPR-B and -C subtypes. To determine which receptor is involved in the vasorelaxant responses to CNP (and potentially EDHF) in the mesenteric vasculature, we used the NPR-A/NPR-B antagonist HS-142-1 and the selective NPR-C agonist cANF4–23. Responses to both EDHF and CNP were unaffected by HS-142-1, suggesting that NPR-C is the predominant subtype involved in the response. Because an NPR-C antagonist has not been identified, we used the selective agonist cANF4–23 to elicit NPR-C activation. cANF4–23 mimicked responses to both EDHF and CNP and was sensitive to blockade by Ba2+ plus ouabain. These observations intimate that the smooth muscle relaxations elicited by EDHF and CNP depend on activation of NPR-C.
Until recently, NPR-C had been postulated to act simply as a “clearance receptor,” binding natriuretic peptides, then internalizing and removing these vasoactive agents from the circulation (11). However, it is now appreciated that the intracellular C-terminal (37 aa) portion of the protein has a consensus sequence that interacts with Gi (29), and this has been shown to regulate adenylate cyclase and phospholipase C activity (30, 34, 35). To determine whether such an effector pathway was responsible for EDHF- and CNP-mediated vascular smooth muscle relaxation, we conducted experiments in the presence of the Gi inhibitor PTx. Responses to EDHF, CNP, and cANF4–23 were all inhibited in the presence of PTx, supporting our hypothesis that Gi-coupled NPR-C activation is involved in EDHF/CNP-mediated smooth muscle relaxation. Moreover, this finding provided further evidence that the mechanism of smooth muscle relaxation produced by EDHF and CNP is indistinguishable.
The observation that responses to EDHF and CNP are inhibited by PTx, which selectively inhibits Gi [endothelial M3 receptors activated by ACh to release EDHF remain active because they are coupled to Gq (36, 37)], intimates that EDHF- and CNP-mediated relaxations are brought about by NPR-C activation and Gi coupling to a smooth muscle K+ channel. The phenomenon of GIRKs, which are regulated by the βγ subunits of Gi, is well established in neuronal cells (38) and cardiomyocytes (39); such channels have similar characteristics to those mediating vascular smooth muscle hyperpolarization in response to EDHF and are inhibited by Ba2+. Thus, we hypothesized that activation of NPR-C by EDHF/CNP results in Gi-dependent activation of a (potentially novel) GIRK in the vascular smooth muscle to bring about hyperpolarization and, thereby, relaxation. To explore this thesis, we used the selective GIRK inhibitor tertiapin (31). Relaxations induced by EDHF, CNP, and cANF4–23 were significantly attenuated in the presence of tertiapin, confirming our proposal that a GIRK is involved in EDHF/CNP-mediated vascular smooth muscle relaxation. Such observations provide yet further evidence that EDHF and CNP are identical in mesenteric resistance arteries.
To investigate whether the hyperpolarizations evoked by CNP were identical to the well characterized hyperpolarizations to EDHF (1, 4), membrane potential measurements were made in situ in intact rat mesenteric vessels. Exogenous CNP caused a marked hyperpolarization of the tissue that was accompanied by smooth muscle relaxation. However, in the presence of Ba2+ plus ouabain, both the relaxation and hyperpolarization elicited by CNP were significantly attenuated. An identical response profile was observed for EDHF-dependent hyperpolarization and smooth muscle relaxation, in terms of time-course, magnitude, and sensitivity to Ba2+ plus ouabain. These data demonstrate that EDHF- and CNP-induced vascular smooth muscle hyperpolarization are indistinguishable.
If the biological activity of EDHF is accounted for by the actions of CNP, then ACh must be able to evoke the release of CNP from vascular endothelial cells. To establish whether this criterion was fulfilled, perfused rat mesenteric preparations were set up in vitro in the presence of NO synthase and cyclooxygenase inhibitors. ACh elicited EDHF-dependent relaxations that were dependent on the release of CNP. In the absence of endothelium or in the presence of 18α-GA [this compound has been used widely in the study of EDHF biological activity and is thought to prevent the movement of EDHF itself, or the electrotonic current elicited by EDHF, between the endothelium and vascular smooth muscle (27, 33)], the release of CNP was abolished and the EDHF-dependent relaxation attenuated. These observations confirmed that ACh elicits the release of CNP from the vascular endothelium and that agents/procedures that prevent CNP efflux likewise inhibit EDHF-mediated responses. Moreover, the inhibitory activity of 18α-GA suggests that the mechanism underlying release of EDHF/CNP from the endothelium is coupled intrinsically to the activity of the myoendothelial gap junctions.
The identification of EDHF as CNP has significant implications not only for understanding of the regulation of cardiovascular homeostasis but also knowledge and treatment of cardiovascular pathologies. CNP possesses a number of cardiovascular actions that combine to exert a potent antiatherogenic influence. This study has defined an important role for endothelial-derived CNP in the regulation of vascular smooth muscle tone and local blood flow; CNP is also a potent inhibitor of vascular smooth muscle mitogenesis (16, 40), and NPR activation has been shown to regulate the reactivity of leukocytes (41, 42). Moreover, CNP mRNA is increased in response to shear stress (43, 44), and plasma levels of CNP are elevated in inflammatory cardiovascular pathologies (45). Thus, EDHF/CNP is likely to maintain a substantial cytoprotective effect on the blood vessel wall, similar to that exerted by EDRF (i.e., NO). Consequently, loss of endothelial-derived CNP may be as an important contributor to the pathogenesis of diseases such as atherosclerosis as deficiencies in NO. Moreover, addition of exogenous CNP, or therapeutics mimicking its biological activity, may prove important new medicines for the treatment of cardiovascular disorders.
In sum, our observations demonstrate that the vascular smooth muscle hyperpolarization and vasorelaxation elicited by EDHF and CNP are indistinguishable and therefore assign a pivotal role to endothelial-derived CNP in the regulation of vascular tone and blood flow. Moreover, we have identified a mechanism responsible for EDHF/CNP-mediated hyperpolarization involving activation of NPR-C and the opening of a smooth muscle GIRK. The conclusion that CNP accounts for the biological activity of EDHF in rat arteries is likely to represent a specieswide phenomenon, because CNP has been identified in the vascular endothelium, and shown to hyperpolarize vascular smooth muscle, of several species including humans (11). Thus, the identification of EDHF, and selective activators or inhibitors of its biological activity, is likely to have a major impact on our understanding of cardiovascular homeostasis and may prove as significant as the discovery of NO and PGI2.
Acknowledgments
A.J.H. is the recipient of a Wellcome Trust Senior Research Fellowship. S.D.C. was supported by a British Heart Foundation Ph.D. Studentship.
Abbreviations
- CNP
C-type natriuretic peptide
- EDHF
endothelium-derived hyperpolarizing factor
- GIRK
G protein-gated inwardly rectifying K+ channel
- NPR
natriuretic peptide receptor
- ACh
acetylcholine
- CTx
charybdotoxin
- PTx
pertussis toxin
- l-NAME
NG-nitro-l-arginine methyl ester
- 18α-GA
18α-glycyrrhetinic acid
- PGI2
prostacyclin
- Gi
Gi G protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fisslthaler B, Popp R, Kiss L, Potente M, Harder D R, Fleming I, Busse R. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 2.Garland C J, Plane F, Kemp B K, Cocks T M. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Suzuki H, Weston A H. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards G, Dora K A, Gardener M J, Garland C J, Weston A H. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 5.Randall M D, Alexander S P, Bennett T, Boyd E A, Fry J R, Gardiner S M, Kemp P A, McCulloch A I, Kendall D A. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- 6.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte P M, Weston A H. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 7.Garland C J, Plane F. In: Endothelium-Derived Hyperpolarizing Factor. Vanhoutte P M, editor. London: Harwood; 1996. pp. 173–179. [Google Scholar]
- 8.Hoeffner U, Feletou M, Flavahan N A, Vanhoutte P M. Am J Physiol. 1989;257:H330–H333. doi: 10.1152/ajpheart.1989.257.1.H330. [DOI] [PubMed] [Google Scholar]
- 9.Zygmunt P M, Hogestatt E D. Br J Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimokawa H, Yasutake H, Fujii K, Owada M K, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, et al. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Chen H H, Burnett J C., Jr J Cardiovasc Pharmacol. 1998;32:S22–S28. [PubMed] [Google Scholar]
- 12.Maack T. Kidney Int. 1996;49:1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 13.Melo L G, Veress A T, Ackermann U, Sonnenberg H. Am J Physiol. 1998;275:H1826–H1833. doi: 10.1152/ajpheart.1998.275.5.H1826. [DOI] [PubMed] [Google Scholar]
- 14.Wei C M, Hu S, Miller V M, Burnett J C., Jr Biochem Biophys Res Commun. 1994;205:765–771. doi: 10.1006/bbrc.1994.2731. [DOI] [PubMed] [Google Scholar]
- 15.Wei C M, Aarhus L L, Miller V M, Burnett J C., Jr Am J Physiol. 1993;264:H71–H73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- 16.Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1991;177:927–931. doi: 10.1016/0006-291x(91)90627-j. [DOI] [PubMed] [Google Scholar]
- 17.Hunt P J, Richards A M, Espiner E A, Nicholls M G, Yandle T G. J Clin Endocrinol Metab. 1994;78:1428–1435. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- 18.Igaki T, Itoh H, Suga S, Hama N, Ogawa Y, Komatsu Y, Mukoyama M, Sugawara A, Yoshimasa T, Tanaka I, et al. Kidney Int. 1996;55:S144–S147. [PubMed] [Google Scholar]
- 19.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, et al. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 20.He X, Chow D, Martick M M, Garcia K C. Science. 2001;293:1657–1662. doi: 10.1126/science.1062246. [DOI] [PubMed] [Google Scholar]
- 21.Honing M L, Smits P, Morrison P J, Burnett J C, Jr, Rabelink T J. Hypertension. 2001;37:1179–1183. doi: 10.1161/01.hyp.37.4.1179. [DOI] [PubMed] [Google Scholar]
- 22.Mulvany M J, Halpern W. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Mulvany M J, Nilsson H, Flatman J A. J Physiol. 1982;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garland J G, McPherson G A. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doughty J M, Plane F, Langton P D. Am J Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- 26.Edwards G, Feletou M, Gardener M J, Thollon C, Vanhoutte P M, Weston A H. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaytor A T, Marsh W L, Hutcheson I R, Griffith T M. Endothelium. 2000;7:265–278. doi: 10.3109/10623320009072213. [DOI] [PubMed] [Google Scholar]
- 28.Dora K A, Garland C J. Am J Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- 29.Murthy K S, Makhlouf G M. J Biol Chem. 1999;274:17587–17592. doi: 10.1074/jbc.274.25.17587. [DOI] [PubMed] [Google Scholar]
- 30.Pagano M, Anand-Srivastava M B. J Biol Chem. 2001;276:22064–22070. doi: 10.1074/jbc.M101587200. [DOI] [PubMed] [Google Scholar]
- 31.Jin W, Lu Z. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- 32.Taylor H J, Chaytor A T, Evans W H, Griffith T M. Br J Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandes R P, Schmitz-Winnenthal F H, Feletou M, Godecke A, Huang P L, Vanhoutte P M, Fleming I, Busse R. Proc Natl Acad Sci USA. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy K S, Teng B Q, Zhou H, Jin J G, Grider J R, Makhlouf G M. Am J Physiol. 2000;278:G974–G980. doi: 10.1152/ajpgi.2000.278.6.G974. [DOI] [PubMed] [Google Scholar]
- 35.Anand-Srivastava M B, Sehl P D, Lowe D G. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- 36.Boulanger C M, Morrison K J, Vanhoutte P M. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammarstrom A K, Parkington H C, Coleman H A. Br J Pharmacol. 1995;115:717–722. doi: 10.1111/j.1476-5381.1995.tb14992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leaney J L, Tinker A. Proc Natl Acad Sci USA. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura H, Yokoyama M, Akita H, Matsushita K, Kurachi Y, Yamada M. J Pharmacol Exp Ther. 2000;293:196–205. [PubMed] [Google Scholar]
- 40.Furuya M, Aisaka K, Miyazaki T, Honbou N, Kawashima K, Ohno T, Tanaka S, Minamino N, Kangawa K, Matsuo H. Biochem Biophys Res Commun. 1993;193:248–253. doi: 10.1006/bbrc.1993.1616. [DOI] [PubMed] [Google Scholar]
- 41.Kiemer A K, Vollmar A M. J Biol Chem. 1998;273:13444–13451. doi: 10.1074/jbc.273.22.13444. [DOI] [PubMed] [Google Scholar]
- 42.Osawa H, Yamabe H, Kaizuka M, Tamura N, Tsunoda S, Baba Y, Shirato K, Tateyama F, Okumura K. Nephron. 2000;86:467–472. doi: 10.1159/000045836. [DOI] [PubMed] [Google Scholar]
- 43.Okahara K, Kambayashi J, Ohnishi T, Fujiwara Y, Kawasaki T, Monden M. FEBS Lett. 1995;373:108–110. doi: 10.1016/0014-5793(95)01027-c. [DOI] [PubMed] [Google Scholar]
- 44.Chun T H, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K, et al. Hypertension. 1997;29:1296–1302. doi: 10.1161/01.hyp.29.6.1296. [DOI] [PubMed] [Google Scholar]
- 45.Hama N, Itoh H, Shirakami G, Suga S, Komatsu Y, Yoshimasa T, Tanaka I, Mori K, Nakao K. Biochem Biophys Res Commun. 1994;198:1177–1182. doi: 10.1006/bbrc.1994.1166. [DOI] [PubMed] [Google Scholar]