Abstract
Background
Plasmodium vivax has a dormant hepatic stage, called the hypnozoite, which can cause relapse months after the initial attack. For 50 years, primaquine has been used as a hypnozoitocide to radically cure P. vivax infection, but major concerns remain regarding the side-effects of the drug and adherence to the 14-day regimen. This study examined the effectiveness of using the directly-observed therapy (DOT) method for the radical treatment of P. vivax malaria infection, to prevent reappearance of the parasite within the 90-day follow-up period. Other potential risk factors for the reappearance of P. vivax were also explored.
Methods
A randomized trial was conducted from May 2007 to January 2009 in a low malaria transmission area along the Thai-Myanmar border. Patients aged ≥ 3 years diagnosed with P. vivax by microscopy, were recruited. All patients were treated with the national standard regimen of chloroquine for three days followed by primaquine for 14 days. Patients were randomized to receive DOT or self-administered therapy (SAT). All patients were followed for three months to check for any reappearance of P. vivax.
Results
Of the 216 patients enrolled, 109 were randomized to DOT and 107 to SAT. All patients recovered without serious adverse effects. The vivax reappearance rate was significantly lower in the DOT group than the SAT group (3.4/10,000 person-days vs. 13.5/10,000 person-days, p = 0.021). Factors related to the reappearance of vivax malaria included inadequate total primaquine dosage received (< 2.75 mg/kg), duration of fever ≤ 2 days before initiation of treatment, parasite count on admission ≥ 10,000/µl, multiple P. vivax-genotype infection, and presence of P. falciparum infection during the follow-up period.
Conclusions
Adherence to the 14-day primaquine regimen is important for the radical cure of P. vivax malaria infection. Implementation of DOT reduces the reappearance rate of the parasite, and may subsequently decrease P. vivax transmission in the area.
Background
Globally, over 3 billion people live in areas at risk of malaria infection; about one billion of these live in countries outside Africa, where malaria transmission is low and Plasmodium vivax is most prevalent [1,2]. Unlike Plasmodium falciparum, P. vivax infection rarely develops into complicated malaria and death is unusual. However, P. vivax has a dormant stage (the hypnozoite) that persists in the human liver and may cause relapse weeks, months, or even years later. Therefore, P. vivax infection is considered to have greater impact on morbidity than mortality, resulting in significant social and economic burdens [1]. Moreover, it is very difficult to control P. vivax transmission, because gametocytes appear almost simultaneously with schizonts.
In Thailand, most malaria cases are caused by P. falciparum and P. vivax. The incidence ratio of P. falciparum malaria to P. vivax malaria is almost 1:1 [3]. A three-day chloroquine regimen followed by 14 days of primaquine is the standard treatment regimen to eradicate P. vivax parasites in the bloodstream and hypnozoites in the liver. Although chloroquine resistance has been reported in Papua New Guinea and Indonesia [4], it is still effective in Thailand [5-8]. Occasional failure of the standard primaquine therapy (15 mg daily for 14 days) to prevent relapse has been observed [9-15]. However, primaquine resistance has not been confirmed. In Thailand, the relapse rates at day 28 are about 50% without primaquine therapy [16], and about 20% with standard primaquine therapy [17]. Relapse has not been observed among patients receiving high-dose primaquine therapy (30 mg daily for 14 days) [16].
A number of factors are reportedly associated with relapse, or the reappearance of P. vivax, including inadequate primaquine dosage [12,14], high parasitaemia at diagnosis, short duration of symptoms prior to diagnosis [14], presence of gametocytes on admission [18], age, and gender [6,14,19,20]. Because the radical cure of P. vivax hypnozoites requires 14 days of primaquine therapy, adherence to the drug regimen may greatly affect the prevention of relapse. Unfortunately, the effect of patient adherence on 14-day primaquine treatment, and its relation to preventing parasite reappearance, is not well-documented.
Directly-observed therapy (DOT) is an effective strategy to ensure patient adherence to long-term chemotherapy. Currently, DOT is the main strategy for treating tuberculosis (TB) in many countries and its success has been widely reported [21]. However, the effectiveness of DOT for 14-day primaquine therapy in vivax malaria has not been studied thoroughly, particularly in malaria-tuberculosis co-endemic countries. Here, a randomized control trial was conducted to determine the effectiveness of DOT for 14-day primaquine administration, and the reappearance of P. vivax in a low malaria transmission area along the Thai-Myanmar border. Other potential risk factors for the reappearance of P. vivax after radical treatment were also explored.
Methods
Study site
The study was conducted between May 2007 and January 2009 at the Rajanagarindra Tropical Disease International Centre (RTIC), Tanaosri Subdistrict, Suan Phung District, Ratchaburi Province, Thailand. The area is situated 100 km west of Bangkok near the Myanmar border. The study area comprised seven hamlets with about 3,500 residents. The population in the area is composed of four ethnic groups, Karen (85%), Thai (14%), Mon and Burmese (1%). For a decade, the RTIC clinic has provided free malaria diagnosis and treatment for the local community. Patients from other hamlets also routinely seek malaria treatment here.
Study participants
Patients aged ≥ 3 years with microscopically-confirmed P. vivax infection were included in the study provided they or their guardians gave informed consent. Patients with microscopically confirmed mixed infections of P. vivax with other Plasmodium species, severe malaria, pregnant or lactating were excluded. G6PD deficient patients were included in this study, since the most common G6PD mutations in Thailand are G6PD Mahidol and G6PD Viangchan [22], which have been reported to be relatively safe for primaquine standard therapy [23]. This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University.
Randomization and treatment
To compare the effectiveness of directly observed therapy (DOT) with self-administered therapy (SAT), study participants were randomly assigned to either a DOT group or a SAT group using the block randomization method with a block size of four. Patients assigned to the SAT group, upon diagnosis, were given anti-malarials for self-administration with standard instructions in taking the drugs. Patients assigned to the DOT group were visited daily at home by RTIC staff until the full course was taken.
All patients were treated with the national standard regimen for vivax malaria, as recommended by the Malaria Control Program of Thailand (chloroquine 1,500 mg base over three days, followed by primaquine 15 mg daily for 14 days). G6PD screening is not required before giving the primaquine treatment. Chloroquine and primaquine doses were adjusted accordingly for patients under 14 years of age: for 8-13 years old, chloroquine 900 mg base over three days, and primaquine 10 mg daily for 14 days; for 3-7 years old, chloroquine 750 mg base over three days, and primaquine 5 mg daily for 14 days. If P. vivax parasitaemia reappeared within three months, the daily primaquine dose was increased to twice the initial dose.
Data collection
Baseline demographic and clinical data were gathered by interview. Finger-prick blood samples were collected on filter paper to examine for G6PD mutations and parasite genotype. All patients were followed for 90 days with Day 0 defined as the first day of drug administration.
During the follow-up period, patients from both groups were visited by RTIC staff at Days 7, 14, 28, 60, and 90, to examine for P. vivax and other Plasmodium parasitaemia. Patients were instructed to visit RTIC if they experience any symptoms during the follow-up period. At Days 7 and 14, patients in the SAT group were asked whether they took their anti-malarials as instructed. Upon microscopic confirmation of P. vivax reappearance, a finger-prick blood sample was taken to determine the parasite genotype for comparison against baseline genotype(s).
DNA extraction
DNA was extracted from finger-prick blood samples on filter paper, using a QIAamp DNA Mini Kit according to the manufacturer's instructions (Qiagen, Germany).
G6PD mutation
The two most common G6PD mutations in the Karen population, G6PD Mahidol and G6PD Viangchan, were examined using polymerase chain reaction (PCR) and restriction fragment length polymorphism analysis of the PCR products (PCR-RFLP), as described by Nuchprayoon et al [24] with slight modification, as follows: the PCR reaction was carried out in a 20 µl reaction volume containing 2.5 mM MgCl2; for PCR-RFLP, 10 µl of the PCR product were digested with an appropriate restriction enzyme (New England Biolabs Inc., UK) in a total volume of 20 µl.
Parasite genotyping
The pvcs and pvmsp3-α genes were used. Nested PCR and PCR-RFLP were carried out, following the previously described procedure [25,26]. For the pvcs gene, five size variants: 650, 680, 700, 720, and 750 base pairs were coded as a, b, c, d, and e, respectively. VK type was labelled as VK210 or VK247 according to the results of PCR-RFLP. For the pvmsp3-α gene, three size variants: 1,900, 1,500 and 1,100 base pairs, were labelled A, B, and C, respectively. For PCR-RFLP analysis by Hha I, digestion types were labelled as h1-h14 (as sizes A, B, and C: h1-h10, h11-h12, and h13-h14, respectively). Digestion types by Alu I were labelled as a1-a24 (as size A, B, and C: a1-a16, a17-a19, and a20-a24, respectively).
Multiplicity of infection (MOI) was estimated by genotyping results in two loci: the pvcs gene and the pvmsp3-α gene. It was defined as the minimum number of combinations of MOI at each locus. In each gene, MOI was estimated by the number of bands on a PCR product gel picture. In the case of the pvcs gene, even if only one band was observed on the PCR product picture, it was estimated that the MOI was two when the results of PCR-RFLP showed both VK types: VK210 and VK247.
Statistical analysis
The baseline characteristics of the patients in the SAT and DOT groups were compared using chi-square, Student's t, and Mann-Whitney U tests, depending on the type and distribution of the data. Age was categorized into three groups, 3-7 years, 8-13 years, and ≥14 years, according to the age-adjusted primaquine regimen. The cut-off level of the total primaquine dose per body weight (2.75 mg/kg) was used to indicate inadequate dose (< 2.75 mg/kg), as reported in a previous study [14]. The incidence rates of P. vivax reappearance observed among patients in the SAT and DOT groups were calculated and compared. Cox's proportional hazard regression modelling was used to examine factors related to the first reappearance of P. vivax malaria parasitaemia. The analysis was performed using Stata 8.0 computer package (StataCorp., USA).
Results
Enrolment and baseline characteristics of the patients
A total of 216 patients were enrolled between 26 May 2007 and 31 October 2008 and randomized into either the SAT group (107 patients) or the DOT group (109 patients). Over half of the patients (n = 123; 57%) were enrolled into the study during the high malaria transmission season in the study area (April to June). G6PD mutations were found in 22% of the patients (homozygous, 2%; hemizygous, 9%; heterozygous, 11%). All mutations were G6PD Mahidol. Despite the random allocation, patients in the DOT group were younger than those in the SAT group (Mann-Whitney U test: p = 0.004 for continuous age variable, chi-square test: p = 0.001 for age groups); other baseline characteristics were comparable between DOT and SAT groups (Table 1).
Table 1.
Baseline characteristics, by treatment group (N = 216)
| Variables | DOT | SAT |
|---|---|---|
| Number of patients | n = 109 | n = 107 |
| Female; N (%) | 45 (41) | 41 (38) |
| Age group; N (%) | ||
| 3-7 years | 34 (31) | 20 (19) |
| 8-13 years | 26 (24) | 13 (12) |
| ≥ 14 years | 49 (45) | 74 (69) |
| G6PD mutation status; N (%) | ||
| Wild-type | 81 (75) | 84 (81) |
| G6PD Mahidol: Homozygous | 2 (2) | 2 (2) |
| G6PD Mahidol: Hemizygous | 9 (8) | 11 (10) |
| G6PD Mahidol: Heterozygous | 16 (15) | 7 (7) |
| Body temperature at Day 0 (°C); Mean ± SD | 37.8 ± 1.2 | 37.8 ± 1.2 |
| Duration of fever pre-treatment; N (%) | ||
| 0-2 days | 62 (57) | 50 (47) |
| 3+ days | 46 (43) | 57 (53) |
| Parasite count at Day 0 (/µl); Geometric mean (range) | 1,890 (32-75600) | 2,533 (32-400000) |
| Gametocyte presence at Day 0; N (%) | 45 (41) | 37 (35) |
| Gametocyte count at Day 0 (/µl); Geometric mean (range) | 141 (16-1600) | 141 (16-8160) |
| P. vivax genotype (pvcs gene) infected at Day 0; N (%) | ||
| VK210 | 95 (91) | 84 (80) |
| VK247 | 4 (4) | 12 (11) |
| VK210&VK247 | 5 (5) | 9 (9) |
| Number of P. vivax genotype infections; N (%) | ||
| Single | 85 (82) | 84 (80) |
| Multiple infection | 19 (18) | 21 (20) |
Incidence rate of the reappearance of P. vivax parasitaemia
Of the 216 patients, 187 (87%) completed their 90-day follow-up period; 90 (83%) patients and 97 (91%) patients were in the DOT and SAT groups, respectively. Reappearance of P. vivax parasitaemia during follow-up was observed in 15 patients (8%). The overall incidence rates of P. vivax parasitaemia reappearance (per 10,000 person-days) at Days 28, 60, and 90, were 1.67, 4.78, and 8.43, respectively. The median (min-max) time to reappearance of P. vivax parasitaemia was 68 (13-90) days.
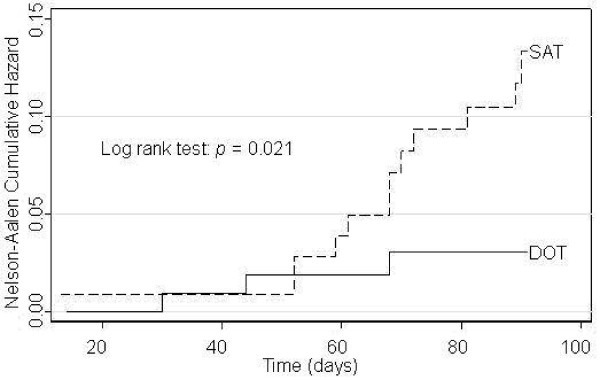
The rate of P. vivax reappearance in the DOT group was significantly lower than the SAT group (Figure 1). The reappearance rates at Days 28, 60, and 90, were 0, 3.18, and 3.37, respectively, in the DOT group, compared with 3.35, 6.39, and 13.49 in the SAT group.
Figure 1.
Nelson-Aalen cumulative hazard curves, by treatment group.
Factors related to the reappearance of P. vivax malaria parasitaemia
In the univariate analysis, treatment group, total primaquine dose per bodyweight, parasite count on admission, and multiple P. vivax-genotype infection were significantly associated with reappearance of P. vivax malaria (Table 2). There was a borderline positive association between appearance of P. falciparum parasitaemia during the follow-up period and risk of P. vivax reappearance (crude HR 4.01, 95% CI 0.90-17.77). G6PD mutation status, infected P. vivax genotype (VK type), gender, presence of gametocyte at baseline, and P. vivax acquisition month did not show statistically significant associations.
Table 2.
Crude and adjusted hazard ratio (HR) and 95% confidence interval (CI) for factors related to P. vivax reappearance
| Variables | Reappearance N (%) |
Crude HR (95% CI) |
Adjusted HR* (95% CI) |
|---|---|---|---|
| Treatment group | |||
| DOT | 3 (2.8) | 1 | 1 |
| SAT | 12 (11.2) | 3.98 (1.12-14.09) | 6.21 (1.39-27.79) |
| Age group | |||
| 3-7 years | 6 (11.1) | 1 | 1 |
| 8-13 years | 1 (2.6) | 0.21 (0.03-1.72) | 0.02 (0.0008-0.54) |
| ≥14 years | 8 (6.5) | 0.60 (0.21-1.72) | 0.62 (0.17-2.32) |
| Duration of fever pre-treatment | |||
| 0-2 days | 11 (9.8) | 1 | 1 |
| 3+ days | 4 (3.9) | 0.38 (0.12-1.21) | 0.16 (0.04-0.68) |
| Total primaquine dose/body weight | |||
| ≥ 2.75 mg/kg | 13 (6.1) | 1 | 1 |
| < 2.75 mg/kg | 2 (50.0) | 8.39 (1.89-37.22) | 10.45 (2.02-54.03) |
| Parasite count at Day0 | |||
| < 10000 /µl | 8 (4.3) | 1 | 1 |
| ≥ 10000 /µl | 6 (20.0) | 4.87 (1.69-14.04) | 5.43 (1.59-18.54) |
| Multiplicity of P. vivax genotype infection | 8 (4.7) | 1 | 1 |
| Single | 6 (15.8) | 5.97 (2.07-17.23) | 4.81 (1.53-15.16) |
| Multiple | |||
| P. falciparum infection post-treatment for P. vivax | |||
| No | 13 (6.3) | 1 | 1 |
| Yes | 2 (22.2) | 4.01 (0.90-17.77) | 13.66 (1.36-137.64) |
| Gender | |||
| Male | 10 (7.7) | 1 | - |
| Female | 5 (5.8) | 0.78 (0.27-2.28) | |
| Presence of gametocyte | |||
| No | 10 (7.5) | 1 | - |
| Yes | 4 (4.9) | 0.63 (0.19-2.01) | |
| pvcs gene genotype | |||
| VK210 | 12 (6.7) | 1 | - |
| VK247 | 1 (6.3) | 1.10 (0.14-8.49) | |
| VK210&VK247 | 1 (7.1) | 1.06 (0.14-8.13) | |
| G6PD mutation status | |||
| Wild-type | 12 (7.3) | 1 | - |
| G6PD Mahidol Homozygote | 0 | - | |
| G6PD Mahidol Hemizygote | 2 (10.0) | 1.33 (0.30-5.93) | |
| G6PD Mahidol Heterozygote | 1 (4.3) | 0.62 (0.081-4.78) |
*Adjusted for variables included in the multivariate Cox regression model
The significant variables from the univariate analysis, with two additional variables (age group and number of days with fever before start of treatment), were included in the multivariate model (Table 2). The reappearance rate for P. vivax was significantly higher in the SAT group, compared with the DOT group (adjusted HR 6.21, 95% CI 1.39-27.79). Children aged 8-13 years had the lowest risk of P. vivax reappearance. Interestingly, the reappearance rate appeared to be about six times lower among patients who received late treatment, i.e. pre-treatment duration of fever ≥3 days, compared with early treatment (adjusted HR 0.16, 95% CI 0.04-0.68). Inadequate total primaquine dose was also associated with reappearance as those who received a total primaquine dose < 2.75 mg/kg were about 10 times more likely to have a subsequent P. vivax attack than those who received a total primaquine dose ≥ 2.75 mg/kg. In addition, parasite-related factors including baseline parasite count ≥ 10,000 /µl, multiple P. vivax-genotype infection, and P. falciparum detected during follow-up were positively associated with risk of P. vivax reappearance (Table 2).
Adherence to 14-day primaquine treatment among the SAT group
Adherence to primaquine treatment was determined by interviewing patients in the SAT group (response rate = 94%) at Days 7 and 14. Fifteen of 101 patients (15%) reported that they have not taken the drug at least once during the 14 days of treatment. Patients who missed a primaquine dose or doses tended to have a higher risk of reappearance of P. vivax parasitaemia than those who took a complete14-day course (13% vs. 9%), although this difference was not statistically significant.
Non-adherence during Week 1 of treatment was reported by 6% of patients, whereas 12% of patients reported that they missed at least one dose of primaquine during Week 2. The median number of missed doses was more than four times higher in the Week 2 of treatment than Week 1. Interestingly, patients who received the treatment within two days of an acute attack tended to non-adhere to treatment in Week 2, compared with Week 1 (17% vs. 2%; Fisher's exact test, p = 0.030), whereas those who came to the clinic > 2 days after the initial attack did not show any difference in treatment adherence for Weeks 1 or 2 (7% vs. 9%). Non-adherence was more likely to be reported by males and in children aged 8-13 years, although the differences were not statistically significant.
Comparison of genotype in primary attack and reappearance of parasitaemia
The parasite genotypes in the primary and subsequent attacks for 15 patients who had reappearance of P. vivax were analysed. Blood samples from two patients failed to amplify, and three blood samples of either primary or subsequent attacks from three patients were missing. The P. vivax genotype patterns of the remaining 10 patients are summarized in Table 3. Three patients had the same P. vivax genotypes in both primary attack and reappearance. Five patients showed different genotype patterns in the primary episode and subsequent attack.
Table 3.
Comparison of P. vivax genotypes in primary attack and reappearance(s) (N = 10)
| Case | Primary attack | First reappearance | Second reappearance | |||
|---|---|---|---|---|---|---|
| Parasite count (/µl) pvcs, pvmsp3α gene | Day | Parasite count (/µl) pvcs, pvmsp3α gene | Day | Parasite count (/µl) pvcs, pvmsp3α gene | ||
| Same* | 1 | > 400000 c_VK210, A_h8a7 | 68 | 160 c_VK210, A_h8a7 | 91 (159) | 800 c_VK210, A_h8a7 |
| 2 | 8800 be_VK210, A_h6a7 | 30 | 6640 be_VK210, A_h6a7 | 29 (59) | 7440 Missing data | |
| 3 | 3080 c_VK210, A_h1a2 | 68 | 16480 c_VK210, A_h1a2 | |||
| Different‡ | 4 | 29360 c_VK247, A_hm†am† | 89 | 400 b_VK210, A_h1a15 | ||
| 5 | 30640 b_VK210, A_h1a4 | 90 | 22880 b_VK210, A_h4a6 | |||
| 6 | 1280 d_VK210&247, A_h2a3 | 52 | 112 c_VK210, A_h5a8 | |||
| 7 | 7440 ab_VK210, AC§ | 52 | 12320 b_VK210, A_h7a2 | |||
| 8 | 19360 be_VK210, A_h3a1 | 81 | 33440 b_VK210, A_h1a4 | |||
| Ambiguous | 9 | 16560 a_VK210, AC§ | 68 | 2040 c_VK210, C_h1a3 | ||
| 10 | 16000 c_VK210, A_hm†a2 | 59 | 2720 c_VK210, A_h5a2 | 60 (119) | 64 c_VK210, A_h1a2 | |
* For two markers, all of the gel picture patterns in the primary-attack parasite and reappearance parasite were the same.
† Digestion pattern not classified, because sum of PCR-RFLP fragment sizes exceeded uncut gene size.
‡ For two markers, at least one band in the gel picture patterns in the primary attack and in the reappearance parasite was different.
§ Digestion pattern not classified because of multiple-genotype infection classified by PCR result for the pvmsp3-α gene.
Among the patients who experienced reappearance of P. vivax malaria, the type B pvmsp3-α gene was not found in either the parasites responsible for the primary attack nor the reappearance. The proportion of pvcs gene genotype, VK type (regardless of gene size), and the pvmsp3-α gene size of the P. vivax parasite in the primary attack and in the reappearance of parasitaemia did not reveal any significant difference.
Discussion
Adherence to a 14-day primaquine regimen for the radical treatment of P. vivax hypnozoites, especially after symptoms have subsided, is a serious concern. In this study we found the non-adherence rate in treatment Week 2 was double compared to Week 1. The 15% non-adherence rate is probably an underestimate since some patients may not have reported missing a dose. Moreover, the home visit on Day 7 among the SAT group may increase the adherence rate among patients in that group, which further underestimates the reported non-adherence rate, compared with the real situation. The DOT method ensures that patients complete the full course of treatment. The findings of this study suggest that, compared with self-administration, DOT increases the likelihood of radical cure in P. vivax malaria infection. Patients with supervised therapy were about six times less likely to have P. vivax reappearance within the 90-day follow-up period. The protective effect of the DOT is likely to be larger than the estimate, if the underestimation of non-adherence rate in the SAT group was taken into account.
This study also showed that total primaquine dose per body weight < 2.75 mg/kg was associated with reappearance of P. vivax parasitaemia. This indicates that an inadequate dose of primaquine during the primary attack may increase the risk of a subsequent P. vivax attack. In practice, weight-independent doses are used because of the difficulties of using weight-adjusted doses in the field. Therefore, total primaquine dose per body weight varies considerably; e.g., patients who weigh 60 kg and 80 kg receive 3.5 mg/kg and 2.6 mg/kg, respectively, adhering to the adult regimen of "15 mg of primaquine daily for 14 days". Treatment failure due to an inadequate dose may be misinterpreted as drug resistance. This misinterpretation may occur not only between individuals, but also between areas because average body weight differs between countries and ethnic groups. The average bodyweight of patients in this study aged ≥ 14 years was 52.7 kg compared to 67.3 kg in a study in Brazil [14]. Therefore, weight-adjusted doses should be considered when assessing whether primaquine-resistant P. vivax is present. In addition, weight-based dosing schedules for primaquine should be more appropriate than the existing nationwide age-adjusted guideline for primaquine treatment due to a wide range of average body weight among different populations.
Poor adherence was more likely to be reported by males or children aged 8-13 years. While children aged 8-13 years showed the highest rate of non-adherence to primaquine treatment, they had the lowest rate of reappearance of P. vivax parasitaemia. This may be explained by the relatively high dose of primaquine that children aged 8-13 years received compared with the other age groups (age group 3-7 years: 4.74 ± 0.95 mg/kg, 8-13 years: 5.07 ± 0.95 mg/kg, ≥ 14 years: 4.19 ± 0.98 mg/kg). Even if these children skipped a dose once or twice, the total primaquine dose would still be sufficient to constitute a "therapeutic dose".
Higher multiple P. vivax-genotype infections were associated with increased risk of the reappearance of P. vivax parasitaemia, which may be the result of acquired genotype-specific host immunity [27]. Patients who suffer from mixed genotype infections may develop genotype-specific acquired immunity to only the dominant genotype in the primary attack. As a consequence, the low-level genotype in the primary attack could reappear more easily than the dominant genotype which may be suppressed by acquired genotype-specific immunity. In contrast, patients with a single genotype infection, whose acquired immunity develops during primary infection, can suppress the reappearing parasites that are caused by hypnozoites with the same genotype as those in the primary attack. In this study, at least six mixed-genotype infections were found among patients who had P. vivax reappearance; however, the conventional PCR used to analyse the genotype in this study was unable to identify the dominant genotype within an individual host. Using real-time PCR to determine the proportion of each genotype in the primary attack may help understand whether the dominant or the low-level genotype actually reappears.
The risk of the reappearance of P. vivax in this study was related to the level of parasitaemia on admission, consistent with a previous study from Brazil [14]. In Thailand, the major strain of the P. vivax parasite is the Chesson strain, which can produce about equal numbers of hepatic schizonts and hypnozoites [28]. This ratio remains constant regardless of the initial number of sporozoites inoculated [29]. Therefore, the absolute number of hypnozoites should increase with parasite count, which subsequently increases the probability of relapse.
The duration of fever before initial treatment was significantly associated with the reappearance of P. vivax. Patients who received early treatment (≤ 2 days) were more likely to develop a repeat P. vivax attack. This finding is possibly explained by the relationship between the timing of primaquine administration and the growth and stabilization of the hypnozoites. Research has not yet confirmed exactly how primaquine works on hypnozoites. However, primaquine is thought to affect the parasite mitochondrial electron transport chain [30]. Because hypnozoites are "dormant", they cannot replace their damaged mitochondria and become extinct [31]. The hypnozoites can be recognized three days after the host has been infected with P. vivax, and the size of hypnozoites becomes stable 7-15 days after infection [32-34]. However, it is unclear when the hypnozoites' function becomes "dormant". Since the incubation period of P. vivax is 12-17 days, some hypnozoites may not have stabilized yet during the first few days after the acute attack. Therefore, if primaquine administration is started earlier after an initial attack, the drug's effects may be less than expected because the hypnozoites are not "dormant" enough. The association between relapse rate and timing of treatment may be explained by the difference in the non-adherence rate between patients treated early and those who had treatment delay. In this study, non-adherence was found in 13% of patients who received late treatment, as compared with 17% of those with early treatment. However, this difference was not statistically significant.
The appearance of P. falciparum parasitaemia during the follow-up period was found to be a risk factor. However, the sample size was too small to obtain reliable results. Further research is needed to confirm these findings.
The pvcs and pvmsp3-α gene loci were used to compare the parasite genotypes in the primary attack and the reappearance of parasitaemia. The results showed a similar genotype pattern in the primary attack and the reappearance of parasitaemia. No particular genotypes were found to be associated with reappearance. Recently, P. vivax genome sequencing has revealed some genes that are likely related to dormancy or differentiation [35], and may be associated with risk of relapse. However, future research using these genes is needed to explore the association. In terms of comparison of genotypes between primary and subsequent attacks, most of the genotypes observed in the parasites in a primary attack were not exactly the same as the reappearance parasites. The genotypic difference in the reappearing parasite cannot distinguish relapse from heterozygous hypnozoite or re-infection [27]. Since the episodes of reappearance were observed within 90 days of the initial attack, when the risk of relapse was highest in this low malaria-transmission area, the parasitaemia reappearances observed were most likely due to relapse.
Conclusions
This study supports the supposition that poor adherence to 14-day primaquine treatment can affect the effectiveness of radical therapy for P. vivax malaria infection; DOT should be considered for improving compliance to 14-day primaquine treatment, since it reduces the risk of relapse. In areas where both tuberculosis and vivax malaria are prevalent, integrating DOT for P. vivax with existing DOT system for treatment of tuberculosis, where health workers had been trained for house visit to deliver drugs, should have a major impact on reducing disease transmission and on the financial burden of these two diseases. Inadequate dose of primaquine is also one of the most important factors for radical cure of P. vivax; weight-adjusted doses should be considered and the most practical way to apply weight-adjusted doses should be sought.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RT, MI, JKo, JKa, SPuk and PS designed the study protocol; SPua, NT, and WM collected the data; RT and MI conducted the PCR analysis; RT, JKa and PS analysed and interpreted the data; RT, SL, and NJD drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rie Takeuchi, Email: zhuneilihui@gmail.com.
Saranath Lawpoolsri, Email: tmslw@mahidol.ac.th.
Mallika Imwong, Email: noi@tropmedres.ac.
Jun Kobayashi, Email: j-kobayashi@it.ncgm.go.jp.
Jaranit Kaewkungwal, Email: tmjkk@mahidol.ac.th.
Sasithon Pukrittayakamee, Email: yon@tropmedres.ac.
Supalap Puangsa-art, Email: tmspa@mahidol.ac.th.
Nipon Thanyavanich, Email: tmnty@mahidol.ac.th.
Wanchai Maneeboonyang, Email: tmwmn@mahidol.ac.th.
Nicholas PJ Day, Email: nickd@tropmedres.ac.
Pratap Singhasivanon, Email: tmpsh@mahidol.ac.th.
Acknowledgements
This work was supported by the Grant for Research on Global Health and Medicine (21A-5) from the Ministry of Health, Labour and Welfare, Japan. We thank Dr. Nicholas J. White (Mahidol-Oxford Tropical Medicine Research Unit, Mahidol University) for his help and advice during the study. We are grateful to all staff of the Rajanagarindra Tropical Disease International Centre, Suan Phung, Ratchaburi, Thailand, for their contribution to data collection. We also acknowledge the support for laboratory work provided by the Wellcome Trust, Mahidol-Oxford Tropical Medicine Research Unit. Thanks to Mr. Paul Adams and Mr. Irwin F Chavez for editing the English language of the final manuscript.
References
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Malaria Report 2008. http://www.who.int/malaria/publications/atoz/9789241563697/en/index.html Accessed 09/05, 2009.
- World Health Organization. WHO SEARO, Malaria situation in SEAR countries. http://www.searo.who.int/EN/Section10/Section21/Section340_4027.htm Accessed 09/05, 2009.
- World Health Organization. Guidelines for the treatment of malaria 2006. 2006.
- Looareesuwan S, Wilairatana P, Krudsood S, Treeprasertsuk S, Singhasivanon P, Bussaratid V, Chokjindachai W, Viriyavejakul P, Chalermrut K, Walsh DS, White NJ. Chloroquine sensitivity of Plasmodium vivax in Thailand. Ann Trop Med Parasitol. 1999;93:225–230. doi: 10.1080/00034989958474. [DOI] [PubMed] [Google Scholar]
- Luxemburger C, van Vugt M, Jonathan S, McGready R, Looareesuwan S, White NJ, Nosten F. Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg. 1999;93:433–438. doi: 10.1016/S0035-9203(99)90149-9. [DOI] [PubMed] [Google Scholar]
- Congpuong K, Na-Bangchang K, Thimasarn K, Tasanor U, Wernsdorfer WH. Sensitivity of Plasmodium vivax to chloroquine in Sa Kaeo Province, Thailand. Acta Trop. 2002;83:117–121. doi: 10.1016/S0001-706X(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Vijaykadga S, Rojanawatsirivej C, Congpoung K, Willairatana P, Satimai W, Uaekowitchai C, Pumborplub B, Sittimongkol S, Pinyorattanachote A, Prigchoo P. Assessment of therapeutic efficacy of chloroquine for vivax malaria in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:566–569. [PubMed] [Google Scholar]
- Jelinek T, Nothdurft HD, Von Sonnenburg F, Loscher T. Long-term efficacy of primaquine in the treatment of vivax malaria in nonimmune travelers. Am J Trop Med Hyg. 1995;52:322–324. doi: 10.4269/ajtmh.1995.52.322. [DOI] [PubMed] [Google Scholar]
- Smoak BL, DeFraites RF, Magill AJ, Kain KC, Wellde B. Plasmodium vivax infections in U.S. army troops: failure of primaquine to prevent relapse in studies from Somalia. Am J Trop Med Hyg. 1997;56:231–234. doi: 10.4269/ajtmh.1997.56.231. [DOI] [PubMed] [Google Scholar]
- Yi KJ, Chung MH, Kim HS, Kim CS, Pai SH. A relapsed case of imported tertian malaria after a standard course of hydroxychloroquine and primaquine therapy. Korean J Parasitol. 1998;36:143–146. doi: 10.3347/kjp.1998.36.2.143. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Regev-Yochay G, Kurnik D. Short report: a consideration of primaquine dose adjustment for radical cure of Plasmodium vivax malaria. Am J Trop Med Hyg. 2000;62:393–395. doi: 10.4269/ajtmh.2000.62.393. [DOI] [PubMed] [Google Scholar]
- Kitchener SJ, Auliff AM, Rieckmann KH. Malaria in the Australian Defence Force during and after participation in the International Force in East Timor (INTERFET) Med J Aust. 2000;173:583–585. doi: 10.5694/j.1326-5377.2000.tb139349.x. [DOI] [PubMed] [Google Scholar]
- Duarte EC, Pang LW, Ribeiro LC, Fontes CJF. Association of subtherapeutic dosages of a standard drug regimen with failures in preventing relapses of vivax malaria. Am J Trop Med Hyg. 2001;65:471–476. doi: 10.4269/ajtmh.2001.65.471. [DOI] [PubMed] [Google Scholar]
- Spudick JM, Garcia LS, Graham DM, Haake DA. Diagnostic and therapeutic pitfalls associated with primaquine-tolerant Plasmodium vivax. J Clin Microbiol. 2005;43:978–981. doi: 10.1128/JCM.43.2.978-981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silachamroon U, Krudsood S, Treeprasertsuk S, Wilairatana P, Chalearmrult K, Mint HY, Maneekan P, White NJ, Gourdeuk VR, Brittenham GM, Looareesuwan S. Clinical trial of oral artesunate with or without high-dose primaquine for the treatment of vivax malaria in Thailand. Am J Trop Med Hyg. 2003;69:14–18. [PMC free article] [PubMed] [Google Scholar]
- Pukrittayakamee S, Imwong M, Looareesuwan S, White NJ. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria. Acta Trop. 2004;89:351–356. doi: 10.1016/j.actatropica.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Pukrittayakamee S, Imwong M, Singhasivanon P, Stepniewska K, Day NJ, White NJ. Effects of different antimalarial drugs on gametocyte carriage in P. vivax malaria. Am J Trop Med Hyg. 2008;79:378–384. [PubMed] [Google Scholar]
- Prasad RN, Virk KJ, Sharma VP. Relapse/reinfection patterns of Plasmodium vivax infection: A four year study. Southeast Asian J Trop Med Public Health. 1991;22:499–503. [PubMed] [Google Scholar]
- Srivastava HC, Sharma SK, Bhatt RM, Sharma VP. Studies on Plasmodium vivax relapse pattern in Kheda district, Gujarat. Indian J Malariol. 1996;33:173–179. [PubMed] [Google Scholar]
- World Health Organization. Global tuberculosis control: surveillance, planning, financing: WHO report 2006. http://www.who.int/tb/publications/global_report/2006/en/index.html Accessed 10/08, 2009.
- Nuchprayoon I, Sanpavat S, Nuchprayoon S. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Thailand: G6PD Viangchan (871G > A) is the most common deficiency variant in the Thai population. Hum Mutat. 2002;19:185–190. doi: 10.1002/humu.9010. [DOI] [PubMed] [Google Scholar]
- Buchachart K, Krudsood S, Singhasivanon P, Treeprasertsuk S, Phophak N, Srivilairit S, Chalermrut K, Rattanapong Y, Supeeranuntha L, Wilairatana P, Brittenham G, Looareesuwan S. Effect of primaquine standard dose (15 mg/day for 14 days) in the treatment of vivax malaria patients in Thailand. Southeast Asian J Trop Med Public Health. 2001;32:720–726. [PubMed] [Google Scholar]
- Nuchprayoon I, Louicharoen C, Charoenvej W. Glucose-6-phosphate dehydrogenase mutations in Mon and Burmese of southern Myanmar. J Hum Genet. 2008;53:48–54. doi: 10.1007/s10038-007-0217-3. [DOI] [PubMed] [Google Scholar]
- Imwong M, Pukrittayakamee S, Gruner AC, Renia L, Letourneur F, Looareesuwan S, White NJ, Snounou G. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J. 2005;4:20–32. doi: 10.1186/1475-2875-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MC, Galinski MR, Barnwell JW, Snounou G, Day KP. Polymorphism at the merozoite surface protein-3α locus of Plasmodium vivax: Global and local diversity. Am J Trop Med Hyg. 1999;61:518–525. doi: 10.4269/ajtmh.1999.61.518. [DOI] [PubMed] [Google Scholar]
- Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann JP, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NP, Anderson TJ, White NJ. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–932. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- Hollingdale MR, Collins WE, Campbell C. In vitro culture of exoerythrocytic parasites of the North Korean strain of Plasmodium vivax in hepatoma cells. Am J Trop Med Hyg. 1986;5:275–276. doi: 10.4269/ajtmh.1986.35.275. [DOI] [PubMed] [Google Scholar]
- Ungureanu E, Killick-Kendrick R, Garnham PCC, Branzei P, Romanescu C, Shute PG. Prepatent periods of a tropical strain of Plasmodium vivax after inoculations of tenfold dilutions of sporozoites. Trans R Soc Trop Med Hyg. 1976;70:482–483. doi: 10.1016/0035-9203(76)90133-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge WE, Dave D, Richards WHG. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Warhurst DC. Short communication: Why are primaquine and other 8-aminoquinolines particularly effective against the mature gametocytes and the hypnozoites of malaria? Ann Trop Med Parasitol. 1984;78:165. doi: 10.1080/00034983.1984.11811790. [DOI] [PubMed] [Google Scholar]
- Krotoski WA, Bray RS, Garnham PC, Gwadz RW, Killick-Kendrick R, Draper CC, Targett GA, Krotoski DM, Guy MW, Koontz LC, Cogswell FB. Observations on early and late post-sporozoite tissue stages in primate malaria. II. The hypnozoite of Plasmodium cynomolgi bastianellii from 3 to 105 days after infection, and detection of 36-to 40-hour pre-erythrocytic forms. Am J Trop Med Hyg. 1982;31:211–225. [PubMed] [Google Scholar]
- Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, Killick-Kendrick R, Wolf R, Sinden R, Koontz LC, Stanfill PS. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- Krotoski WA, Garnham PC, Cogswell FB, Collins WE, Bray RS, Gwasz RW, Killick-Kendrick R, Wolf RH, Sinden R, Hollingdale M. Observations on early and late post-sporozoite tissue stages in primate malaria. IV. Pre-erythrocytic schizonts and/or hypnozoites of Chesson and North Korean strains of Plasmodium vivax in the chimpanzee. Am J Trop Med Hyg. 1986;35:263–274. doi: 10.4269/ajtmh.1986.35.263. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang'a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]