Abstract
IL-25 (IL-17E) is a member of IL-17 cytokine family. IL-25-deficient mice exhibit impaired Th2 immunity against nematode infection, implicating IL-25 as a key component in mucosal immunity. The sources of IL-25 and mechanisms responsible for the induction of Th2 immunity by IL-25 in the gastrointestinal tract remain poorly understood. There is also little information on the regulation of IL-25 during inflammation or its role in gut function. In the current study, we investigated the regulation of IL-25 during Nippostrongyrus brasiliensis (N. brasiliensis) infection and the contribution of IL-25 to the infection-induced alterations in intestinal function. We found that epithelial cells, but not immune cells, are the major source of IL-25 in the small intestine. N. brasiliensis infection-induced up-regulation of IL-25 depends upon IL-13 activation of STAT6. IL-25−/− mice had diminished intestinal smooth muscle and epithelial responses to N. brasiliensis infection, associated with an impaired Th2 protective immunity. Exogenous IL-25 induced characteristic changes similar to those following nematode infection, but was unable to restore the impaired host immunity against N. brasiliensis infection in IL-13−/− mice. These data show that IL-25 plays a critical role in nematode infection-induced alterations in intestinal function that are important for host protective immunity, and IL-13 is the major downstream Th2 cytokine responsible for the IL-25 effects.
IL-25, also called IL-17E, is a cytokine member of IL-17 family that includes IL-17A through F. While other members of the IL-17 family have biological activities similar to Th1 inflammatory cytokines, IL-25 appears to be involved primarily in the promotion of Th2 immunity, including allergy, asthma, and enteric nematode infection (1). Of equal importance is that IL-25 also inhibits pro-inflammatory Th1 and Th17 cytokine responses (2). IL-25−/− animals develop severe intestinal inflammation during nematode infection or are highly susceptible to experimental autoimmune encephalomyelitis (3,4). In addition, IL-25 is down-regulated in the colon in patients with inflammatory bowel disease (IBD) and exogenous IL-25 ameliorates experimental colitis in mice (5). Studies in isolated cell populations showed that IL-25 is produced by a variety of immune cells such as activated Th2 cells (1), mast cells (6), macrophages (7), and CD4+ and CD8+ T cells (2). More recently, it was demonstrated that peripheral human eosinophils and basophils secrete IL-25 (8). There is a low constitutive expression of IL-25 in several tissues, with the highest expression in GI tract, lung, and testis (1). IL-25 mRNA is up-regulated in the gut following Nippostrongylus brasiliensis (N. brasiliensis) infection or in the lung in response to Aspergillus fumigatus infection (9), but the principal source of IL-25 in infection remains to be identified. In addition, the mechanisms underlying the regulation of IL-25 expression during nematode infection are relatively unexplored.
Enteric nematode infection induces a polarized Th2 immune response, including elevated production of IL-4, IL-5, and IL-13, which contributes to worm expulsion. There is strong evidence supporting a role for IL-25 in the Th2-mediated protective immunity, as mice with IL-25 deficiency cannot expel worms efficiently and develop a Th1- and Th17-related chronic inflammation (2). Additionally, IL-25 is proposed to act in the initiation and amplification of allergic airway pathology that has striking similarities to nematode infection (2,10,11). Expulsion of enteric nematodes is associated with stereotypic changes in intestinal function including smooth muscle hyper-contractility, epithelial cell hypo-secretion, and increased mucosal permeability. These changes are dependent primarily on IL-4/IL-13 and receptor-mediated activation of STAT6 signaling pathways (12,13). The effects of IL-25 on gut function are unknown.
Given the potential anti-inflammatory effects of IL-25 and its importance in the development of Th2 immunity, the present study was designed to: (i) investigate the role of IL-4/IL-13 and STAT6 signaling in N. brasiliensis infection-induced up-regulation of IL-25; (ii)identify the specific cell population that produce/express IL-25 in the small intestine; (iii) determine the contribution of IL-25 in the N. brasiliensis infection-induced alterations in intestinal smooth muscle and mucosal function; and (iv) elucidate the downstream molecules that mediate the IL-25 effect on intestinal smooth muscle function. This study is the first to show that epithelial cells are the major sources of IL-25 in the small intestine during infection, that nematode-induced up-regulation of IL-25 depends upon IL-13 activation of STAT6, and that IL-25 has effects on gut function that are mediated primarily by IL-13.
Materials and Methods
Mice
BALB/c, C57BL/6, and severe compromised immunodeficiency (SCID) mice were purchased from the Small Animal Division of the National Cancer Institute or Jackson laboratory (Bar Harbor, ME 04609). Mice deficient in STAT6 (STAT6−/−), IL-4 (IL-4−/−), and IL-13 (IL-13−/−) on either BALB/c or C57BL/6 background were obtained from the breeding colonies at the University of Cincinnati (a generous gift from Dr. Fred Finkelman). Mice deficient in IL-25 (IL-25−/−) were generated by Regeneron Pharmaceuticals (Tarrytown, NY) and were backcrossed to the C57BL/6 background for 10 generations. These studies were conducted in accordance with principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Health and Human Services Publication (National Institutes of Health 85-23, revised 1996), and the Beltsville Animal Care and Use Committee, 2003.
Administration of IL-25 or IL-13
Mice (n=5/group) were injected i.v. with 0.7μg of mouse recombinant IL-25 or 10μg of IL-13 (R&D Systems, Minneapolis, MN) in 100μl of saline daily for 7 days or as indicated otherwise. Control mice were given an injection of saline (for IL-13 treatment) or 35μg BSA (for IL-25 treatment). The amount of cytokine administered was based on the observation that daily injection of immune competent BALB/c mice with this dose of IL-13 enhances worm expulsion (14), or the minimum dose of IL-25 that induced a prominent Th2 immune response from a previous study (1).
Enteric nematode infection and worm expulsion
Infective, third stage larvae of N. brasiliensis (L3) (specimens on file at the U.S. National Parasite Collection, U.S. National Helminthological Collection, Collection 81930, Beltsville, MD) were propagated and stored at room temperature in fecal/charcoal/peat moss culture plates until used (13). Groups of mice were inoculated subcutaneously with 500 L3. Except the time-course experiment, mice were euthanized and studied at day 9 (BALB/c) or 10 (C57BL/6) post-infection. The timing of the studies following infection with N. brasiliensis correlated with the time of the maximal effects on gut function and coincided with worm expulsion, based on the results from our previously- (13) or currently-performed time-course experiment on WT mice. Heligmosomoides polygyrus (H. polygyrus) and Trichuris muris (T. muris) infection were described previously (13,15). Appropriate age-matched controls were performed for each infection. Adult worms were detected quantitatively by scanning the intestinal surface with a dissecting scope. In general, WT mice completely expelled worms by day 9 in BALB/c or day 10 in C57BL/6 after inoculation, and therefore, the presence of adult worms in the intestine indicates delayed expulsion.
In vitro smooth muscle contractility in organ baths
In vitro smooth muscle contractility was measured as described previously (13). Smooth muscle responses to electric field stimulation (EFS), 5-HT, or acetylcholine, and the amplitude of spontaneous contractions were determined. Tension was expressed as force per cross sectional area (16).
In vitro epithelial cell ion transport in Ussing chambers
Muscle-free segments of small intestine were mounted in Ussing chambers as described previously (17). After a 15-minute period, concentration-dependent changes in short-circuit current (Isc) were determined in response to the cumulative addition of acetylcholine to the serosal side. Responses from all tissue segments exposed to acetylcholine from an individual animal were averaged to yield a mean response per animal.
Micro-snap well assay for mucosal transepithelial electrical resistance (TEER)
The modified micro-snap well system is a miniaturized version of the standard Ussing chamber that has been engineered to measure the TEER of intestinal fragments exposed to various stimuli (18). A decrease in TEER reflects increased tissue permeability. Briefly, segments of mouse intestine stripped of both muscle and serosal layers were placed in the micro-snap well system. Two hundred fifty μl of DMEM containing 4.5g/L glucose, 4mM L-glutamine, 50U/ml penicillin, 50μg/ml streptomycin, and MEM with 1mM non-essential amino acids was added to the mucosal side. Three milliliter of the same medium is added to the serosal side. The system was incubated at 37°C with 5%CO2 for 30 minutes to stabilize the pH and the baseline TEER measurement was measured.
Preparation of frozen tissue blocks and sectioning for laser-capture microdissection (LCM)
Frozen tissue blocks were prepared as described (19). Four-micrometer tissue sections used for LCM were obtained from frozen blocks using plain uncoated slides and a HM505E cryostat (Richard-Allan Scientific). Sections of frozen tissue were stained with hematoxylin and eosin (H&E) to assess changes in tissue morphology. Cryosectioned tissue was stained with H&E and dehydrated. LCM was performed with a PicCell II (Arcturus Engineering). Cells were captured from the region of the epithelium, lamina propria, and smooth muscle in the small intestine and transferred to CapSure LCM Caps (Arcturus Engineering).
RNA extraction, cDNA synthesis and real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from intestine whole tissue or from LCM samples as described previously (19). RNA samples (2μg) were reverse-transcribed to cDNA using the First Strand cDNA Synthase Kit (MBI Fermentas, Hanover, MD) with random hexamer primer. Real-time quantitative PCR was performed on an iCycler detection system (Bio-Rad, CA). PCR was performed in a 25μl volume using SYBR green Supermix (Bio-Rad, Hercules, CA). Amplification conditions were: 95°C for 3 min, 50 cycles of 95°C for 15s, 60°C for 15s, and 72°C for 20s. The fold-changes in mRNA expressions for targeted genes were relative to the respective vehicle groups of mice after normalization to 18s rRNA. Primer sequences (Table 1) were designed by using Beacon Designer 7.0 (Premier Biosoft International, Palo Alto, CA), and synthesized by the Biopolymer Laboratory of the University of Maryland.
Table 1.
Primer sequences for real-time quantitative PCR.
| Gene | Primer sequences (5’ to 3’) |
|---|---|
| IL-25 | Forward, CAGCAAAGAGCAAGAACC |
| Reverse, CCCTGTCCAACTCATAGC | |
| IL-17RA | Forward, ACTGTGGAGACCTTGGAC |
| Reverse, CTTGCTTAGAGTGAATGTGAC | |
| IL-17RB | Forward, ACCGTCTGTCGCTTCACTG |
| Reverse, CCACTTTATCTGCCGCTTGC | |
| IL-12p40 | Forward, AGATGAAGGAGACAGAGGAG |
| Reverse, GCACGAGGAATTGTAATAGC | |
| IL-23a | Forward, CAACTTCACACCTCCCTAC |
| Reverse, CCACTGCTGACTAGAACTC | |
| IFN-γ | Forward, TGGCTGTTTCTGGCTGTTACTG |
| Reverse, AGGTGTGATTCAATGACGCTTATG | |
| GATA3 | Forward, CTGAGCAGCCACCACACC |
| Reverse, GTGAAGAGATGAGGACTGGAGTG | |
| T-bet | Forward, GTATCCTGTTCCCAGCCGTTTC |
| Reverse, ACTGTGTTCCCGAGGTGTCC |
Immunofluorescence staining
Frozen blocks of mid-jejunum were prepared using the Swiss-roll technique and stored at −80°C. Tissue sections (4 μm) were cut from frozen blocks using an HM505E cryostat (Richard-Allan Scientific, Kalamazoo, MI). Tissue slides were fixed in cold acetone for 30 min and blocked with 10% normal rat serum in PBS for 1 hr at room temperature, incubated with anti-IL-25 antibody (1:50, R&D Systems, Minneapolis, MN) overnight at 4 °C, and then incubated with Alexa 488 rabbit anti-goat IgG. The slides were cover-slipped with Vectorshield (Vector Laboratories, Burlingame, CA) and digitally photographed using an Olympus microscope and Fluo View confocal software (Olympus America Inc., Centeral Valley, PA). The images were taken by establishing settings for the samples from the individual vehicle groups and using the same conditions to evaluate the samples from the infected or treated groups. Comparisons were made only among slides prepared on the same day.
Solutions and drugs
Krebs buffer contained (in mM) 4.74 KCl, 2.54 CaCl2, 118.5 NaCl, 1.19 NaH2PO4, 1.19 MgSO4, 25.0 NaHCO3, and 11.0 glucose. All drugs were obtained from Sigma (St Louis, MO) unless indicated otherwise. On the day of the experiment, 5-HT was dissolved in water and appropriate dilutions were made.
Data analysis
Agonist responses were fitted to sigmoid curves (Graphpad, San Diego, CA). Statistical analysis was performed using one-way ANOVA followed by Neuman-Keuls test to compare the responses and gene expression among the different treatment groups.
Results
Nematode-induced up-regulation of IL-25 is IL-13- and STAT6-dependent
Previous studies showed that N. brasiliensis infection increased mRNA expression of IL-25 in the small intestine (9). To determine the temporal changes in IL-25 expression during nematode infection, we infected WT BALB/c mice with N. brasiliensis and collected tissue at day 5, 7, 9, and 14 post infection. Real-time PCR analysis showed that IL-25 mRNA expression in the small intestine began to rise at day 7 (1.0±0.2 in vehicle versus 3.1±1.0 in N. brasiliensis, n=5, p<0.05), peaked at day 9 (Figure 1A), and returned to control levels by day 14, post infection (data not shown). Notably, the infection-induced up-regulation of IL-25 in mice was not specific to N. brasiliensis, nor was it restricted to the small intestine, as similar results were observed in H. polygyrus-infected small intestine (1.0±0.1 in vehicle versus 8.0±2.0 in H. polygyrus, n=5, p<0.05) or T. muris-infected colon (1.0±0.2 in vehicle versus 2.8±0.6 in T. muris, n=5, p<0.05). In addition, the expression of IL-25 was independent of the genetic background of the mouse, since similar effects were seen in both BALB/c and C57BL/6 mice (data not shown).
Figure 1.
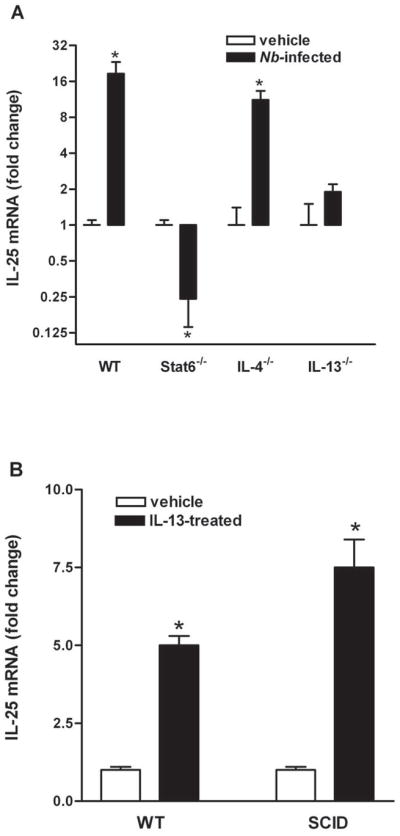
Nematode-induced IL-25 is IL-13- and STAT6-dependent. Mice were infected with N. brasiliensis (Nb) and studied at day 9 post-infection (A), or injected i.v. with 10μg of mouse recombinant IL-13 daily and studied at day 7 post-injection (B). qPCR was performed to measure mRNA expression in the small intestine. The fold changes were relative to the individual vehicle groups after normalization to 18s rRNA. * p<0.05 versus the respective vehicle group (n≥5 for each group).
We next determined the role of IL-4/IL-13 activation of STAT6 on the up-regulation of IL-25 using IL-4−/−, IL-13−/−, or STAT6−/− mice following N. brasiliensis infection. As the time-course experiment on WT BALB/c mice showed that IL-25 mRNA expression peaked at day 9 post-infection, this time-point was therefore selected for the studies to investigate the immune regulation of IL-25 expression. There was a similar mRNA expression of IL-25 in all strains of uninfected mice (data not shown). The infection-induced up-regulation of IL-25 in WT mice was present in IL-4−/− mice, but absent in STAT6−/− and IL-13−/− mice, indicating a dependence on IL-13 activation of STAT6 (Figure 1A). The importance of IL-13 is supported further by the finding that exogenous administration of IL-13 up-regulated IL-25 expression (Figure 1B).
Epithelial cells, but not immune cells, are the major cellular source of IL-25 in the small intestine
To determine the source of IL-25 in the small intestine, we used qPCR in epithelial, smooth muscle, and lamina propria captured by LCM. As described previously, mRNA expression of the specific molecular markers for epithelial (villin), smooth muscle (α smooth muscle actin), and immune cells (leukocyte common antigen) was analyzed first on the respective LCM insure the purity of the samples (19). Intestinal epithelial cells from control mice expressed detectable levels of IL-25 mRNA that were further up-regulated by N. brasiliensis infection (Figure 2A). Of interest is that expression of IL-25 was undetectable in either lamina propria or smooth muscle of small intestine from control or nematode-infected mice, suggesting that immune or smooth muscle cells do not express measurable levels of IL-25 mRNA (Figure 2A). As a positive control for RNA recovery, IL-13 was detectable in LCM-captured lamina propria from both control and infected mice. There was also no IL-25 mRNA expression in mesenteric lymph nodes in either control or N. brasiliensis-infected mice. Consistent with a non-T/non-B cell source of IL-25, we found that exogenous administration of IL-13 up-regulated IL-25 expression in SCID mice (Figure 1B).
Figure 2.
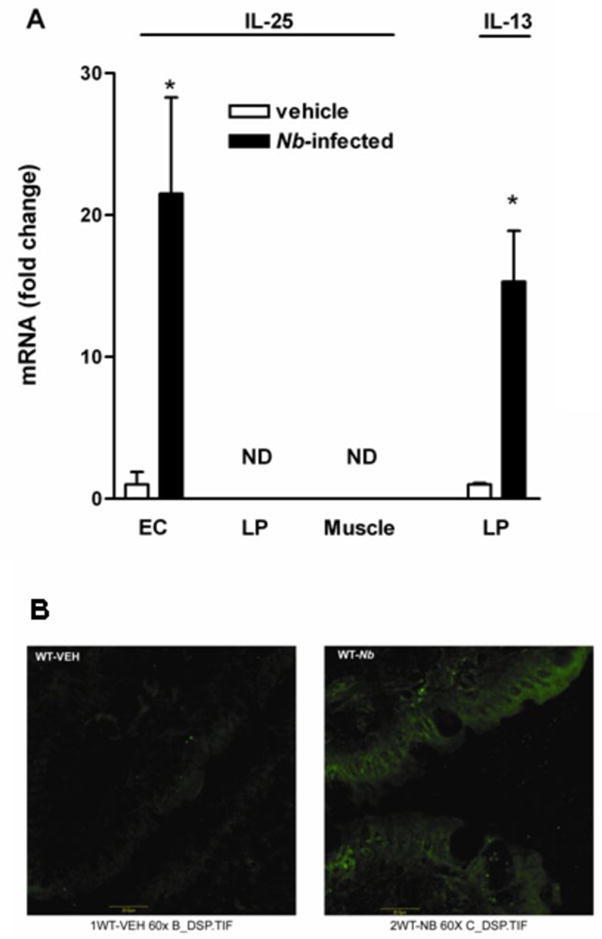
Epithelial cells, but not immune cells, are the major cellular source of IL-25 in the small intestine. Mice were infected with N. brasiliensis and studied 9 days later. (A) LCM combined with qPCR technique was performed to determine mRNA expression in specific cell populations from captured intestinal epithelial (EC), lamina propria (LP), and smooth muscle (Muscle). (B) Frozen tissue blocks of mid-jejunum were prepared and the sections were cut for immunofluoresence staining with anti-IL-25mAb. Pictures are representative of each group of at least 5 mice. Original magnification, x600.
To further confirm that IL-25 was expressed selectively on intestinal epithelial cells, we used immunofluorescent staining of frozen sections of small intestine with anti-IL-25 mAb. Similar to mRNA data from LCM samples, IL-25 staining was evident only in epithelial cells, but not in the lamina propria or smooth muscle. Staining of surface cells was significantly increased in intensity in N. brasiliensis-infected mice (Figure 2B). The specificity of the antibody to IL-25 was validated by a preliminary experiment showing no staining on tissue slide when the antibody was omitted. Moreover, no staining was revealed when the same antibody was used for the intestinal tissue slides from either IL-25KO control or infected mice.
Immune-regulated IL-25 receptor expression
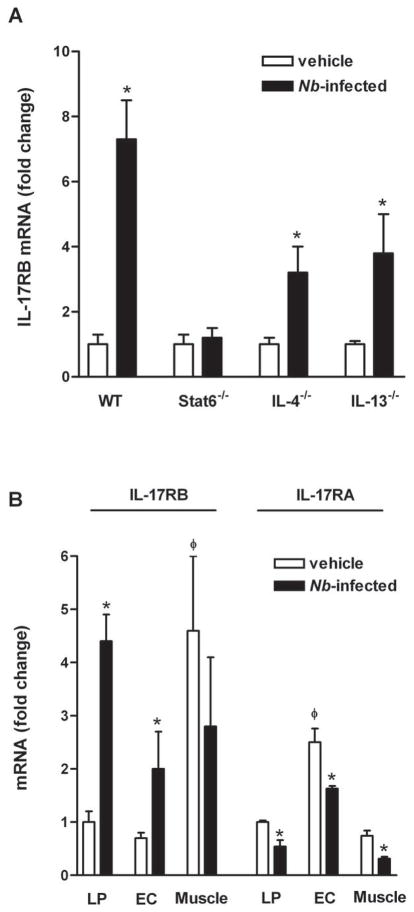
The activity of IL-25 requires the receptor subunit IL-17RB, which binds IL-25, as well as IL-17RA. Interestingly, however, IL-17RA does not appear to interact directly with IL-25, but does bind other members of the IL-17 family (20). To determine the potential IL-25-responsive cells in the small intestine, we measured IL-25 receptor mRNA expression at baseline and after nematode infection. In whole tissue, only IL-17RB was up-regulated significantly in response to infection by a mechanism that was STAT6-dependent (Figure 3A). In addition, the elevated expression was observed in infected IL-4−/− or IL-13−/− mice, indicating that either Th2 cytokine alone can elevate the expression (Figure 3A). LCM revealed constitutive expression of both IL-17RA and IL-17RB in epithelial and smooth muscle cells, with the highest expression of IL-17RA in epithelial cells and the highest expression of IL-17RB in smooth muscle cells (Figure 3B). Unlike IL-25, both IL-17RA and IL-17RB were expressed constitutively on immune cells in the lamina propria. Nematode infection increased expression of IL-17RB in epithelial cells and in the lamina propria, but did not further increase expression in smooth muscle (Figure 3B). In contrast, expression of IL-17RA was reduced in the entire cross section of the small intestine, including cells from lamina propria, epithelial, and smooth muscle (Figure 3B).
Figure 3.
Immune-regulated IL-25 receptor expression. Mice were infected with N. brasiliensis and studied 9 days later. qPCR was performed to examine mRNA expression of IL-17RA and IL-17RB on samples from small intestine (A), or LCM-captured intestinal lamina propria (LP), epithelial (EC), or smooth muscle cells (Muscle) (B). The fold changes were relative to the individual vehicle groups (A) or vehicle LP (B) after normalization to 18s rRNA. * p<0.05 versus the respective vehicle group; φp<0.05 versus the respective LP (n≥5 for each group).
Contribution of IL-25 to nematode infection-induced intestinal smooth muscle and mucosal function
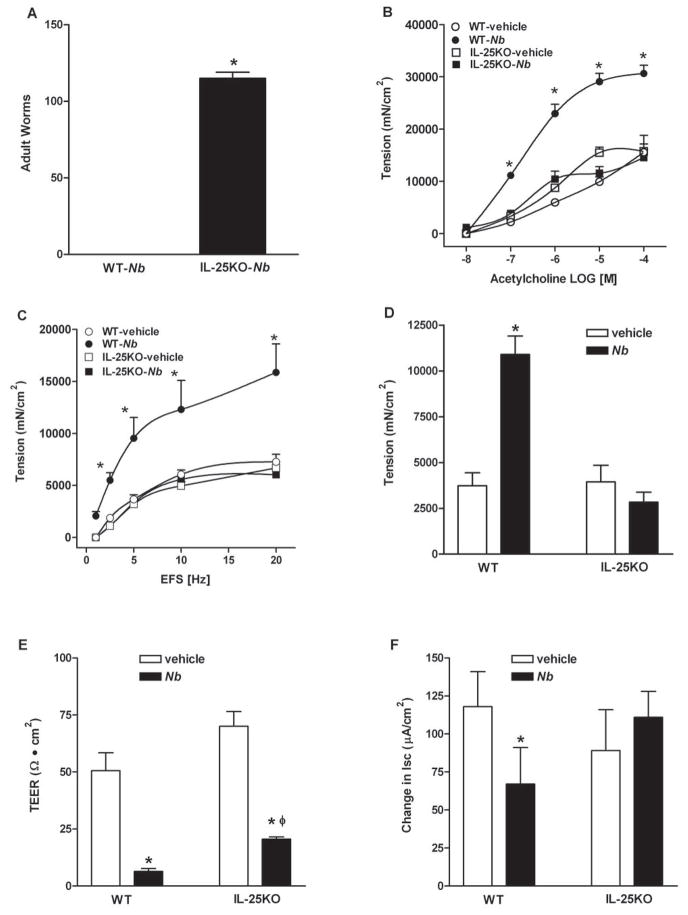
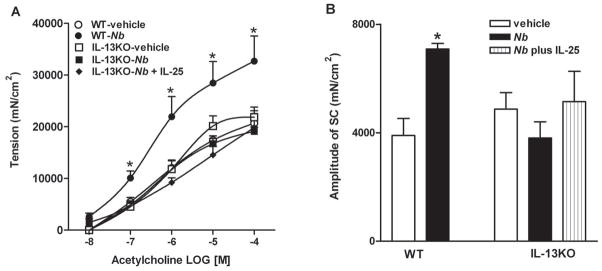
In animals with nematode infection, a measurement of successful Th2 protective immunity is the effective clearance of adult worms, which is completed by day 9 in BALB/c and day 10 in C56BL/6 WT mice after inoculation. Expulsion was impaired in IL-25−/− mice on the C56BL/6 background, as significant numbers of worms remained in the small intestine at day 10 (Figure 4A). Changes in gut function facilitate worm clearance; therefore, we compared smooth muscle and epithelial cell function in WT and IL-25−/− mice. N. brasiliensis infection induced a characteristic intestinal smooth muscle hyper-contractility in WT mice as shown previously (13,21–23). The enhanced smooth muscle responses to acetylcholine, electric field stimulation, and 5-HT were absent completely in infected IL-25−/− mice (Figure 4B–D). In addition, the increased amplitude of spontaneous contractions in infected WT mice (4247±1275 in vehicle versus 8517±1851 in infected, p<0.05, n≥5) was abrogated as well in IL-25−/− mice (4476±1378 in vehicle versus 4037±424 in infected, n≥3).
Figure 4.
Impaired host immunity against N. brasiliensis infection in IL-25−/− mice was associated with a diminished intestinal smooth muscle and epithelial responses to infection. C57BL/6 mice (WT) or mice with IL-25 deficiency (IL-25−/−) were infected with N. brasiliensis (Nb) and studied at day 10 post-infection. Numbers of adult worms were counted (A). Intestinal strips were suspended longitudinally in organ baths for in vitro contractility studies in response to (B) acetylcholine (ACH, 10nM-0.1mM), (C) EFS (1–20Hz, 80V), or (D) 5-HT (100μM). For epithelial functional studies, muscle-free mucosa was mounted in (E) microsnap-well for the measurement of TEER, or in Ussing chambers for epithelial cell response to (F) acetylcholine (1mM). *p<0.05 versus WT-Nb (A), or the respective vehicle (B–F); φp<0.05 versus the respective WT (n≥3 for each group).
IL-25 also plays a conspicuous role in infection-induced changes in epithelial cell function. Although the increase in mucosal permeability (decrease in TEER) was attenuated modestly in IL-25−/− mice (Figure 4E), the stereotypic reduction in epithelial secretion in response to acetylcholine was not observed in IL-25−/− mice (Figure 4F).
Exogenous IL-25 induced similar changes in intestinal epithelial and smooth muscle function to nematode infection
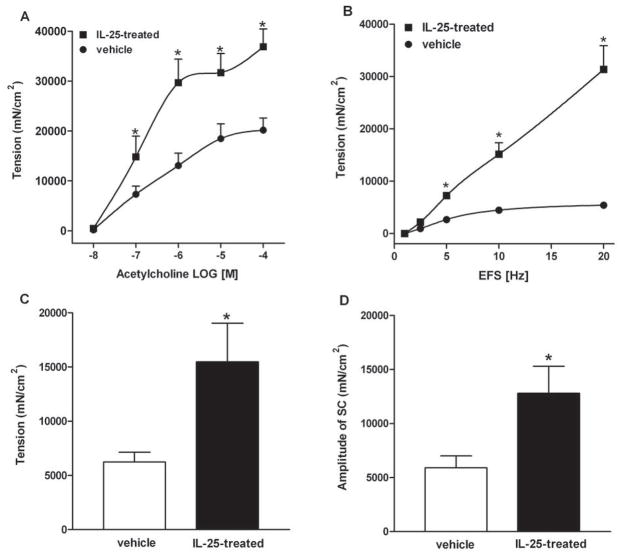
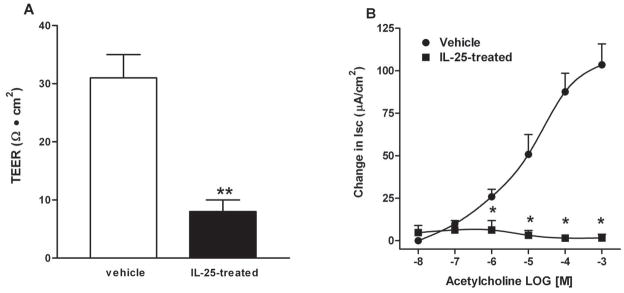
To further investigate the effects of IL-25 on gut function, we administered recombinant IL-25 to mice daily for 7 days. Exogenous IL-25 induced a smooth muscle hyper-contractility in response to acetylcholine (Figure 5A), electrical field stimulation (Figure 5B), and 5-HT (Figure 5C) and increased the amplitude of spontaneous contractions (Figure 5D). Epithelial responses were also altered by IL-25, including increased mucosal permeability (Figure 6A) and hypo-secretion in response to acetylcholine (Figure 6B). These changes in gut function were similar to those reported previously in response to nematode infection or exogenous administration of IL-4/IL-13 (13,17,21,24,25).
Figure 5.
Exogenous IL-25 induced intestinal smooth muscle hyper-contractility in mice. BALB/c mice were injected i.v. with 0.7μg of IL-25 daily and studied at day 7 post-injection. Intestinal strips were suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH, 10nM-0.1mM), (B) EFS (1–20Hz, 80V), (C) 5-HT (100μM), or (D) for spontaneous contraction. *p<0.05 versus the respective vehicle (n≥5 for each group).
Figure 6.
Exogenous IL-25 increased mucosal permeability and induced stereotypic changes in mucosal epithelial function in mice. BALB/c mice were injected i.v. with 0.7μg of IL-25 daily and studied at day 7 post-injection. Muscle-free mucosa was mounted in (A) microsnap-well for the measurement of TEER, or in (B) Ussing chambers for epithelial secretion in response to acetylcholine (10nM-1mM). *p<0.05 versus the respective vehicle (n≥5 for each group).
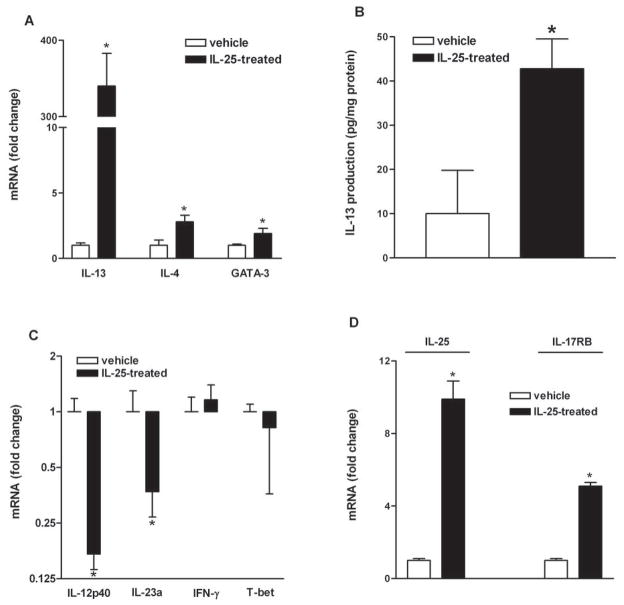
IL-25-induced changes in gut function were associated with a significant up-regulation of the Th2 cytokines, IL-4 and IL-13, and master Th2 transcription factor, GATA-3 (Figure 7A). The ability of IL-25 to increase IL-13 mRNA was validated further by ELISA, which showed an elevation in in situ IL-13 production in the intestine of mice treated with IL-25 (Figure 7B). The major Th1 cytokines, IL-12p40 and IL-23a, were significantly down-regulated in IL-25-treated mice, while IFN-γ and the Th1 master transcription factor, T-bet, remained unchanged (Figure 7C). Interestingly, IL-25 administration up-regulated the expression of IL-25 itself and its receptor, IL-17RB, suggesting a positive feedback mechanism that could significantly amplify the effect of IL-25 during infection (Figure 7D).
Figure 7.
Exogenous IL-25 selectively influenced T helper (Th) cell immune responses in the small intestine in mice. BALB/c mice were injected i.v. with 0.7μg of IL-25 daily and studied at day 7 post-injection. qPCR was performed to measure the mRNA expression of major cytokines of Th2 (A), Th1 (C), and IL-25 and IL-17RB (C). ELISA was performed to validate in situ IL-13 production in the small intestine (B). The fold increases for mRNA expression were relative to the individual vehicle groups after normalization to 18s rRNA. * p<0.05 versus the respective vehicle group (n≥5 for each group).
Exogenous IL-25 was unable to restore the impaired host immunity against N. brasiliensis infection in IL-13−/− mice
Previous studies proposed that the biological activities of IL-25 are mediated by the downstream effects on Th2 cytokines IL-4, IL-5, IL-9, and IL-13 (11), but whether this involves a specific cytokine or a combination of cytokines is unknown. To address this question, we administered IL-25 daily to N. brasilienisis-infected IL-13−/− mice, using the same dose that evoked a significant increase in smooth muscle contractility in WT mice (Figure 5). The infection-induced intestinal smooth muscle hyper-contractility to acetylcholine and the increased amplitude of spontaneous contractions, observed in WT mice (13,21,23), was absent in infected IL-13−/− mice and was not restored by IL-25 (Figure 8). Finally, exogenous IL-25 did not reinstate the host defense against N. brasiliensis infection in IL-13−/− mice, as both vehicle- and IL-25-treated IL-13−/− mice harbored comparable numbers of adult worms in the small intestine, whereas C57BL/6 WT mice expelled worms completely at day 10 post-infection (data not shown).
Figure 8.
Exogenous IL-25 was unable to restore the impaired host immunity against N. brasiliensis infection in mice with IL-13 deficiency. Mice were infected with N. brasiliensis (Nb) and studied at day 10 post-infection. One separate group of IL-13−/− mice received daily injection of IL-25 simultaneously with N. brasiliensis infection, starting from day 1 until day 8 post-infection. Intestinal strips were suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH, 10nM-0.1mM), or (B) for spontaneous contraction. *p<0.05 versus the respective vehicle (n≥5 for each group).
Discussion
IL-25 is emerging as a key regulator of inflammation in the gut mucosa because of its ability to promote Th2, while suppressing Th1 and Th17, cytokine responses. In the present study, we demonstrated that there is a constitutive expression of IL-25 in intestinal epithelial cells, but not in immune cells, which is up-regulated significantly during nematode infection. IL-17RB, the receptor that binds IL-25, is located on both structural and immune cells and is also up-regulated during infection. Infection-induced increases in both IL-25 and IL-17RB expression involved activation of STAT6. Furthermore, IL-13 is the major downstream Th2 cytokine responsible for the IL-25-mediated changes in gut function that are important for protective immunity against nematode infection.
Low levels of IL-25 mRNA are found in many tissues with the highest expression in gastrointestinal tract (1). There are, however, controversial reports regarding the cell types that produce IL-25. For example, Fort et al. showed that IL-25 was expressed exclusively by Th2 cells (1), whereas Wang et al. demonstrated that eosinophils and basophils, but not other immune cells (T cells, B cells, monocytes, DC cells, etc), secreted IL-25 (8). In response to N. brasiliensis infection, various immune cells including macrophages, dendritic cells, eosinophils, and Th2 cells, are activated and recruited to the intestinal lamina propria. Previous results showed that certain isolated immune cells, including highly polarized Th2 cells, could be induced to express IL-25 (1,2,6,7). Notably, we observed that cells from intestinal lamina propria and mesenteric lymph nodes had undetectable levels of IL-25 mRNA constitutively and during nematode infection, suggesting that immune cells in the gut are not the major source of IL-25 in vivo. The finding that epithelial cells produce IL-25 is consistent with previous reports in both the colon (4) and lung (8). Of interest is the ability of nematode infection or exogenous IL-13 to up-regulate epithelial cell expression of IL-25, which was coincident with a reduction in pro-inflammatory cytokines IL-23 and IL-12p40. Others showed a down-regulation of IL-25 in colonic epithelial cells in the absence of commensal bacteria, associated with an over-expression of IL-23 and IL-12p35 (4). These data support an important role for epithelial-derived IL-25 in immune homeostasis in both the small intestine and colon, acting in particular to control the development of Th17-driven pathologies. This is the first report showing that epithelial cells are the major source of IL-25 in the small intestine during infection; however, we cannot rule out the possibility that immune cells from other portions of the gut, or under other conditions in the small intestine, can express IL-25.
The receptor for IL-25 consists of IL-17RB, which binds IL-25 and is a 56-kDa single transmembrane protein expressed abundantly in kidney, intestine, and other peripheral organs (26), and IL-17RA, which appears to be necessary for the biological activity of IL-25 in the lung (20) and is expressed in both structural and immune cells (27). While IL-25 mRNA was found only in epithelial cells, both structural and immune cells in the small intestine express IL-17RA and IL-17RB. Infection up-regulated IL-17RB expression by a mechanism involving IL-4/IL-13 and STAT6, an effect similar to that observed for IL-25. The observation that exogenous IL-25 also up-regulated its own expression, as well as IL-17RB, suggests a novel positive feedback mechanism in which epithelial-derived IL-25 can maintain its own expression. In the lung, part of the IL-25 effect was attributed to up-regulation of IL-17A, which binds to IL-17RA (20). The role of IL-17RA in IL-25-mediated effects in the small intestine is less evident, as neither nematode infection nor exogenous IL-25 altered the already constitutively low expression of IL-17A (our unpublished data). Indeed, IL-25 down-regulated IL-17RA, suggesting that the major effects of IL-25 in the intestine appear to be mediated through IL-25 binding to IL-17RB.
In the present study, we extend previous findings and show that the up-regulation of IL-25 is perhaps a common feature in nematode infection, as comparable increases were observed in N. brasiliensis and H. polygyrus infection of the small intestine as well as T. muris infection of the colon. IL-25 is proposed to act as both an initiating and amplification factor for the Th2 immune response in allergic airway inflammation (10). During the course of N. brasiliensis infection, IL-25 mRNA expression peaked at day 9 post-infection, which follows the up-regulation of IL-13 and is coincident with the maximal changes in intestinal function (Zhao Gastro 2008). These data indicate that IL-25 may act more as an amplification factor than an initiator of the Th2 response during nematode infection. The mechanisms underlying the regulation of IL-25 remain relatively unexplored. In the present study, exogenous IL-25 increased IL-4/IL-13 expression, yet nematode infection or exogenous IL-13 also up-regulated IL-25 expression by a STAT6-dependent mechanism. This reciprocal control between IL-25 and IL-13 constitutes a double positive feedback mechanism that acts to amplify the Th2 immune response in vivo, and may play an important role in developing and maintaining the host defense against nematode infection. This is a novel role for IL-13, as IL-25 is thought to be important primarily for the initiation of the Th2 response, an effect confirmed in the current study by the increased generation of IL-13 in response to exogenous IL-25. The biological activity of IL-13 is mediated by IL-13Rα1, which forms the type 2 IL-4R complex with IL-4Rα, linked to STAT6 (28). Both IL-4Rα and IL-13Rα1 are expressed constitutively on epithelial cells (29). Taken together, these data indicate that epithelial-derived IL-25 binds to local immune cells leading to the release of IL-13, which binds to the type 2 IL-4R to activate STAT6, resulting in an increased epithelial cell production of IL-25.
Nematode infection is associated with STAT6-dependent changes in both epithelial and smooth muscle function that facilitate worm expulsion (13,17,24,25). IL-13 is the dominant effector molecule that coordinates the host immune response to N. brasiliensis infection and mice deficient in IL-13 exhibit impaired worm clearance (30). Indeed, many of the infection-induced changes in gut function are dependent largely on IL-13 (13). In the present study, however, the infection-induced changes in smooth muscle function were dependent entirely on IL-25. Although smooth muscle contains receptors for IL-25, IL-17RA and IL-17RB, the fact that exogenous IL-25 could not induce worm clearance or hyper-contractility in IL-13−/− mice indicated that these effects were mediated by the downstream up-regulation of IL-13. This is consistent with data showing that IL-13 was required for IL-25-mediated protection against experimental autoimmune encephalomyelitis and Th17 suppression (3). Since the primary source of IL-25 appears to be epithelial cells, a direct effect of IL-25 on smooth muscle in vivo is unlikely. These data emphasize the importance of IL-25 up-regulation of IL-13, which then induces a hyper-contractility of smooth muscle directly via STAT6-dependent gene expression, as well as indirectly through alternatively activated macrophages (23). The ability of IL-25 to down-regulate expression of pro-inflammatory Th1 and Th17 cytokines, which are associated with a hypo-contractility of smooth muscle, may also contribute to the indirect effects of IL-25 on smooth muscle. The function of IL-17RB on smooth muscle and its interaction with IL-17RA is unclear, as the downstream signaling of these receptors has not been fully elucidated, but may function to influence the activity of other cytokines that bind these subunits on smooth muscle.
IL-13 is not expressed by structural cells; therefore, the effects of IL-25 on IL-13 can be attributed to IL-17RA/IL-17RB on immune cells. Identification of the cell types that respond to IL-25 and generate Th2 cytokines, especially in the gut, is an area of active research. Early studies indicated these cells were perhaps a novel population of IL-4/5/13-producing non-B non-T cells (1). Four more recent papers showed that several previously-unidentified cell populations may also respond to IL-25, including “multipotent progenitor cell population” (31), “natural helper cells” (32), “nuocytes” (33), and “Ih2 cells” (34). It remains to be determined whether any of these cell types contribute to IL-25-induced production of Th2 cytokines that impact intestinal function.
The current study showed th at exogenous IL-25 mimicked the effects of nematode infection or exogenous IL-4 and IL-13 on epithelial cell permeability and secretion (17,24,25). IL-25 also plays a role in nematode infection-induced alterations in epithelial function, as the infection-induced epithelial hypo-secretion in response to acetylcholine was abrogated in deficient mice, and the increased permeability was modestly attenuated. Previous studies demonstrated that these two nematode-induced effects were STAT6-dependent; therefore, the inability of IL-25 deficiency to completely prevent the infection-induced changes in permeability was surprising. This result may be attributed to a direct effect of worms on intestinal permeability that is independent of IL-25-mediated up-regulation of Th2 cytokines. Previous studies showed that mice deficient in IL-25 were unable to expel worms, while administration of IL-25 restored the host immunity against T. muris in genetically susceptible mice or induced rapid worm expulsion in N. brasiliensis-infected WT mice (2,11). The presence of worms in the intestine of deficient mice supports the hypothesis of a direct effect.
In conclusion, this is the first study to show the importance of IL-25 in nematode infection-induced changes in intestinal function that contribute to worm expulsion. While IL-25 is implicated in the host immune response to nematode infection through regulation of Th2 cytokines (2,11), the expression of IL-25 and the receptors are also regulated by Th2 cytokine activation of STAT6. This reciprocal regulation plays an important role in initiation and maintenance of the Th2 protective immunity during nematode infection. It is likely that IL-25 produced by epithelial cells acts on receptors, primarily IL-17RB, located on immune cells to amplify the local expression of IL-13. Indeed, IL-13 plays a critical role in the biological activities of IL-25 in host immunity against enteric nematode infection. The ability of IL-25 to down-regulate the expression of pro-inflammatory cytokines supports a key role for this epithelial-derived cytokine in mucosal homeostasis and may provide a novel therapeutic target for the treatment of gut pathologies such as IBD.
Acknowledgments
The authors wish to thank Dr. Fred D Finkelman, University of Cincinnati, for providing STAT6- and IL-13-deficient mice, Drs. Margaret Karow and Drew Murphy at Regeneron Pharmaceuticals for providing breeding pairs of IL-25−/− mice, and Dr. Mohan E. Tuiapurkar from University of Maryland School of Medicine for technical assistance on using Olympus microscope.
This work was supported by NIH grants R01-DK083418 (AZ), R01-AI/DK49316 (T.S-D), and USDA CRIS project #1235-51000-055 (JFU). The opinions and assertions in this article are those of the authors and do not necessarily represent those of the U. S. Department of Agriculture or the Department of Defense.
Abbreviations
- N. brasiliensis
Nippostrongylus brasiliensis
- IL-4−/−
IL-4-deficient
- IL-13−/−
IL-13-deficient
- IL-25−/−
IL-25-deficient
- STAT6−/−
STAT6-deficient
- WT
wild type
Footnotes
The authors have no conflicts of interest to disclose.
Reference List
- 1.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 2.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. The Journal of Experimental Medicine. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruso R, Sarra M, Stolfi C, Rizzo A, Fina D, Fantini MC, Pallone F, MacDonald TT, Monteleone G. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270–2279. doi: 10.1053/j.gastro.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 7.Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, Rhim TY, Park CS. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–296. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- 8.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 10.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. The Journal of Experimental Medicine. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells 3. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–G232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 13.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on STAT6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 14.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and STAT6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 15.Urban J, Fang H, Liu Q, Ekkens MJ, Chen SJ, Nguyen D, Mitro V, Donaldson DD, Byrd C, Peach R, Morris SC, Finkelman FD, Schopf L, Gause WC. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J Immunol. 2000;164:4250–4256. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- 16.Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther. 2001;299:768–774. [PubMed] [Google Scholar]
- 17.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF., Jr The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 18.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto M, Zhao A, Sun R, Stiltz J, Madden KB, Mentink-Kane M, Ramalingam T, Wynn TA, Urban JF, Jr, Shea-Donohue T. IL-13 Receptor {alpha}2 Regulates the Immune and Functional Response to Nippostrongylus brasiliensis Infection. J Immunol. 2009;183:1934–1939. doi: 10.4049/jimmunol.0804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickel EA, Siegel LA, Yoon BRP, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of Functional Roles for Both IL-17RB and IL-17RA in Mediating IL-25-Induced Activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 21.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune Regulation of Protease-Activated Receptor-1 Expression in Murine Small Intestine during Nippostrongylus brasiliensis Infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao A, Urban JF, Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Th2 Cytokine-Induced Alterations in Intestinal Smooth Muscle Function Depend on Alternatively Activated Macrophages. Gastroenterology. 2008;135:217–225. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 25.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 27.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto M, Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, Urban JF, Jr, Wynn TA, Shea-Donohue T. Functional Importance of Regional Differences in Localized Gene Expression of Receptors for IL-13 in Murine Gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF. CYTOKINE REGULATION OF HOST DEFENSE AGAINST PARASITIC GASTROINTESTINAL NEMATODES:Lessons from Studies with Rodent Models*1. Annual Review of Immunology. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 31.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa Ji, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 33.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie ANJ. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13ΓÇôexpressing cells in type 2 immunity. Proceedings of the National Academy of Sciences. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]