Abstract
An octathymidylate was synthesized with the alpha anomer of thymidine instead of the naturally occurring beta anomer. This oligonucleotide binds to complementary sequences containing beta-nucleosides. Binding to ribose-containing oligomers and polymers is much stronger than binding to deoxyribose-containing analogs. A derivative of acridine (9-amino-6-chloro-2-methoxyacridine) was covalently attached either to the 5' phosphate or to the 3' phosphate of the alpha-octathymidylate. A pentamethylene linker was used to bridge the phosphate group and the 9-amino group of the acridine derivative. In both cases the complexes with the complementary sequences were strongly stabilized due to the additional binding energy provided by intercalation of the acridine ring within the miniduplex structure formed by the oligonucleotide with its target sequence. The acridine-substituted alpha-oligothymidylates did not lose their discrimination between ribose and deoxyribose-containing complementary sequences. The alpha-oligothymidylates were much more resistant towards endonucleases than their beta analogs, independently of whether they were linked to the acridine derivative. Acridine substitution provided additional protection against the corresponding exonucleases. alpha-Oligodeoxynucleotides covalently linked or not to intercalating agents represent families of molecules that open possibilities to block mRNA translation or viral RNA expression in vitro and in vivo.
Full text
PDF
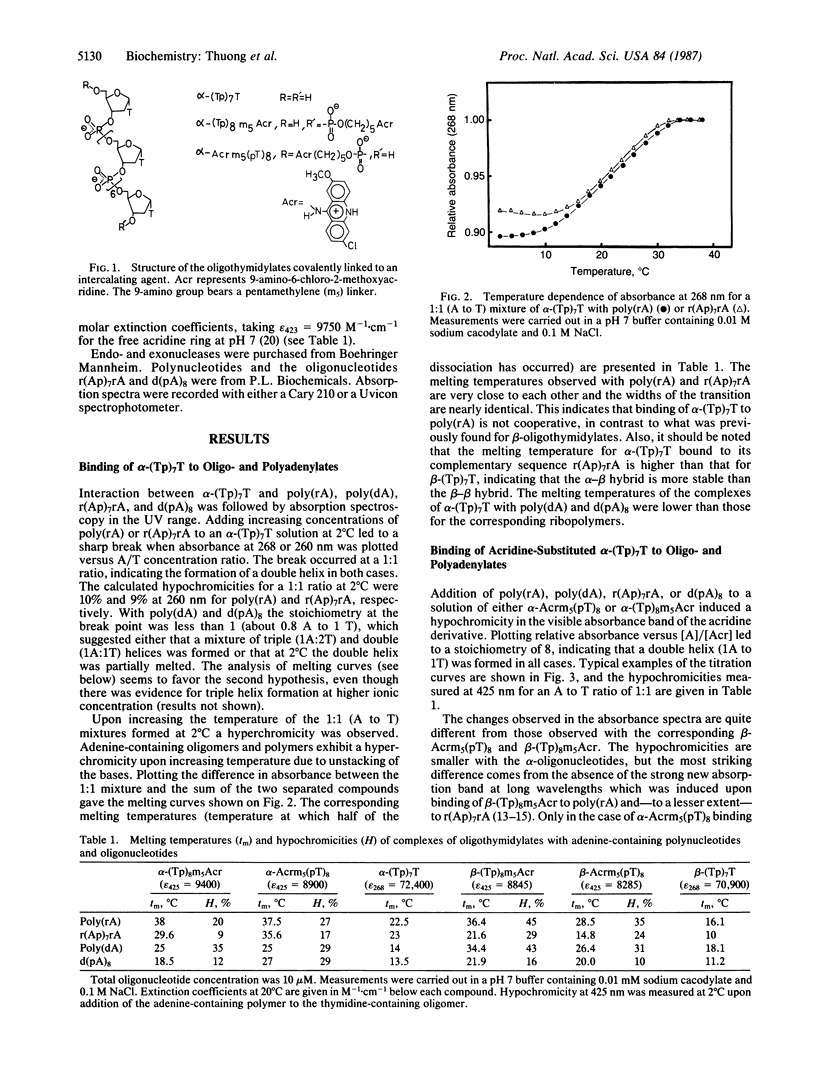
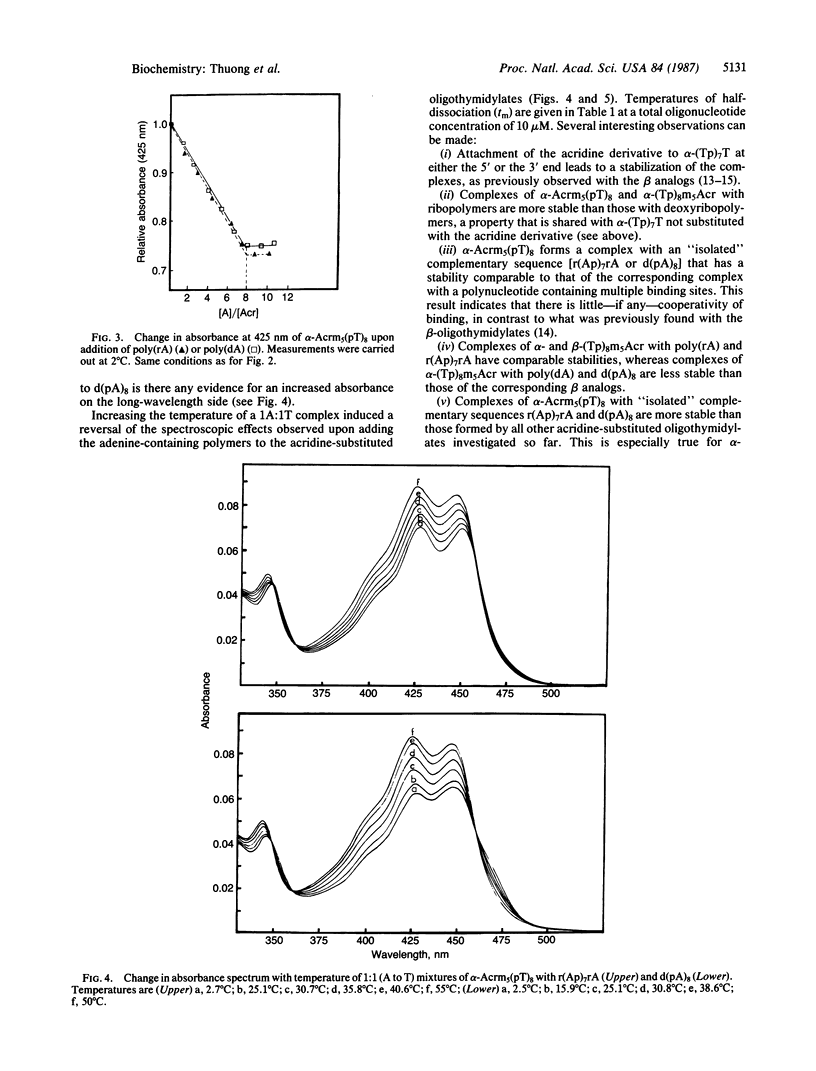
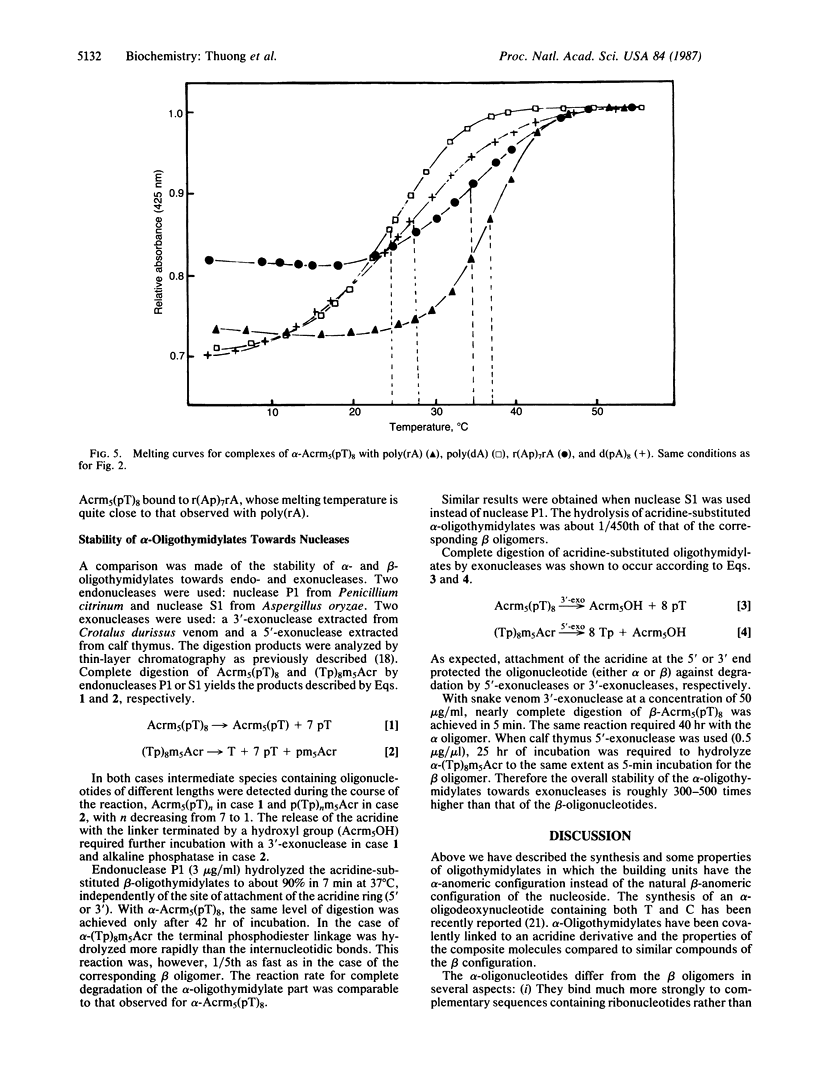

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Toulmé J. J., Hélène C. Anti-messenger oligodeoxynucleotides: specific inhibition of rabbit beta-globin synthesis in wheat germ extracts and Xenopus oocytes. Biochimie. 1986 Sep;68(9):1063–1069. doi: 10.1016/s0300-9084(86)80180-8. [DOI] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA. I. Synthesis, characterization by high field 1H-NMR, and base-pairing properties of the unnatural hexadeoxyribonucleotide alpha-[d(CpCpTpTpCpC)] with its complement beta-[d(GpGpApApGpG)]. Nucleic Acids Res. 1986 Jun 25;14(12):5019–5035. doi: 10.1093/nar/14.12.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. L'ARN non sens (nsARN): un outil pour inactiver spécifiquement l'expression d'un gène donné in vivo. C R Acad Sci III. 1984;299(8):271–274. [PubMed] [Google Scholar]
- Stephenson M. L., Zamecnik P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séquin U. Nucleosides and nucleotides. 5. The stereochemistry of oligonucleotides consisting of 2'-deoxy-alpha-D-ribosides, a study with Drieding stereomodels. Experientia. 1973 Sep 15;29(9):1059–1062. doi: 10.1007/BF01946717. [DOI] [PubMed] [Google Scholar]
- Thuong N. T., Chassignol M., Lancelot G., Mayer R., Hartmann B., Leng M., Hélène C. Synthesis and structural studies of a self-complementary decadeoxynucleotide d(AATTGCAATT). I.-Synthesis and chemical characterization of the decanucleotide. Biochimie. 1981 Oct;63(10):775–784. doi: 10.1016/s0300-9084(81)80037-5. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. D., Lopp I. G. Analysis of cooperativity and ion effects in the interaction of quinacrine with DNA. Biopolymers. 1979 Dec;18(12):3025–3041. doi: 10.1002/bip.1979.360181210. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Saneyoshi M. Synthetic nucleosides and nucleotides. XXI. On the synthesis and biological evaluations of 2'-deoxy-alpha-D-ribofuranosyl nucleosides and nucleotides. Chem Pharm Bull (Tokyo) 1984 Apr;32(4):1441–1450. doi: 10.1248/cpb.32.1441. [DOI] [PubMed] [Google Scholar]


