Abstract
Background
Alternative arrangements of chromosome 2 inversions in Anopheles gambiae are important sources of population structure, and are associated with adaptation to environmental heterogeneity. The forces responsible for their origin and maintenance are incompletely understood. Molecular characterization of inversion breakpoints provides insight into how they arose, and provides the basis for development of molecular karyotyping methods useful in future studies.
Methods
Sequence comparison of regions near the cytological breakpoints of 2Rb allowed the molecular delineation of breakpoint boundaries. Comparisons were made between the standard 2R+b arrangement in the An. gambiae PEST reference genome and the inverted 2Rb arrangements in the An. gambiae M and S genome assemblies. Sequence differences between alternative 2Rb arrangements were exploited in the design of a PCR diagnostic assay, which was evaluated against the known chromosomal banding pattern of laboratory colonies and field-collected samples from Mali and Cameroon.
Results
The breakpoints of the 7.55 Mb 2Rb inversion are flanked by extensive runs of the same short (72 bp) tandemly organized sequence, which was likely responsible for chromosomal breakage and rearrangement. Application of the molecular diagnostic assay suggested that 2Rb has a single common origin in An. gambiae and its sibling species, Anopheles arabiensis, and also that the standard arrangement (2R+b) may have arisen twice through breakpoint reuse. The molecular diagnostic was reliable when applied to laboratory colonies, but its accuracy was lower in natural populations.
Conclusions
The complex repetitive sequence flanking the 2Rb breakpoint region may be prone to structural and sequence-level instability. The 2Rb molecular diagnostic has immediate application in studies based on laboratory colonies, but its usefulness in natural populations awaits development of complementary molecular tools.
Background
Anopheles gambiae sensu stricto, the most important vector of human malaria in Africa, is the nominal member of a group of at least seven morphologically indistinguishable and closely related mosquito species [1,2]. Polytene chromosome analysis of this group, the An. gambiae complex, revealed an abundance of paracentric inversions [1,3], characterized by the breakage and 180 degree rearrangement of chromosome segments excluding the centromere. More than 130 paracentric inversions have been detected across the group as a whole, with 10 inversions distinguishing six of these species. Given the absence of morphological differences, these fixed chromosomal landmarks provided the first routine basis for species identification [1]. However, fixed inversion differences between species and populations are the exception in the An. gambiae complex. The majority of inversions remain polymorphic in natural populations. Although most species in the complex carry at least one polymorphic inversion, only two species--An. gambiae s. s. (hereafter, An. gambiae) and Anopheles arabiensis--possess most of the known inversion polymorphisms, potentially helping to explain their vast geographic and ecological distributions across much of tropical Africa [1,3].
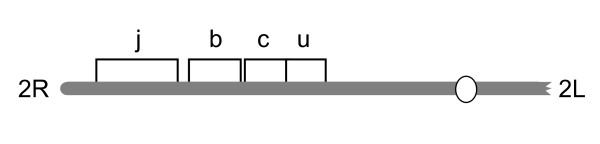
Cytological studies have also demonstrated the highly non-random occurrence of inversions along the five arms of the polytene complement in An. gambiae [1,3,4]. Contrary to the null expectation that they should be distributed among chromosome arms in proportion to arm length, inversions are highly concentrated on the right arm of chromosome 2 (2R) (Figure 1). Although 2R represents less than 30% of the complement, this arm is the source of 6/7 (86%) common, and 67/82 (82%) rare, polymorphic chromosomal inversions in An. gambiae and 18/31 (58%) common polymorphic inversions in the species complex as a whole--a highly significant bias [3,4]. Moreover, the distribution of inversion breakpoints along the 2R arm is not uniform. Inversion breakpoints not only cluster in particular regions, but also appear coincident at the cytological level at a much higher rate than expected by chance [4], suggesting that a nonrandom process could be responsible for their origin and/or maintenance. Intriguingly, Coluzzi et al [3] identified the central part of chromosome arm 2R, the area corresponding to polymorphic inversions 2Rb, bc and u in An. gambiae, as being involved in independent interspecific inversions in the sibling species Anopheles merus, Anopheles melas and An. arabiensis, leading him to speculate that these parallel chromosomal changes may underlie adaptations to ecologically distinct larval breeding sites characteristic of these different species.
Figure 1.
Schematic diagram (after [13]) of common inversions 2Rj, 2Rb, 2RC and 2Ru on chromosome 2R in An. gambiae.
The abundance of inversions in the An. gambiae complex, and their nonrandom taxonomic, genomic and ecological distributions suggested that they may be playing more than a passive role in the diversification of these mosquitoes. Indeed, chromosomal inversions have been viewed as a mechanism for ecotypic differentiation in An. gambiae [3,5,6]. A recent model that could explain the spread and distribution of inversions proposes that through suppressed recombination in inversion heterozygotes, allelic combinations beneficial in particular environments are protected from recombination with other genetic backgrounds adapted to alternative ecological settings [7,8]. The maintenance of polymorphic inversions would thereby confer greater ecological flexibility to the species. Evidence consistent with an adaptive role for inversion polymorphisms in An. gambiae are the stable geographic clines in inversion frequency associated with climatic factors, such as aridity, replicated across Africa [1,9-11]; the microhabitat differences in inversion frequencies of indoor- or outdoor-resting populations, also associated with aridity [12]; the temporal cycling of inversion frequencies in concert with rainy and dry seasons [1,13]; and the heterotic maintenance of inversions in laboratory colonies [14]. Of particular relevance to malaria transmission and control is the connection between inversions and indoor/outdoor resting, as this mosquito behavioral response to aridity impacts the probability of vector-human contact and vector contact with insecticide-treated walls or bed nets [15]. Moreover, the process of ecotypic differentiation, potentially leading to speciation [5], implies restricted gene flow between populations and may be accompanied by other physiological and behavioral differences that could alter the efficiency of vector control efforts in unanticipated ways.
To date, only three inversions have been molecularly characterized in An. gambiae s.l., one on 2L (2La [16]) and two on 2R (2Rj [17]; 2Rd' [18]). Based on detailed molecular analysis of the breakpoint junctions, no clear consensus has emerged on the likely mechanism of breakage, nor have precise mechanisms responsible for inversion maintenance yet been identified. However, characterization of these breakpoint regions led to the development of molecular diagnostics for 2La [19] and 2Rj [20], which allow much higher throughput karyotyping of natural populations than allowed by laborious cytological methods, and have facilitated ongoing functional analysis [21-23]. Toward the long-term goal of understanding mechanisms responsible for the origin and maintenance of inversions in An. gambiae, the breakpoint structure of inversion 2Rb is presented along with a rapid molecular karyotyping method.
Methods
Assembly of 2Rb breakpoint proximal sequences
Using PCR primers designed from the chromosomally standard An. gambiae PEST reference genome [24]; [25], a fosmid library prepared from the An. gambiae BKO strain (2Rj+bcu/j+bcu; [17]) was screened for clones that possessed unique (non-repetitive) sequence corresponding to the centromere-proximal breakpoint region of the 2R+b arrangement. (This strategy was part of a larger, ongoing effort to molecularly characterize other inversion breakpoints on chromosome 2R). Based on localization with in situ hybridization and sequence comparison, Fosmid clone 332D was identified as going to the breakpoints, and end-sequenced using the Big Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) and the ABI PRISM 3700 DNA Analyzer (Applied Biosystems). Sequences verified using Lasergene Seqman software (DNASTAR) were submitted to GenBank (accession numbers HN153245-HN153246).
End-sequence of fosmid 332D was used to walk toward the 2Rb proximal breakpoint using trace sequence reads generated from whole genome sequencing of the An. gambiae S form (Pimperena strain; Lawniczak et al, submitted). The Pimperena strain carries the opposite (inverted) orientation of the 2Rb arrangement (i.e., 2Rb/b). Pimperena trace sequences matching sequence from fosmid 332D were used to initiate an iterative BLAST procedure in which the Pimperena trace archive was queried and contigs from this 2Rb background were built using mate pair information and sequence similarity. These 2Rb contigs were compared to the PEST (2R+b) assembly to find evidence of the rearrangement breakpoint. Highly repetitive regions that could not be assembled manually were excluded. Manually assembled 2Rb sequences flanking the putative breakpoint were used to identify scaffolds assembled independently during the An. gambiae M (Mali-NIH, 2Rbc/bc) and S genome sequencing project [26,25] and compared to the PEST genome.
Mosquito sampling and cytological determination of karyotype
Collections of indoor resting An. gambiae were made by spray catch from Mali and Cameroon. Samples from Mali were collected from seven villages in the southern part of the country in Aug-Sep 2004, as previously described [20]. Samples from Cameroon were collected from five villages in a forest/savanna mosaic zone in the eastern part of the country in Sep-Oct 2009: Gado-Badzere and Zembe Borango (4°58.435'N, 13°2.403'E), Nkoumadjap (4°21.575'N, 13°38.391'E), Mayos I (4°20.505'N, 13°33.490'E), and Daiguene (4°46.608'N, 13°50.668'E). Mosquitoes were sorted morphologically to An. gambiae s.l. and by gonotrophic stage. Ovaries of semi-gravid females were dissected and placed into a micro-tube containing Carnoy's solution (1 part glacial acetic acid, 3 parts ethanol); the carcass was placed in a correspondingly numbered micro-tube and stored over desiccant for DNA-based analysis. Preparation and scoring of polytene chromosomes followed [20].
PCR determination of karyotype
Genomic DNA was isolated from individual mosquitoes using the DNeasy Extraction Kit (Qiagen, Valencia, CA). Anopheles gambiae sensu stricto and its molecular forms were identified using an rDNA-based PCR diagnostic assay [27].
Sequence comparisons between the PEST (2R+b), Pimperena (2Rb), and Mali-NIH (2Rbc) genome assemblies were used to design primers to differentially amplify alternative arrangements of the b inversion. Diagnostic primers include: b-For (5'-CGGGAGCAAAGATAAGTAGCA), b+-Rev (5'-CCGGATAATCGACGCTCTAC), and b-Rev (5' AACCCTACCATATACCAGTACCAACG 3'). The 25 μl PCR reaction contained 20 mM Tris-Cl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP; 5 pmol b-For primer, 2.5 pmol b-Rev primer, 5 pmol b+-Rev primer, 1 U Taq polymerase, 5% DMSO and 1% BSA. PCR was performed on 1 μl genomic DNA template using an initial incubation at 94°C for 2 min, followed by 35 cycles of 94°C for 30s, 58°C for 30s, and 72°C for 45s, concluding with a 10 min incubation at 72°C and a 4°C hold. Mosquitoes from established colonies with known karyotypes were used as PCR controls. These included Mali-NIH M form (2Rbc; 2La) and Pimperena S form (2Rb; 2La/+a) available from MR4[28], and colonies selected at the University of Notre Dame to be homokaryotypic for chromosomal inversions on 2R: CAM M form (2R+; 2L+, derived from parental colony Yaounde from Cameroon), KIST S form (2R+; 2L+, derived from parental colony KISUMU from Kenya), and SUCAM M form (2R+; 2La/+a, derived from a cross between CAM and Sua2La [22]).
Results and Discussion
Sequence assembly across the 2Rb breakpoints
Three genome assemblies were compared to infer the molecular organization of the 2Rb rearrangement breakpoints. The An. gambiae PEST reference is homokaryotypic standard (uninverted) for all chromosomal arrangements including 2R+b [24,29], while the S and M form assemblies were derived from colonies (Pimperena and Mali-NIH, respectively) carrying the opposite (2Rb inverted) arrangement [26]. Sequence spanning the breakpoint regions of the 2Rb inversion were assembled manually, using Sanger trace reads and mate pair information available from S form genome sequencing on Vectorbase [25]. The query sequence used to seed iterative searches of S traces was end-sequence determined from a fosmid clone (332D) shown to hybridize in situ to the centromeric end of the 2R+b arrangement. The two resulting manual trace assemblies (available as additional file 1), representing the last ~10 kb of sequence at both ends of the rearrangement, were validated by comparison with scaffolds generated from independent automated whole genome shotgun (WGS) assemblies of M, S PEST (Figure 2).
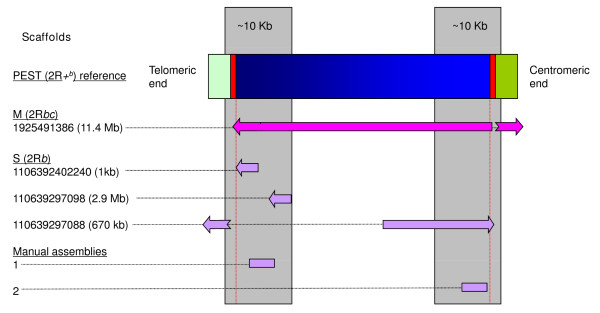
Figure 2.
Schematic diagram of scaffolds analysed in M and S relative to the An. gambiae PEST reference. Horizontal rectangle (labeled at left) represents genomic sequence from the PEST reference genome, with green shaded sections indicating flanking sequence at the telomeric and centromeric ends, red sections indicating repetitive sequence at both breakpoints, and the blue section indicating the 2R+b arrangement. Horizontal arrows indicate the relative position (not to scale) and orientation of assembly or manual scaffolds used in the analysis. The scaffold number (if applicable), approximate length, and source (An. gambiae M or S genome sequence) is indicated at left. Vertical gray rectangles highlight the ~10 kb breakpoint regions whose sequence was compared between the PEST, M and S genomes.
Mate-pair information verified the linkage of sequence within the inversion to flanking sequence outside both breakpoints in the manual assemblies. However, neither breakpoint could be manually assembled without gaps, due to the presence of highly repetitive sequences (Figure 3). Gap-lengths were estimated based on mate pair and clone insert-size information. Both breakpoint regions also contain gaps in all three WGS assemblies (M, S and PEST). Scaffolds 1106392397088 (S assembly) and 1925491386 (M assembly) span the proximal (centromeric) 2R+b breakpoint across gaps and into unique flanking sequence. Neither M nor S WGS assemblies produced scaffolds that spanned the distal (telomeric) breakpoint.
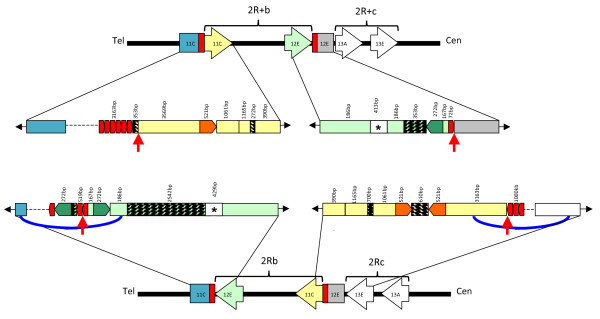
Figure 3.
Structure of the 2Rb/+b breakpoint junctions. At top and bottom is a schematic overview of the 2Rb/+b and 2Rc/+c arrangements as represented by the PEST and M reference sequences, respectively. Horizontal black bar represents a segment of chromosome 2R. The relative position and orientation of chromosomal arrangements is indicated by labeled brackets, and centromeric/telomeric ends of 2R are indicated as Cen and Tel, respectively. Shaded arrows indicate the orientation of the arrangements, and labels inside the arrows (e.g., 11C) provide the cytogenetic subdivision in which the breakpoint junction occurs. Blue and gray boxes labeled with the corresponding cytogenetic subdivision represent flanking sequence outside of chromosomal rearrangements; red boxes represent repetitive DNA. The central part of the diagram provides a more detailed structural analysis of the color-coded breakpoint regions. Throughout, color is used to indicate homologous sequences between alternative arrangements, except for rectangles filled by patterns, which represent exclusive insertion events. Horizontal blunt arrows shaded in olive green and orange are sequences present once in the 2R+b arrangement that have been duplicated into a palindrome in the alternative 2Rb arrangement. The red vertical arrows represent the putative breakpoints, positions where unique sequence ends and repetitive sequence framing both ends of the arrangement (red blunt arrows) begins. Black arrows at the ends of each diagram represent continuing chromosomal sequence; dotted lines represent gaps in the assembly. Blue curved lines represent sequence linked by mate-pair information. Asterisk framed by a white box indicates the chromosomal region targeted by the PCR diagnostic assay; see text and Figure 4 for details. Not to-scale.
Molecular organization of the 2Rb inversion breakpoints
Assemblies of the 2Rb inversion breakpoints from Mali-NIH (2Rbc) and Pimperena (2Rb) traces were nearly identical, with only minor SNP and insertion-deletion differences. A schematic diagram of their molecular structure, in comparison to the corresponding regions of the uninverted chromosome, is provided in Figure 3. Together, these data reveal that the 2Rb inversion encompasses 7.55 Mb and extends from subdivision 11C to 12E on the cytogenetic map of PEST (position 19,023,925 to 26,758,676 on 2R; red arrows in Figure 3).
Outside of and immediately flanking these breakpoints on both 2R+b and 2Rb arrangements is a repetitive structure comprising tandemly arrayed copies of unit length ~30 bp, (ACTTTTGCGATTGTCGCAAAAACTTCTGCGA)N. At the telomeric end of the 2R+b arrangement of PEST, this tandem repeat structure extends for at least 3.2kb (i.e., >100 tandem repetitions) and is flanked by a ~10 kb assembly gap. At the centromeric end of 2R+b, the repetitive structure is 72 bp in length. The alternative 2Rb arrangement in both Mali-NIH and Pimperena also was flanked by the same tandem repeat sequence. At the telomeric breakpoint of 2Rb, the tandem repeat sequence spans 519 bp and is embedded in a palindrome, flanked by a ~2.5 kb assembly gap. At its centromeric end, the 2Rb breakpoint also abuts at least 1 kb of the same tandem repeat, but the highly repetitive nature of this sequence caused gaps in both WGS and manual assemblies.
The position of these tandem repeats, presumably at the precise breakpoints of both arrangements (2Rb and 2R+b), implicates the tandem repeat in the generation of this chromosomal inversion, although the ancestral-descendant relationship between the alternative gene orders based on these data is ambiguous. Other molecularly characterized inversion breakpoints on 2R in An. gambiae also possess flanking repetitive sequences, but only on the derived arrangement: 2Rj is flanked by nearly identical 14.6 kb complex inverted repeat structures reminiscent of segmental duplications, while 2L+a is bordered by homologous repetitive elements [17,19]. In neither case were these repetitive sequences found adjacent to either breakpoint of the ancestral arrangement, in contrast to the situation for 2Rb. Either the 2Rb or 2R+b arrangements could have arisen via non-allelic homologous recombination between flanking tandem repeats on the ancestral chromosome, leading to inversion of the intervening sequence. Notably, two unrelated palindromes with short internal spacers were present at the breakpoints of the 2Rb inversion in both Pimperena and Mali-NIH 2Rb arrangements, one at each end. As the arms of both palindromes involve sequences apparently present only once in the PEST genome (near each breakpoint), it is tempting to suggest that the 2R+b arrangement in PEST may be ancestral.
Gene annotations adjacent to the 2Rb breakpoints
Gene predictions were compared between the 2Rb and 2R+b arrangements, within the ~10 kb region immediately internal to the breakpoints. Most of the sequence consisted of transposons and low complexity sequence. There were no genes annotated in the 10 kb region proximal to the centromeric breakpoint of 2R+b in PEST. At the telomeric end of this arrangement, one gene has been predicted of unknown function (AGAP002299, annotated as a conserved hypothetical protein). This gene has putative orthologs in other mosquitoes (Culex quinquefasciatus, CPIJ008031; Aedes aegypti, AAEL007792) and Drosophila melanogaster (CG18635). The corresponding gene at the centromeric end of 2Rb contained several SNP differences and a deletion in the predicted 5' UTR. Although limited EST evidence suggests that this gene may have alternative transcripts, potential functional consequences of sequence differences in AGAP002299 between alternative arrangements (if any) remain to be investigated.
Molecular karyotyping by PCR
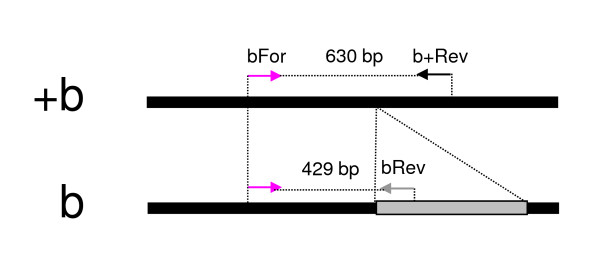
Extensive lengths of repetitive DNA and associated assembly gaps precluded a molecular karyotyping strategy that depends upon PCR amplification across inversion breakpoints. Instead, a PCR assay was developed that exploits an insertion-deletion difference between arrangements, as close as possible to the breakpoint (~1 kb; Figure 3). The assay employs three primers, one of which (bFor) is a universal primer that anneals to both arrangements (Figure 4). The second primer, bRev, was designed to anneal to a 2.5 kb sequence exclusive to the 2Rb arrangement. Together, bFor and bRev should amplify a 429 bp fragment when 2Rb is present. Although the third primer, +bRev, can anneal to both arrangements, successful PCR amplification with this primer is expected only from the 2R+b arrangement, on which the distance spanned by +bRev and bFor is 630 bp (the corresponding distance between these primers on the 2Rb arrangement exceeds 3 kb).
Figure 4.
Schematic diagram of the three-primer PCR assay for molecular karyotyping of 2Rb. The white box with an asterisk in Figure 3 is represented here. Areas common to both arrangements are connected by dotted lines. The grey box represents an insertion exclusive to the 2Rb arrangement, to which primer bRev anneals. Primer bFor is a universal primer that anneals to a region common to both arrangements. Although +bRev can anneal to both arrangements at different distances from bFor, size limitations on successful PCR amplification restrict the product to a 2R+b-specific fragment of 630 bp. In combination with bFor,primer bRev amplifies a 429 bp sequence diagnostic of the 2Rb arrangement.
As a first step in the validation of this assay, its performance was tested on at least 25 mosquitoes (50 chromosomes) sampled from each of five different An. gambiae laboratory colonies of known and monomorphic 2Rb karyotype (determined from polytene chromosome banding pattern), originating from geographic locations as diverse as Mali, Cameroon, Liberia, and Kenya: Mali-NIH M form (2Rbc/bc), CAM M form (2R+b/+b), SUCAM M form (2R+b/+b), KIST S form (2R+b/+b), and Pimperena S form (2Rb/b). Without exception, PCR amplicons of the expected size were generated. Moreover, when DNA was mixed in 1:1 proportion from mosquitoes carrying 2Rb or 2R+b karyotypes prior to PCR, both bands were amplified (suggesting that the assay is capable of detecting 2Rb/+b heterozygotes). Additionally, at least 15 mosquitoes from an An. arabiensis colony (Dongola) selected to be homokaryotypic for 2Rb (i.e., 2Rb/b) were tested, and each generated the expected 429 bp PCR fragment (and only this fragment), consistent with other evidence that the 2Rb inversion in An. arabiensis and An. gambiae shares a common origin (e.g., see [30]).
As a second step of validation, the PCR assay was performed using An. gambiae sampled from natural populations in southern Mali and eastern Cameroon. Females at the appropriate gonotrophic stage were karyotyped based on polytene chromosome banding pattern, and these cytogenetic results were compared to those obtained from molecular karyotyping of the same specimen. Of the 267 mosquitoes whose karyotype could be determined both cytologically and molecularly in the overall sample, 223 (84%) yielded congruent results (Table 1). In the Cameroon collections were five An. arabiensis, of which two were successfully karyotyped as 2Rb/b. Their molecular karyotype was congruent, as judged by the expected 429 bp amplicon observed for both mosquitoes.
Table 1.
Performance of the 2Rb molecular karyotyping PCR assay in field collections of An. gambiae
| Molecular karyotypes congruent with banding pattern | ||
|---|---|---|
| Banding pattern | Cameroon | Mali |
| 2R+b/+b | 67/70 (96%) | 14/31 (45%) |
| 2R+b/b | 55/65 (85%) | 16/22 (73%) |
| 2Rb/b | 30/37 (81%) | 42/42 (100%) |
In the Cameroon sample, departures from the expected molecular results revealed no obvious trend apart from the fact that the PCR assay appeared to be less successful at accurately diagnosing the 2Rb arrangement, especially in 2Rb/b homokaryotypes. The frequency of other 2R inversions observed in this sample (2Rc, 2Rd, and 2Ru) was very low, ~3%. By contrast, cytologically defined 2Rb/b mosquitoes from Mali were invariably recognized as such by the PCR assay. However, the accuracy of the molecular method apparently declined precipitously for 2Rb/+b heterokaryotypes and even more so for standard (2R+b/+b) homokaryotypes from Mali. A more in-depth analysis of the full, cytologically determined 2R karyotypes of mosquitoes responsible for the discrepant molecular results revealed a remarkable insight. This insight is founded on the recognition by Coluzzi and coworkers that inversion 2Rc is found almost exclusively in combination with 2Rb and 2Ru, as 2Rbc or 2Rcu in Mali, where chromosomal polymorphism on 2R is very high [3,13]. Taking this observation into account, mosquitoes from Mali yielding incongruent molecular results--all 17 (100%) of the 2R+b/+b homozygotes and all 6 (100%) of the 2Rb/+b heterozygotes-- also carried the 2Rcu arrangement (Table 2). By convention, mosquitoes scored as 2Rcu/cu based on chromosomal banding pattern carry the banding pattern typical of the standard arrangement with respect to other 2R inversions (i.e., 2R+j+bcu/+j+bcu; [13]), and thus should be diagnosed molecularly as 2R+b (i.e., presumably two copies of the 630 bp PCR amplicon, given the expected 2R+b/+b karyotype). Similarly, mosquitoes scored as 2Rb/cu (i.e., 2R+jb+c+u/+j+bcu) should be diagnosed molecularly as 2Rb/+b heterozygotes. Instead, the presence of the 2Rcu arrangement was perfectly correlated with presence of a 429 bp amplicon that is normally diagnostic of the 2Rb arrangement. Sequencing of this unexpected amplicon verified that it matched the 2Rb sequence between the bFor and bRev primers (100% sequence identity), thus ruling out the possibilities that the fragment was an unrelated sequence fortuitously close to 429 bp in length, or a foreshortened segment of the 2R+b chromosome. Taken together, these results are consistent with the hypothesis that the 2Rcu arrangement may be derived from an ancestral 2Rb chromosomal background. This implies a secondary rearrangement from 2Rb back to 2R+b on chromosomes whose banding pattern appears to be 2R+bcu through the microscope. The arrangement of tandem repeats containing the same core sequence at opposite sides of the 2Rb breakpoint could provide the substrate for successive rearrangements through breakpoint reuse. However, it should be noted that this hypothesis depends at least in part upon an interpretation of some karyotypes that are not cytologically distinguishable (e.g., 2Rbc/u versus 2Rb/cu; Table 2), and it requires validation by further molecular investigation.
Table 2.
Molecular karyotyping discrepancies in relation to banding patterns on 2R in Mali
| Source of anomalies based on 2Rb karyotype | Complete 2R karyotype | Exp. molecular karyotype | Obs. molecular karyotype |
|---|---|---|---|
| 2R+b/+b (N = 17) | 2Rj+bcu/j+bcu (N = 13) | 2R+b | 2Rb |
| 2R+j+bcu/+j+bcu (N = 3) | 2R+b | 2Rb | |
| 2R+j+bcu/+j+b+c+u (N = 1) | 2R+b | 2Rb | |
| 2R+b/b (N = 6) | 2Rj+bcu/jbcu (N = 4) | 2R+b/b | 2Rb |
| 2R+jb+c+u/+j+bcu (N = 2) | 2R+b/b | 2Rb | |
Application of this PCR assay in Mali in conjunction with cytogenetic analysis, raised the intriguing possibility of 2Rb homoplasy through breakpoint reuse. However, it appears that the PCR assay as presented here has limited application by itself, for molecular karyotyping of 2Rb in natural populations of An. gambiae, even in Cameroon where the degree of 2R chromosomal polymorphism is low, due to a relatively high rate of 2Rb "miscalls". The rate of miscalls is based on the assumption that the cytogenetic analysis was error-free, which is unlikely. Thus, the miscall rate may be over-estimated. Nevertheless, it is possible that high repeat content near the 2Rb breakpoints is associated with relatively high genetic instability and sequence rearrangement, which can result in elimination or alteration of primer binding sites as well as unexpected changes in amplicon length due to insertions and deletions. On the other hand, the PCR assay yields results that are perfectly congruent with cytology in all laboratory colonies tested thus far, suggesting that the diagnostic assay will be useful in experimental manipulations and crosses where rapid karyotype analysis of living mosquitoes, both males and females across all developmental stages, is desired. Moreover, the 2Rb PCR assay may prove useful in Mali in the future, in combination with yet-to-be-developed molecular diagnostic assays for other arrangements on 2R.
Conclusions
Elucidation of the molecular breakpoint structure of the 7.5 Mb 2Rb inversion points to the involvement of repetitive DNA-- specifically, extensive tandem arrays of short unit length flanking both breakpoints-- in the rearrangement process. Although the ancestral-descendant relationship between standard and inverted arrangements is uncertain, molecular karyotyping based on a newly developed PCR diagnostic assay suggests two things. First, the 2Rb inversion shared between the sibling species An. gambiae and An. arabiensis has a common origin. Second, the polytene chromosome banding pattern indicative of the 2R+b standard arrangement may have arisen twice through breakpoint reuse. Sequence instability and high repeat content near the breakpoints complicate the application of the PCR diagnostic assay for molecular karyotyping of natural populations, although the assay represents a novel and powerful tool for functional genomic studies of 2Rb in laboratory colonies, and may hold promise for future field application in combination with other molecular tools. The current impediments to analysis of inversion breakpoints posed by repetitive DNA may be overcome by powerful new technologies [31], such as single DNA molecule platforms capable of mapping and even sequencing repetitive DNA, enabling further insights into the origin and stability of 2R rearrangements in natural populations of An. gambiae.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
FHC and DMS conceived the study. NFL and FHC designed and coordinated the study, with the assistance of SJE, SFT, CC and NJB. NFL, DMS, AAR, KRR, DAB, and MVS performed molecular, cytogenetic and/or computational experiments. NFL, DMS, and FHC analysed the results. NFL wrote the manuscript, with contribution from NJB. All authors read and approved the final manuscript.
Supplementary Material
that provides manual assemblies prepared from An. gambiae S proximal and distal 2Rb breakpoint regions as described in the Methods section, and the trace mate-pairs from the S genome, which cross both breakpoints.
Contributor Information
Neil F Lobo, Email: nlobo@nd.edu.
Djibril M Sangaré, Email: djibril_mrtc@yahoo.fr.
Allison A Regier, Email: aregier@nd.edu.
Kyanne R Reidenbach, Email: kreidenb@nd.edu.
David A Bretz, Email: bretz.2@nd.edu.
Maria V Sharakhova, Email: msharakh@vt.edu.
Scott J Emrich, Email: semrich@nd.edu.
Sekou F Traore, Email: cheick@mrtcbko.org.
Carlo Costantini, Email: carlo.costantini@ird.fr.
Nora J Besansky, Email: nbesansk@nd.edu.
Frank H Collins, Email: frank@nd.edu.
Acknowledgements
M. Kern and M. Belton provided laboratory assistance, O. Grushko and J. Agbor assisted with karyotyping, M. Coulibaly and S. Traore' managed the 2004 field collections in Mali. Thanks to the entomological teams of MRTC (Mali) and OCEAC (Cameroon) for fieldwork. The Mali-NIH and Pimperena genome sequence data used to assemble breakpoint junctions were produced by The Genome Center at Washington University School of Medicine, St. Louis and J. Craig Venter Institute, Rockville with funding from NHGRI (08UHG003079). Research was supported by NIH R01 AI076584 to NJB. DMS was supported by NIH Clinton Funds.
References
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- White GB. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68:278–301. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Della Torre A, Di Deco MA, Petrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Pombi M, Caputo B, Simard F, Di Deco MA, Coluzzi M, Della Torre A, Costantini C, Besansky NJ, Petrarca V. Chromosomal plasticity and evolutionary potential in the malaria vector Anopheles gambiae sensu stricto: insights from three decades of rare paracentric inversions. BMC Evol Biol. 2008;8:309. doi: 10.1186/1471-2148-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluzzi M. In: Mechanisms of Speciation. Barigozzi C, editor. New York: Alan R. Liss, Inc; 1982. Spatial distribution of chromosomal inversions and speciation in anopheline mosquitoes; pp. 143–153. [PubMed] [Google Scholar]
- Ayala FJ, Coluzzi M. Chromosome speciation: Humans, Drosophila, and mosquitoes. Proc Natl Acad Sci USA. 2005;102(Suppl 1):6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton N. Chromosome inversions, local adaptation and speciation. Genetics. 2006;173:419–434. doi: 10.1534/genetics.105.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Ann Rev Ecol Evol Syst. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard F, Ayala D, Kamdem GC, Etouna J, Ose K, Fotsing JM, Fontenille D, Besansky NJ, Costantini C. Ecological niche partitioning between the M and S molecular forms of Anopheles gambiae in Cameroon: the ecological side of speciation. BMC Ecol. 2009;9:17. doi: 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IHN, Ose K, Fotsing JM, Sagnon NF, Fontenille D, Besansky NJ, Simard F. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 2009;9:16. doi: 10.1186/1472-6785-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca V, Sabatinelli G, Di Deco MA, Papakay M. The Anopheles gambiae complex in the Federal Islamic Republic of Comoros (Indian Ocean): some cytogenetic and biometric data. Parassitologia. 1990;32:371–380. [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Behavioural divergences between mosquitoes with different inversion karyotypes in polymorphic populations of the Anopheles gambiae complex. Nature. 1977;266:832–833. doi: 10.1038/266832a0. [DOI] [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, DiDeco MA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- della Torre A, Merzagora L, Powell JR, Coluzzi M. Selective introgression of paracentric inversions between two sibling species of the Anopheles gambiae complex. Genetics. 1997;146:239–244. doi: 10.1093/genetics/146.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux L, Grammicia G. The Garki Project. Research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- Sharakhov IV, White BJ, Sharakhova MV, Kayondo J, Lobo NF, Santolamazza F, Della Torre A, Simard F, Collins FH, Besansky NJ. Breakpoint structure reveals the unique origin of an interspecific chromosomal inversion (2La) in the Anopheles gambiae complex. Proc Natl Acad Sci USA. 2006;103:6258–6262. doi: 10.1073/pnas.0509683103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly MB, Lobo NF, Fitzpatrick MC, Kern M, Grushko O, Thaner DV, Traore SF, Collins FH, Besansky NJ. Segmental duplication implicated in the genesis of inversion 2Rj of Anopheles gambiae. PLoS ONE. 2007;2:e849. doi: 10.1371/journal.pone.0000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiopoulos KD, della Torre A, Predazzi V, Petrarca V, Coluzzi M. Cloning of inversion breakpoints in the Anopheles gambiae complex traces a transposable element at the inversion junction. Proc Natl Acad Sci USA. 1998;95:12444–12449. doi: 10.1073/pnas.95.21.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Santolamazza F, Kamau L, Pombi M, Grushko O, Mouline K, Brengues C, Guelbeogo W, Coulibaly M, Kayondo JK, Sharakhov I, Simard F, Petrarca V, Della Torre A, Besansky NJ. Molecular karyotyping of the 2La inversion in Anopheles gambiae. Am J Trop Med Hyg. 2007;76:334–339. [PubMed] [Google Scholar]
- Coulibaly MB, Pombi M, Caputo B, Nwakanma D, Jawara M, Konate L, Dia I, Fofana A, Kern M, Simard F, Conway DJ, Petrarca V, della Torre A, Traoré S, Besansky NJ. PCR-based karyotyping of Anopheles gambiae inversion 2Rj identifies the BAMAKO chromosomal form. Malar J. 2007;6:133. doi: 10.1186/1475-2875-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Hahn MW, Pombi M, Cassone BJ, Lobo NF, Simard F, Besansky NJ. Localization of candidate regions maintaining a common polymorphic inversion (2La) in Anopheles gambiae. PLoS Genet. 2007;3:e217. doi: 10.1371/journal.pgen.0030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca KA, Gray EM, Costantini C, Besansky NJ. 2La chromosomal inversion enhances thermal tolerance of Anopheles gambiae larvae. Malar J. 2009;8:147. doi: 10.1186/1475-2875-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EM, Rocca KA, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malar J. 2009;8:215. doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298(5591):129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Vectorbase. http://www.vectorbase.org
- Lawniczak MKN, Emrich S, Holloway AK, Regier AP, Olson M, White B, Redmond S, Fulton L, Appelbaum E, Godfrey J, Widespread divergence between incipient Anopheles gambiae species revealed by whole genome sequences. Science. 2010. in press . [DOI] [PMC free article] [PubMed]
- Santolamazza F, Della Torre A, Caccone A. Short report: A new polymerase chain reaction-restriction fragment length polymorphism method to identify Anopheles arabiensis from An. gambiae and its two molecular forms from degraded DNA templates or museum samples. Am J Trop Med Hyg. 2004;70:604–606. [PubMed] [Google Scholar]
- MR4. http://www.mr4.org
- Mukabayire O, Besansky NJ. Distribution of T1, Q, Pegasus and mariner transposable elements on the polytene chromosomes of PEST, a standard strain of Anopheles gambiae. Chromosoma. 1996;104:585–595. doi: 10.1007/BF00352298. [DOI] [PubMed] [Google Scholar]
- White BJ, Cheng C, Sangare D, Lobo NF, Collins FH, Besansky NJ. The population genomics of trans-specific inversion polymorphisms in Anopheles gambiae. Genetics. 2009;183:275–288. doi: 10.1534/genetics.109.105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Kelso J. High-throughput DNA sequencing--concepts and limitations. Bioessays. pp. 524–536. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
that provides manual assemblies prepared from An. gambiae S proximal and distal 2Rb breakpoint regions as described in the Methods section, and the trace mate-pairs from the S genome, which cross both breakpoints.