Abstract
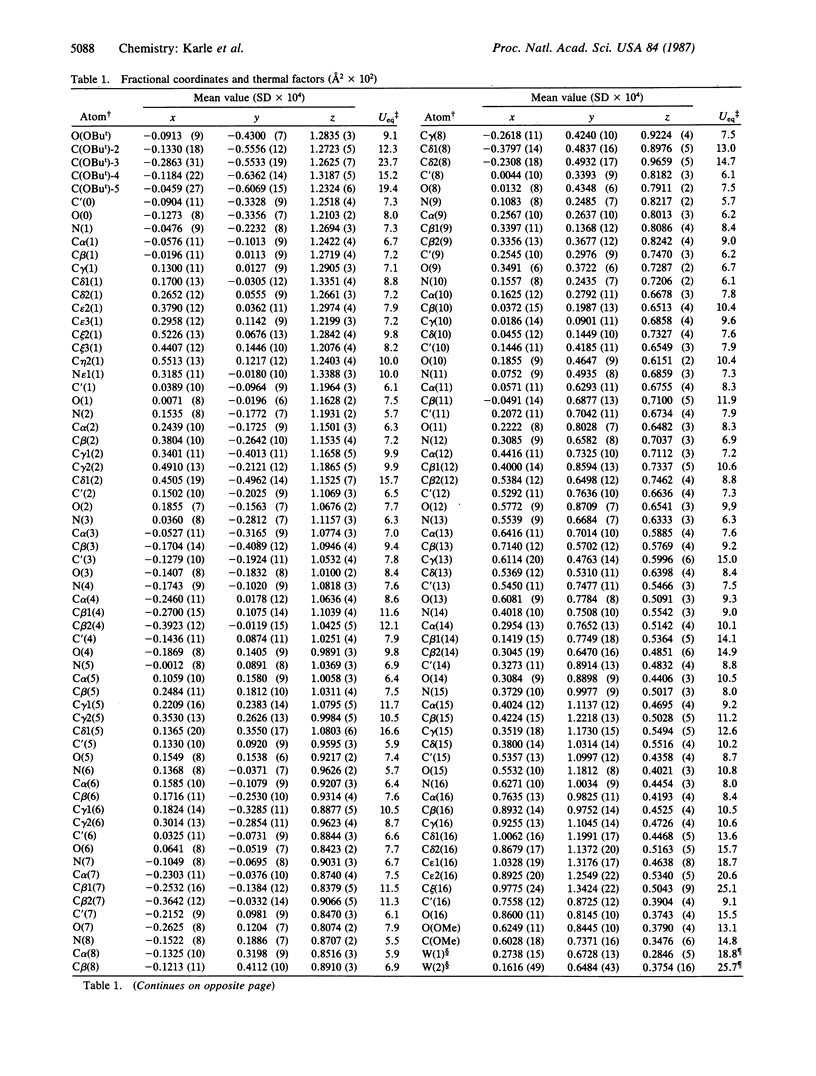
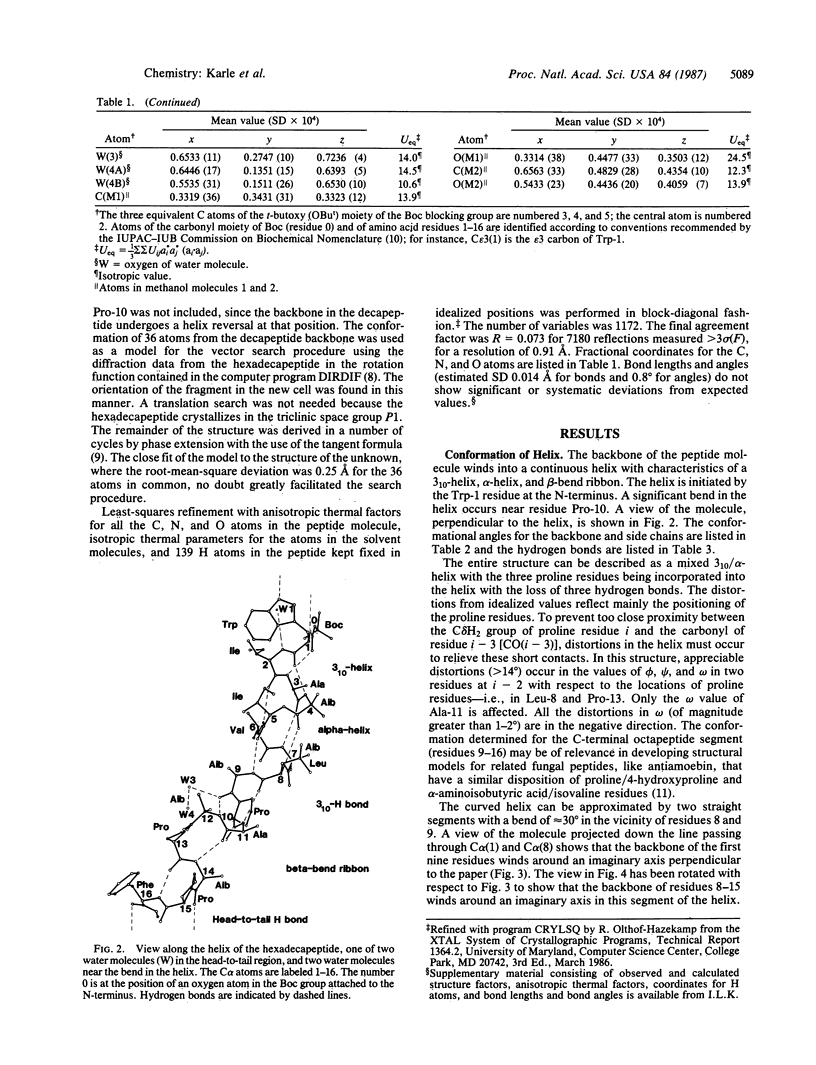
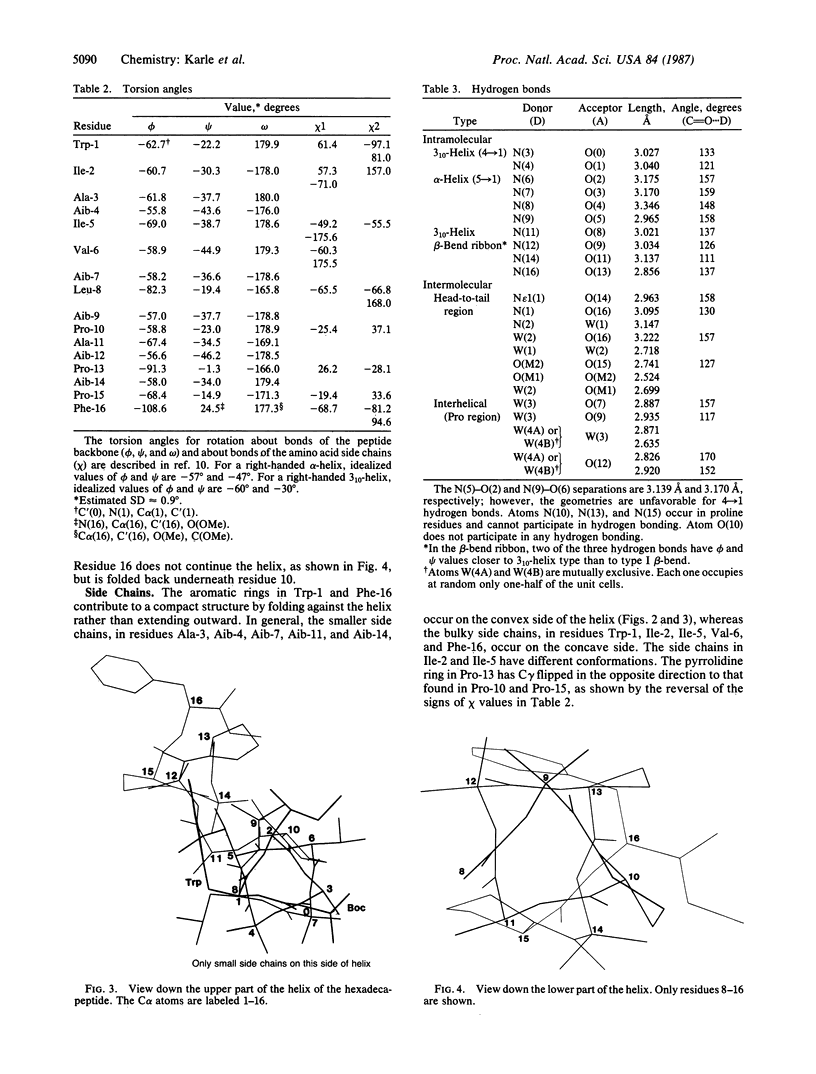
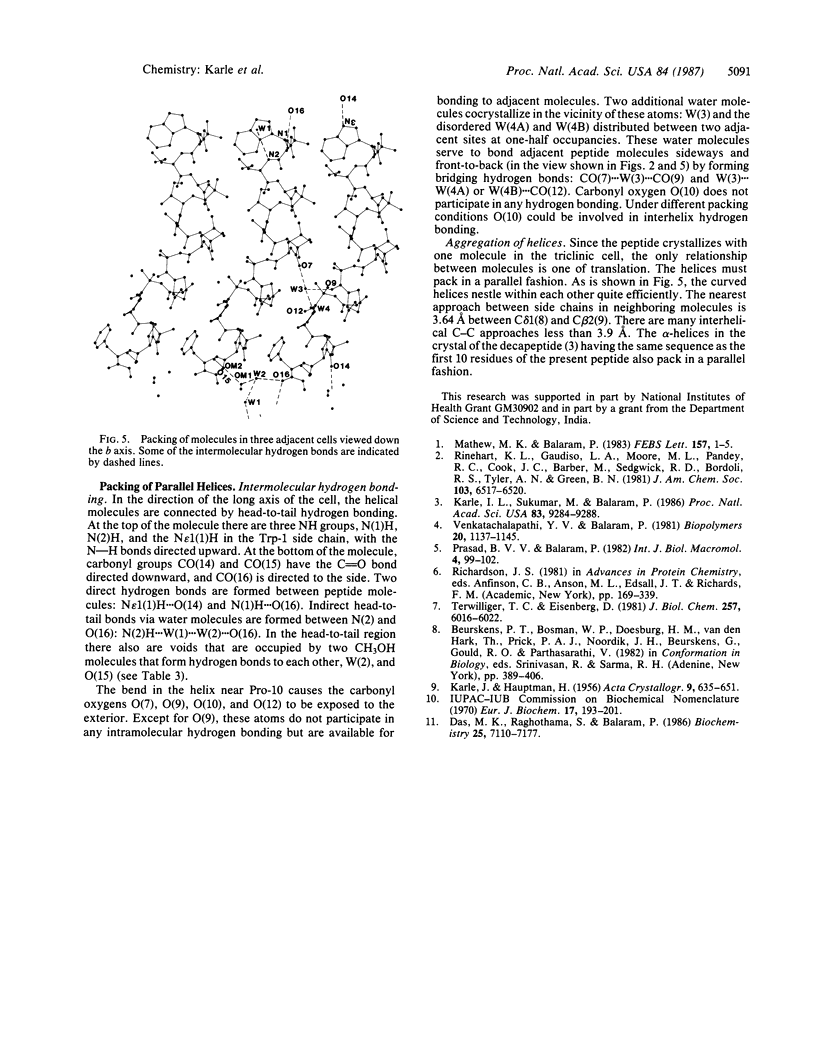
Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-Ala-Aib-Pro-Aib-Pro-Phe-OMe (where Boc is t-butoxycarbonyl and Aib is alpha-aminoisobutyric acid), a synthetic apolar analog of the membrane-active fungal peptide antibiotic zervamycin IIA, crystallizes in space group P1 with Z = 1 and cell parameters a = 9.086 +/- 0.002 A, b = 10.410 +/- 0.002 A, c = 28.188 +/- 0.004 A, alpha = 86.13 +/- 0.01 degrees, beta = 87.90 +/- 0.01 degrees, and gamma = 89.27 +/- 0.01 degrees; overall agreement factor R = 7.3% for 7180 data (F0 greater than 3 sigma) and 0.91-A resolution. The peptide backbone makes a continuous spiral that begins as a 3(10)-helix at the N-terminus, changes to an alpha-helix for two turns, and ends in a spiral of three beta-bends in a ribbon. Each of the beta-bends contains a proline residue at one of the corners. The torsion angles phi i range from -51 degrees to -91 degrees (average value -64 degrees), and the torsion angles psi i range from -1 degree to -46 degrees (average value -31 degrees). There are 10 intramolecular NH...OC hydrogen bonds in the helix and two direct head-to-tail hydrogen bonds between successive molecules. Two H2O and two CH3OH solvent molecules fill additional space with appropriate hydrogen bonding in the head-to-tail region, and two additional H2O molecules form hydrogen bonds with carbonyl oxygens near the curve in the helix at Pro-10. Since there is only one peptide molecule per cell in space group P1, the molecules repeat only by translation, and consequently the helices pack parallel to each other.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Das M. K., Raghothama S., Balaram P. Membrane channel forming polypeptides. Molecular conformation and mitochondrial uncoupling activity of antiamoebin, an alpha-aminoisobutyric acid containing peptide. Biochemistry. 1986 Nov 4;25(22):7110–7117. doi: 10.1021/bi00370a053. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Sukumar M., Balaram P. Parallel packing of alpha-helices in crystals of the zervamicin IIA analog Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe.2H2O. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9284–9288. doi: 10.1073/pnas.83.24.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982 Jun 10;257(11):6016–6022. [PubMed] [Google Scholar]


