Abstract
Plasmodium falciparum-infected erythrocytes (IE) sequester in the intervillous space (IVS) of the placenta causing placental malaria (PM), a condition that increases a woman’s chances of having a low birth weight (LBW) baby. Because IE sequester, they frequently are not observed in peripheral blood smears, resulting in women with PM being misdiagnosed and thus not treated. Since sequestered IE induce inflammation in the IVS, detection of inflammatory mediators in the peripheral blood may provide an approach for diagnosing PM. Two counter-regulatory molecules, TNF-α receptor (TNFR) 1 and TNF-R2, modulate the pathological effects of TNF-α. Levels of these soluble TNFR (sTNFR) are reported to be elevated in children with severe malaria, but it is unclear if they are increased in the peripheral blood of PM-positive women with asymptomatic infections. In this study, sTNFR levels were measured throughout the course of pregnancy, as well as at delivery, in women with asymptomatic infections and those who remained uninfected. Results showed that both sTNFR were significantly increased in the peripheral blood of women with asymptomatic malaria (p<0.0001) and were positively correlated with parasitemia (p<0.0001 for sTNF-R1 and p=0.0046 for sTNF-R2). Importantly, levels of sTNF-R2 were elevated in the peripheral blood of women who were PM-positive but peripheral blood-smear negative (p=0.0017). Additionally, sTNF-R2 levels were elevated in the blood of malaria-positive women who delivered LBW babies. In vitro studies demonstrated that syncytiotrophoblasts were not a major source of sTNFR. These data suggest that sTNF-R2 may be a valuable biomarker for detection of malaria-associated inflammation.
Introduction
In countries where Plasmodium falciparum is present, malaria infections in pregnant women are an important cause of maternal morbidity and neonatal mortality (1). Although women may have clinical cases of malaria during pregnancy, the disease usually remains asymptomatic. Infected erythrocytes (IE) sequester in the intervillous space (IVS) of the placenta, creating a condition known as placental malaria (PM), that is associated with maternal anemia and poor pregnancy outcomes (reviewed in (2)). Since IE sequester, they may not be present in routine peripheral blood smears (3) making diagnosis of malaria difficult. A major consequence of PM is low birthweight (LBW) babies who have a reduced chance of surviving the first year of life (4). Thus, accurate diagnosis and rapid treatment of malaria in pregnant women are of great importance.
Sequestration of IE in the IVS can lead to a change in cytokine balance, including an increase in TNF-α (5, 6). Elevated TNF-α enhances the cytoadherence of IE to trophoblasts (7) and supports the recruitment of monocytes into the IVS (8), an event that correlates with LBW babies (6, 9). TNF-α is an important cytokine in PM and its levels must be carefully regulated. Low levels are necessary for enhancing phagocytic activity and controlling parasite densities (10), but high levels of TNF-α can trigger inhibition of endocrine function and initiate extracellular matrix degradation resulting in poor pregnancy outcomes (11, 12). The biological activity of TNF-α can be modulated, in part, by its soluble receptors, sTNF-R1 and sTNF-R2 (13) that are shed from the surface of a number of different cell types by proteolysis. The soluble form of the receptors can stabilize TNF-α when the cytokine is present in low concentrations in plasma or neutralize TNF-α by competing for occupation of the receptors on the cell surface when the cytokine is in excess in the local environments (14). TNF-R1 is constitutively expressed in most tissues. However, TNF-R2 is strongly regulated during an inflammatory response. Elevated levels of both soluble receptors in plasma have been associated with various pathological conditions (15). During pregnancy, both TNF-α (16) and its receptors are normally expressed and shed by villous trophoblasts (17, 18). The accumulation of IE in the IVS can alter the normal tight immunologic balance at the maternal-fetal interface (19) leading to inflammation, pathological changes in the placenta, and LBW babies. Not all women with PM, however, suffer complications at delivery and this raises the question of whether inhibitory or regulatory systems, such as sTNF-R1 and 2, modulate immunological events within the IVS.
Significant elevation of sTNF-R1 and sTNF-R2 has been reported in plasma of patients with severe malaria (20–25), malaria-positive pregnant women (26), and rhesus monkeys infected with P. coatneyi (27). However the amount of receptors, TNF-α, and their relationship to protection during malaria infections remains unclear (28). An association between the peripheral blood parasitemia and sTNFR levels has been found in some studies, suggesting that higher parasite burdens induce higher levels of inflammation that, in turn, increase the shedding of these receptors. It remains unknown if the extent of inflammation is high enough in asymptomatic P. falciparum infections for sTNFR receptors to be shed and thus detected in peripheral blood. In this study, we sought to determine if sTNF-R1 and sTNF-R2 were elevated in the peripheral blood of asymptomatic pregnant women who had PM. If so, detection of these receptors might be used as a biomarker for diagnosing PM. An in vitro model using trophoblasts, endothelial cells, and monocytes was also developed to identify the source of sTNF-R1 and -R2 in the peripheral blood of women with PM. Finally, we sought to determine if elevated levels of these receptors were predictive of malaria-associated LBW babies.
Materials and Methods
Study Population and Sample Selection
Between 2001 and 2004, a prospective cohort study was conducted in pregnant women in Yaoundé, Cameroon where the transmission of P. falciparum is ~13 infectious bites/person/year (29). The study was approved by the Institutional Review Board, Georgetown University and the National Ethics Committee of Cameroon. The women were recruited <14 weeks of pregnancy and followed monthly through delivery and post-partum. Non-pregnant women of child-bearing age were recruited as controls and followed for 12 months. Before entering the study, informed consent was obtained. At enrollment, information on the women’s health, gravidity, age, and estimated length of pregnancy were recorded: monthly information on use of anti-malarial drugs was recorded; and birth outcome and baby weight were obtained. Blood samples were collected during each monthly visit, the hematocrit (packed cell volume [PCV]) was determined, and blood-smears were prepared and examined for P. falciparum IE as described below. Women who were blood smear- positive for malaria were prescribed antimalarial drug treatment according to the policy of the Ministry of Health.
Samples were selected from the above women. To determine if sTNFR were present in malaria-positive women (i.e., women who were blood-smear positive), 44 plasma samples from 19 pregnant women (2.3 ± 0.8 samples/women) and 37 samples from 15 non-pregnant women (2.5 ± 0.6 samples/women) were evaluated. In order to follow changes in sTNFR levels during pregnancy, 125 plasma samples from 28 pregnant women (4.5 ± 0.7 samples/women) were analyzed. All women included in this study had asymptomatic infections when they were blood-smear positive. In addition, 282 additional paired peripheral-placental IVS plasma samples collected at delivery from a prior study (30) were selected in order to compare the association of peripheral and IVS plasma levels of sTNFR with delivery outcome. Among the 282 samples, 102 samples were selected from women who had LBW babies (i.e., ≤ 2500 g) and 180 women who had normal birth weight (NBW) babies. To assure adequate sample sizes, an approximately equal number of PM-positive (PM+) and PM-negative (PM−) women were included the LBW and NBW groups, and women in the PM+ and MP− groups were further matched for parity, age and parasitemia. Among the 282 samples selected, 35 were from women who were peripheral blood-smear negative but PM+, i.e., women routinely misdiagnosed by peripheral blood smears. Characteristics of the 282 women included in the 4 groups are shown in Table 1. Immediately after delivery, maternal venous blood was drawn in the presence of EDTA and blood from the IVS was collected using the biopsy-pool method (31).
Table I.
Characteristics of the women selected for use in the study*
| Groups | # of women | Age (years) | Birth weight (g) | Primi-gravidae (%) | Length of gestation (weeks) | PCV (%) | Placental malaria (%) | Placental parasitemia (%) |
|---|---|---|---|---|---|---|---|---|
| LBW | n=102 | 22.9 (± 5.1) | 2,038 (± 383)† | 52.9 | 35.4 (± 3.8)† | 30.7 (± 6.6) † | 48 | 6.3 (± 11.9) |
| NBW | n=180 | 22.8 (± 4.6) | 3,165 (± 423)† | 54.0 | 38.6 (± 2.9)† | 33.9 (± 5.7)† | 49 | 6.4 (± 14.7) |
Data are expressed as mean (± SD);
p<0.001
Determination of Parasitemia
Thick and thin smears were made using blood collected during pregnancy and at delivery. Slides were stained with Diff-Quik (Baxter Scientific Products, Miami, FL) and thick smears were examined for parasites. If parasites were not detected after examining 100-high power fields, the woman was considered to be malaria-negative. If parasites were detected, the number of parasites per 200 WBC was determined and the number of parasites/μl was calculated using the women’s WBC count. In order to diagnose PM, a portion of the placental biopsy was used to prepared impression smears that were stained with Diff-Quik and examined for parasites. If present, percent parasitemias were determined by counting the number of IE per 2,000 RBC. The remainder of the tissue was fixed in buffered formalin, embedded in paraffin, sectioned, stained with hematoxylin-eosin and Giemsa, and examined for parasites. PM was defined as detection of parasites in either impression smears or histological sections. In addition, PCR was used to detect the presence of P. falciparum parasites in blood samples from 20 women who were followed throughout the course of pregnancy (32). Women who were blood-smear negative but PCR-positive were classified as having submicroscopic infections.
In vitro culture of Plasmodium falciparum and preparation of parasite extracts
P. falciparum parasites (D-10 and 3D7 strains) were maintained in continuous in vitro cultures using a modification of the method of Trager and Jensen (33). Parasites were cultured using human erythrocytes at a 5% hematocrit in RPMI-1640 supplemented with 4.5g/L of D-glucose, 2.383 g/L of HEPES, 0.02 mg/mL of hypoxanthine, 1.5g/L of sodium bicarbonate, 0.11 g/L of sodium pyruvate, 5% heat-inactivated human serum (PAA, Dartmouth, MA) and 0.25% Albumax II (Invitrogen, Carlsbad, CA). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2, 5% O2 and 90% N2. Parasites were grown to 10–12% parasitemias with or without prior sorbitol synchronization (34). Cells were layered onto 40% to 80% Percoll gradients, centrifuged at 1,350g at room temperature (RT) and fractions enriched for late-stage IE were pooled, washed with phosphate buffered saline (PBS), and frozen at −80°C until used. Normal RBC were also cultured in vitro, washed, and frozen. IE and RBC were freeze/thawed rapidly 3 times, sonicated, and centrifuged at 7,000g for 10 minutes. The resulting supernatant containing soluble parasite antigens, pellet containing membranes and hemozoin pigments, and RBC membranes were used in the study.
In vitro Studies Using BeWo, THP-1 and HBMVEC Cells
Immortalized cell lines were tested in vitro for their ability to shed sTNFR in response to diverse inflammatory signals. The cell lines used are either found in infected placentas or known to be in contact with IE during malaria infections. Specifically, forskolin-induced BeWo (choriocarcinoma cells), which represent a model for syncytiotrophoblast lining the IVS, and phorbol 12-myristate 13-acetate (PMA)-activated THP-1, as a model of activated macrophages known to accumulate in the IVS during PM (35) were used. Human brain microvascular endothelial cells (HBMVEC) were also used as a positive control for receptor shedding. BeWo choriocarcinoma cells (American Type Culture Collection (ATCC) cat# CCL-98, Manassas, VA) were grown in HAM’s F-12 and used until passage 30. HBMEC, a generous gift from V. Nerurkar (University of Hawaii), were maintained in Eagles Complete Medium, grown in flasks coated with attachment factor (Cell Systems, Kirkland, WA), and used between passages 8 and 12. Finally, THP-1 cells (ATCC cat# TIB-202), a human monocyte cell line, were grown in suspension in RPMI-1640 medium. All media were supplemented with 10% FCS (VWR, West Chester, PA), 2 mM L-glutamine (Invitrogen), 100 IU/ml of penicillin and 100 μg/ml of streptavidin (Invitrogen). Cultures were maintained under standard conditions in a humidified incubator with 5% CO2 and 95% air.
To determine if these cells released sTNFR in response to the parasite extracts, optimal assay conditions were determined for each cell line prior to the study. BeWo cells were seeded at 5×105 cells/ml, incubated for 24 h, and then treated with 10 nM forskolin for 24 h to induce the cells to form a syncytium. Cells were then cultured for an additional 24 h in incomplete medium prior to addition of the parasite extracts. HBMVEC were seeded at 1×106 cells/ml 24 h prior to the experiments. THP-1 cells were used either directly or first activated with PMA. Briefly, THP-1 were seeded at 1×106 cells/ml, treated for 18 h with 50 nM PMA that induced the adhesion of the cells, and then cultured for 48 h with fresh medium before the addition of parasite extracts. All cells lines were cultured in triplicate for 24 h with the following: PMA 50 and 200 nM; TNF-α 0.1 and 10 ng/ml; LPS 0.1 and 10 μg/ml; RBC at 103 and 106 cells/well; and parasite extracts equivalent to 103 and 106 IE per well. After 24 h, supernatants from individual wells were collected, centrifuged, and stored at −80°C until used. Cell viability was assessed using the MTT assay (36). Briefly, at the end of the experiment, 100 μl of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide stock solution (MTT, final concentration: 0.5 mg/ml) was added to each well and cells were incubated for an additional 4 h at 37°C. Thereafter, the MTT solution was aspirated from the wells, 200 μl of 10% sodium dodecyl sulfate (SDS) was added to solubilize the cells. The optical density (OD) of the released product was measured at 595 nm using a spectrophotometer (SpectraMax 34, Molecular Devices, Sunnyvale, CA).
Quantifications of TNF-R1 and -R2 in plasma and cell culture supernatants
The levels of soluble TNF-R1 and TNF-R2 in plasma were quantified in a multiplex assay using the Luminex™ suspension array technology with antibody-coated beads obtained commercially with reagents and secondary antibodies (Invitrogen, Cat # LHC3021 and LHC3031). Plasma samples were diluted 1:8 with the diluent provided in the kit (Invitrogen). To determine sTNF-R1 and -R2 concentrations (pg/ml), standard curves were prepared for each plate using reconstituted standards provided in the kits and linear regression analysis. The sensitivity of the assay was 2 pg/ml. In order to determine if the multiplex assay was detecting both free and TNF-bound receptors in plasma, 5, 0.5 and 0.05 ng/ml of TNF-α was added to plasma samples with known amounts of sTNFR, pre-incubated for 1h, assayed, and the amount of sTNFR in paired samples with and without added TNF-α were compared. Levels of sTNFR in cell culture supernatants were measured using commercial ELISA kits from HyCult Biotechnology b.v. (PB Uden, The Netherlands).
Statistical analysis
Results are reported as median or mean (± SD) levels of sTNFR (pg/ml). Spearman correlation coefficient was used to assess correlations with malaria parasitemia and sTNFR in the peripheral and IVS plasma. The between-group differences in the percentage of PM infections, LBW, and other categorical variables were compared using the Chi-squared test. The between-group differences in sTNFR levels at delivery were tested using the Mann-Whitney or Wilcoxon rank-sum test as appropriate. Linear regression analysis was used to evaluate changes in sTNFR levels during the course of pregnancy. The between-group comparisons in repeated measures of sTNFR level during pregnancy were made using a likelihood ratio test based on a mixed model with an unstructured covariance structure among repeated measures. All the statistical analyses were conducted using SAS software (V9.1, Cary, NC).
Results
Levels of sTNF-R1 and sTNF-R2 in the peripheral blood of non-pregnant and pregnant women
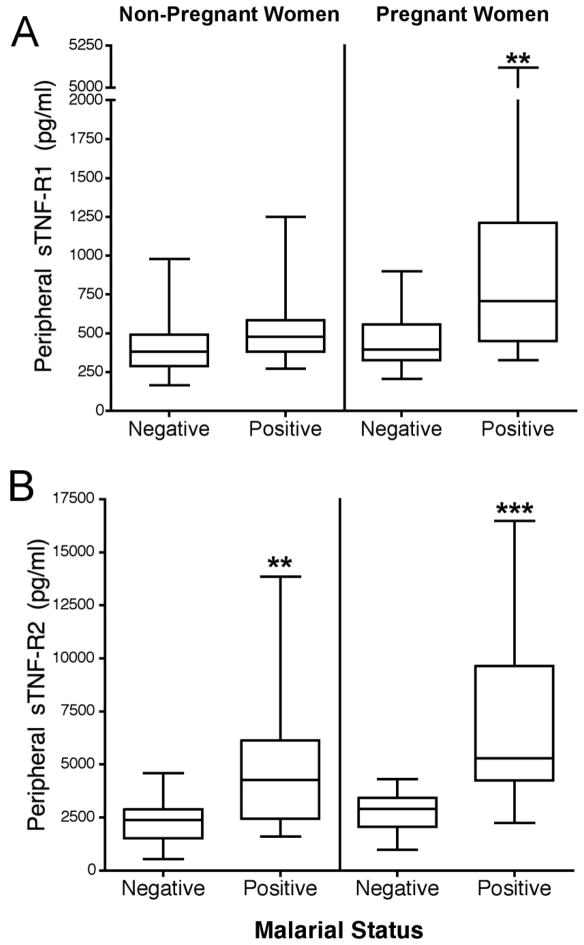
sTNF-R1 and sTNF-R2 levels were measured in peripheral blood of 37 non-pregnant (n=24 malaria-negative, n=13 malaria-positive) and 44 pregnant (n=20 malaria-negative and 24 malaria-positive) women (Fig. 1). Parasitemias were similar between non-pregnant and pregnant women (mean 3,999 ± 9,006 and 2,941 ± 5,322 parasites/μl, respectively)(p=0.49). sTNF-R1 levels were similar in non-pregnant women who were slide-positive and slide-negative for malaria; however, sTNF-R1 levels were significantly higher in pregnant women who were malaria slide-positive compared to pregnant women who were malaria-negative (p<0.01) (Fig. 1A). In contrast, sTNF-R2 levels were significantly increased in both non-pregnant and pregnant women who were slide-positive for malaria (p<0.01 and p<0.001, respectively) (Fig. 1B). These results document that increased sTNF-R1 levels in malaria-positive women is pregnancy-dependent, but that sTNF-R2 is elevated in both pregnant and non-pregnant women who were slide-positive for malaria.
FIGURE 1.
Peripheral sTNF-R1 and 2 levels in non-pregnant and pregnant women who were either malaria-negative (slide-negative) or malaria-positive (i.e., slide-positive). A) sTNF-R1. B) sTNF-R2. Soluble TNFR levels were measured using 37 plasma samples from non-pregnant women (n=24 malaria-negative, n=13 malaria-positive) and 44 plasma samples from pregnant women (n=20 malaria-negative, n = 24 positive). Results are shown in box-and-whisker plot where boxes represent the 25th and 75th percentiles, the horizontal line is the median, and bars indicate the lowest 5th and highest 95th percentiles. (*p<0.05, **p<0.01, ***p <0.001)
Levels of sTNFR throughout pregnancy in malaria-negative women
During the course of pregnancy, serum levels of sTNF-R1 have been reported to increase (37). To evaluate the extent of increase, sTNF-R1 and sTNF-R2 levels were measured in the blood of pregnant women who remained slide-negative for malaria during the course of pregnancy. Linear regression analysis showed a small, but significant, increase in sTNF-R1 levels during the course of pregnancy (p=0.026), with levels of 774 ± 230 pg/ml at <30 wks increasing to 831 ± 135 pg/ml at 36–38 wks. On the other hand, levels of sTNF-R2 remained constant throughout pregnancy in malaria-negative women.
Levels of sTNFR and malaria infections
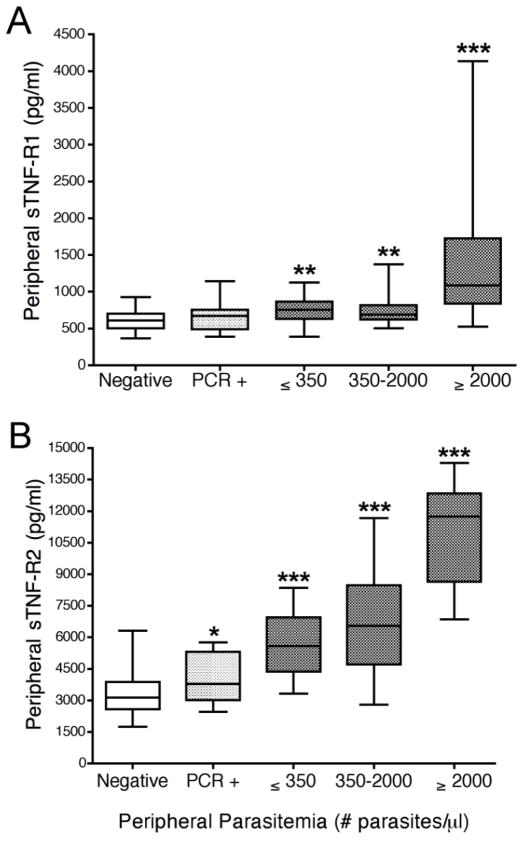
The effect of malarial infections on receptor levels during pregnancy was assessed using 125 samples from 28 women (Fig. 2). Levels of both peripheral sTNF-R1 and sTNF-R2 were significantly elevated in pregnant women with asymptomatic infections and the increase correlated with peripheral parasitemia. sTNF-R1 levels were significantly elevated in all women who were slide-positive (p<0.01), but not in women with submicroscopic infections (Fig. 2A). In contrast, while sTNF-R2 levels were also significantly higher in all women who were infected with P. falciparum compared to uninfected pregnant women (p<0.0001) (Fig. 2B), a marginal, but significant, increase of sTNF-R2 was observed in women who had sub-microscopic infections (p=0.05). Importantly, the amount of sTNF-R1 and sTNF-R2 in plasma was directly correlated with increasing parasitemias (sTNF-R1: r=0.64, p<0.0001; sTNF-R2: r=0.59, p=0.0046).
FIGURE 2.
Levels of sTNF-R1 and sTNF-R2 in the peripheral blood of pregnant women with increasing parasitemias. Panels A and B show sTNF-R1 and TNF-R2 levels, respectively. Results are based on 125 peripheral blood samples from 28 pregnant women who were followed throughout pregnancy. Negative (n=64 samples from women who were slide- and PCR-negative for malaria); PCR+ (n=15 samples from women who were PCR+ but slide-negative); and 46 peripheral blood-smear positive samples, including n=13 samples from women with ≤ 350 parasites/μl (p/μl), n=22 with 350–2000 p/μl, and n=11 with ≥2000 p/μl). Results are shown in box-and-whisker plot where boxes represent the 25th and 75th percentiles, the horizontal line is the median, and bars indicate the lowest 5th and highest 95th percentiles. (*p<0.05, **p<0.01, ***p <0.001)
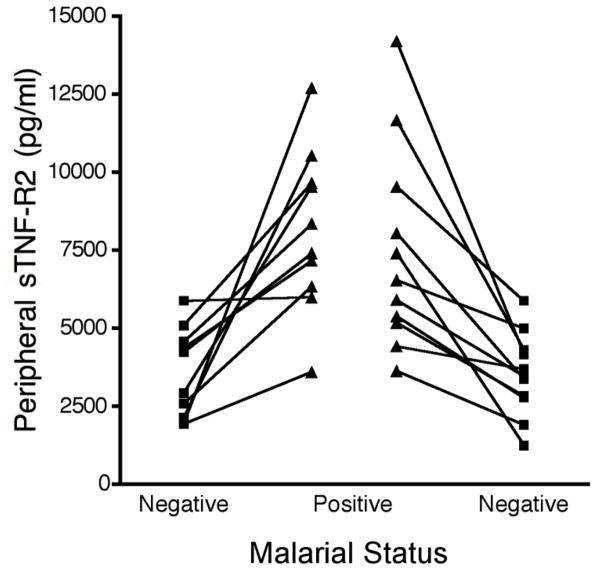
Analysis of samples from women followed longitudinally showed that sTNF-R2 levels increased in all women when they became blood smear-positive compared to the previous blood smear-negative visit (Fig. 3). In addition, sTNF-R2 levels returned to pre-infection levels at the next blood-smear negative visit. Overall, levels of sTNF-R1 were not as sensitive as sTNF-R2 and not all women had a significant increase of sTNF-R1 when they were slide-positive compared to a previous malaria-negative visit (data not shown). The average fold increase for sTNF-R1 was only 1.6 ± 0.7 compared to a 2.5 ± 1.2 fold increase in sTNF-R2 levels (p=0.008).
FIGURE 3.
sTNF-R2 levels of individual women at consecutive prenatal visits. Blood samples were collected at monthly prenatal visits. An increase in sTNF-R2 was seen in all pregnant women (n=10) when they became malaria-positive and levels decreased at the next visit (n = 11 women) when they were malaria-negative.
Levels of sTNFR in the peripheral blood at delivery
Levels of sTNFR in peripheral plasma samples collected at delivery from women with or without PM were determined (n = 144 malaria-negative women, n= 127 women peripheral blood smear-positive and PM+, and n = 35 women peripheral blood smear-negative but PM+) (Fig. 4). Overall, levels of sTNFR were higher and more variable in samples collected following labour than during pregnancy most likely because parturition itself is an inflammatory process (compare Fig. 2 and 4). At delivery, peripheral blood levels of sTNFR were significantly higher in women with peripheral and placental malaria than malaria-negative women (sTNF-R1 p<0.001; sTNF-R2 p<0.001) (Fig. 4). These observations were especially evident for primigravid women (data not shown). Importantly, sTNF-R2 levels were significantly elevated in the peripheral blood of women who were peripheral blood smear-negative but had PM (Per neg/Pla pos) (p=0.0017) (Fig. 4B). These results suggest that one might be able to diagnose PM in blood-smear negative women by detecting sTNR-R2 in their peripheral blood.
FIGURE 4.
Peripheral sTNF-R2 levels at delivery. Panel A and B show results for sTNF-R1 and sTNF-R2, respectively, for 144 malaria-negative women, 127 women who were peripheral and placental positive (Per Pos/Pla Pos), and 35 women who were peripheral blood-smear negative, but PM-positive by impression smear and/or histology (Per Neg/Pla Pos). Results are shown in box-and-whisker plot where boxes represent the 25th and 75th percentiles, the horizontal line is the median, and bars indicate the lowest 5th and highest 95th percentiles. (**p<0.01, ***p <0.001)
Comparison of sTNFR levels in peripheral and IVS (placenta) plasma of PM− and PM+ women
Plasma levels of sTNFR in maternal peripheral and IVS blood were compared using paired samples from 25 PM− and 40 PM+ women. sTNF-R1 levels were significantly higher in the IVS compared to peripheral blood (mean 1,497 pg/ml ± 1,436 pg/ml and 3,278 ± 2,462 pg/ml, respectively) (p<0.0001), which is consistent with syncytiotrophoblasts being a major source of sTNF-R1 in pregnant women. The amount of sTNF-R1, however, was similar in the IVS of PM+ and PM− women (mean 2,871 ± 1,795 pg/ml and 3,930 ± 3,196 pg/ml, respectively), suggesting that IE sequestered in the IVS did not increase shedding of sTNF-R1 by the syncytiotrophoblasts. On the other hand, sTNF-R2 levels were higher in the peripheral blood of women with PM compared to those without (mean 7,907 ± 5,606 pg/ml and 4,322 ± 2,364 pg/ml, respectively)(p=0.005), but no difference in sTNF-R2 levels in the IVS of PM+ and PM− women was found (p=0.19). These results confirm that sTNF-R1 and sTNF-R2 shedding is induced by different immunological stimuli and suggesting that cells in the placenta may not be the major source of sTNF-R2 observed in women with PM.
sTNFR levels in peripheral and IVS plasma of women who deliver NBW and LBW babies
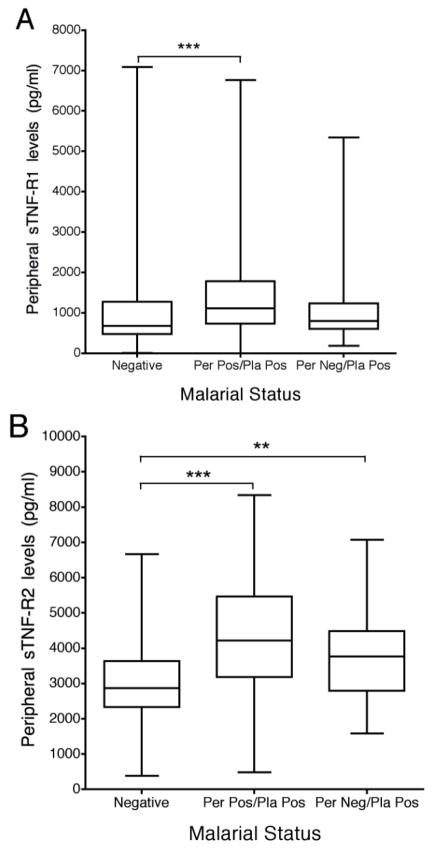
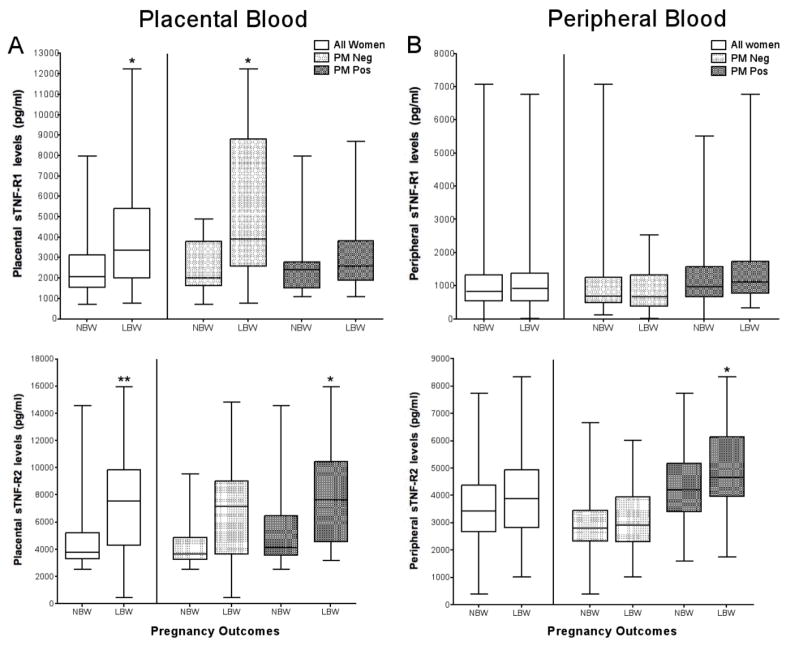
Previous studies have shown that inflammation within the placenta is one of the major causes of premature deliveries of LBW babies (38). Therefore, samples of IVS plasma from 282 women with and without PM who had LBW and NBW babies were tested (Table 1). Results showed that sTNF-R1 levels and sTNF-R2 were significantly elevated in IVS plasma of mothers delivering LBW babies compared to NBW (p=0.02 and p=0.003, respectively) (Fig. 5A: all women). The increase in sTNF-R1 was not associated with PM; in fact, high levels of sTNF-R1 were found in PM- women (p=0.03). In contrast, sTNF-R2 levels in the IVS were significantly increased in women with PM who delivered LBW babies compared to NBW (p<0.05) (Fig. 5A).
FIGURE 5.
Placental and peripheral levels of sTNF-R1 and sTNF-R2 in women with LBW and NBW deliveries. The two figures on the left (A) show sTNFR levels in the IVS and the two on the right (B) show levels in maternal peripheral blood. Each box represents 12 to 180 women. Results are shown in box-and-whisker plot where boxes represent the 25th and 75th percentiles, the horizontal line is the median, and bars indicate the lowest 5th and highest 95th percentiles. (*p<0.05, **p<0.01, ***p <0.001)
To determine if differences in sTNFR levels the IVS could be detected in the peripheral blood, paired peripheral blood samples from the mothers were tested (Fig. 5B). Among PM-negative mothers, peripheral plasma levels of neither sTNF-R1 nor sTNF-R2 were significantly different between women who delivered LBW and NBW babies. However among PM+ mothers, peripheral levels of sTNF-R2, but not sTNF-R1, were significantly higher in women who had LBW compared to NBW babies (p<0.05) (Fig. 5B). These results underline a physiological difference in the shedding of sTNF-R1 and sTNF-R2 in LBW outcomes in infected and uninfected women. They also show that levels in the peripheral blood do not always reflect those occurring within the IVS.
Production of sTNFRs in vitro by different cell types
Although a variety of cell types are known to shed sTNFR, it is unclear if cellular interaction with P. falciparum antigens can stimulate shedding for sTNFR. Accordingly, two extracts containing P. falciparum proteins (i.e., soluble proteins and the remaining particulate pellet) were incubated with BeWo trophoblasts, HBMVEC, and activated THP-1 monocytes, and the resulting supernatants were screened for sTNFR. Results showed that neither extract induced shedding of sTNFR from any of the cell lines; that is, levels were not significantly higher than those found in corresponding control cultures stimulated with normal RBC (data not shown). In comparison, all three cell types released sTNFR in vitro when treated with 50 nM PMA (p<0.001, for both sTNF-R1 and sTNF-R2 for all 3 cell lines), demonstrating that the cells were capable of releasing these receptors. Likewise, LPS induced the shedding of sTNF-R1 from HBMVEC (p=0.01), as well as, sTNF-R1 and sTNF-R2 from HBMVEC (p<0.01, p<0.001, respectively) and sTNF-R2 from activated THP-1 cells (p<0.001). These results confirmed that shedding of these receptors can be induced from placental, endothelial, and immune cells by inflammatory signals; however, shedding was not detected in response to direct contact with soluble parasite or particulate antigens.
Discussion
sTNFR are important counter-regulatory mediators of TNF-α. Their presence in the peripheral blood signals that an inflammatory response is occurring somewhere in the body. When P. falciparum IE sequester in the placental IVS, a cascade of inflammatory events is initiated, including the production of β-chemokines that attract activated macrophages that produce TNF-α. This study hypothesized that sTNFR would be induced as part of the inflammatory response and that their release into the peripheral blood might provide a new approach for diagnosing PM.
Prior to this study, sTNFR have been detected in the peripheral blood of children with severe P. falciparum infections, including those with cerebral malaria (22, 23), demonstrating that malaria can induce the extensive shedding of sTNFR. It remained unclear, however, if asymptomatic infections would be sufficient to stimulate detectable levels of sTNFR, i.e., in cases where parasitemias and inflammatory signals were low or when parasites were sequestered within the IVS environment. In the current study, sTNF-R1 and sTNF-R2 were both detected in the peripheral blood of pregnant women with asymptomatic infections (Fig. 1). Upon infection, a small increase in sTNF-R1 was observed only in pregnant women; whereas, a significant increase in sTNF-R2 was detected in both pregnant and non-pregnant women. Because sTNF-R2 was elevated in asymptomatic infections, detection of sTNF-R2 in the peripheral blood may prove to be a new approach for diagnosing malaria in pregnant women who are infected with P. falciparum.
Today, there is a great need for better diagnosis of women with PM. An estimated 20% to >50% of women with PM are peripheral blood smear-negative (3, 39) and thus remain untreated, putting them risk of anemia, perinatal complications, and delivering LBW babies (1). sTNF-R2 has the characteristics of a good biomarker for diagnosis of malaria in pregnant women, that is, it was detected in all pregnant women when they were slide-positive, absent [or at very low levels] at the next slide-negative visit (Fig. 3), significantly elevated when peripheral parasitemias were low (less than 350p/μ) (Fig. 2), detectable in women with submicroscopic infections (p<0.05)(Fig. 2), positively correlated with parasitemia and present in women with PM who were slide-negative (p=0.0017) (Fig. 4). In Cameroon, as well as elsewhere throughout Africa, peripheral parasitemias in primigravidae and multigravidae average 5,000 ± 1,000p/μl and 1,200 ± 500p/μl, respectively (30). Thus, detecting sTNF-R2 in blood samples should be sensitive enough to detect most malaria cases. Since sTNF-R2 is a marker of inflammation, it is possible that an elevation can result from pathological conditions other than malaria. In many parts of Africa, however, malaria is the most common infectious disease contracted during pregnancy. In fact, malaria is one of the few diseases women become more susceptible to during pregnancy. Detection of sTNF-R2 alone is a good biomarker of inflammation but may not be sufficient to diagnose malaria. However, a combination of currently available antigen-detection rapid diagnostic tests (RDT) for malaria in conjunction with detection of sTNF-R2 may improve diagnosis of malaria, especially in women where the level of inflammation is sufficient to result in placental pathology. Our data show that women with asymptomatic infections have on-going inflammation and produce large amounts of sTNF-R2; and thus should be treated with antimalarial drugs.
The initial hypothesis was that inflammatory cells in the IVS would be the primary source of sTNF-R2 in malaria-infected pregnant women; however, this does not appear to be the case. Additional cell types contribute to sTNF-R2 levels found in pregnant women with PM. IL-10, which is elevated in PM (5), has been shown to induce the release of sTNF-R2 from monocytes (40). Endothelial cells lining blood vessels are also a source of inducible of TNF-R2, as demonstrated by in vitro studies using HUVEC cells (41) and a murine model of experimental cerebral malaria (42). The binding of late-stage IE to endothelial cells in the deep vasculature can induce transient inflammation and contribute to sTNF-R2 levels in the peripheral blood. In addition, CD4+CD25+ regulatory T cells shed sTNF-R2 (43). During pregnancy, the placenta maintains immuno-tolerance of the fetus (reviewed in (44)) and high levels of CD4+CD25+ regulatory T cells, up to 30% of all CD4+ cells in the uterus of mice, have been detected (45). Regulatory T cells have also been found in the peripheral blood of pregnant women (46). In addition, the severity of malaria-associated symptoms has been correlated with number of CD4+ CD25+ regulatory T cells (47). Thus, sTNF-R2 detected in the plasma of pregnant women could have been produced by multiple cell types.
Both hemozoin pigment complexed with parasite DNA and proteins and parasite glycosyl-phosphatidylinositol (GPI) are known to directly stimulate monocytes to release TNF-α(48, 49). Since sTNFRs help regulate TNF-α levels, it seemed logical these factors might also stimulate shedding of sTNFR; however, this does not seem to be the case. Neither receptor was shed when BeWo trophoblasts, HBMVEC, and THP-1 monocytes were cultured with soluble late-stage proteins or the residual pellet containing membrane proteins, parasite-derived hemozoin, and GPI. Since both sTNF-R1 and sTNF-R2 were detected in culture supernatants following culture with inflammatory stimuli (i.e., PMA and LPS), it appears that sTNFR shedding is part of the overall inflammatory process and does not occur following direct contact with parasite-derived factors known to stimulate TNF -α.
Multiple factors contribute to LBW of newborns. In the current study, low infant birth weights were due primarily to prematurity. A major cause of premature deliveries is infections that stimulate inflammation. Since syncytiotrophoblasts are a major source of sTNF-R1 in pregnant women, it was not surprising to find elevated levels of sTNF-R1 in the IVS of women who had LBW babies (p<0.05) (Fig. 5A). Interestingly, however, was the observation that high levels were found in malaria-negative women (p=0.05); whereas, sTNF-R1 levels were not elevated in the IVS of mothers with PM. Previous studies have shown that when circulating TNF-α binds to TNF-R1 expressed on syncytiotrophoblasts, the receptor-complex is not shed, but rather the receptor is internalized by trophoblasts and a signaling pathway is initiated leading to cell death (50). If this happened during PM, a cascade of events would result leading to early parturition and premature deliveries (reviewed in (51)). In addition, if PM triggered internalization of sTNF-R1, a decrease in neutralization of macrophage-produced TNF-α would occur, resulting in higher levels of TNF-α in the IVS, thereby increasing the risk of placental pathology associated with LBW babies. A decrease in shedding of sTNF-R1 would have a significant impact on neutralization of TNF-α since the soluble form of TNF-R1 has a higher affinity for TNF-α than the membrane-bound receptor (52). In contrast, sTNF-R2 levels were elevated in the IVS of women who had LBW babies, especially those with PM (p<0.05)(Fig. 5). The importance of sTNF-R2 in regulating IVS levels of TNF-α is unknown.
In summary, better diagnoses of malaria in pregnant women would greatly reduce maternal morbidity and neonatal mortality in regions where malaria is endemic. sTNF-R1 were detected in pregnant women in response to P. falciparum infections, but differences in the amounts of this receptor in the peripheral blood of infected and non-infected women is too small for sTNF-R1 to serve as a biomarker. However, elevated levels of sTNF-R2 were detected in the peripheral blood of pregnant women as well as non-pregnant women with asymptomatic malaria infections. Further studies are warranted to explore sTNF-R2 as a specific host biomarker of inflammation in women with PM and as a predictor for LBW babies.
Acknowledgments
We would like to express our gratitude to all the Cameroonian women who participated in this study. We are indebted to the immense help of the entire staff of the Biotechnology Center of the University of Yaoundé 1 for the collection and preparation of the samples and the reading of blood smears for parasitemia data.
The work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health grants UO1AI-35839 and 5UO1-AI043888 (field studies and sample collection) and RO1-AI071160 (laboratory studies).
References
- 1.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760–9. doi: 10.1128/CMR.17.4.760-769.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leke RF, Djokam RR, Mbu R, Leke RJ, Fogako J, Megnekou R, Metenou S, Sama G, Zhou Y, Cadigan T, Parra M, Taylor DW. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J Clin Microbiol. 1999;37:2992–2996. doi: 10.1128/jcm.37.9.2992-2996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt HL, Snow RW. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:569–576. doi: 10.1016/s0035-9203(01)90082-3. [DOI] [PubMed] [Google Scholar]
- 5.Suguitan ALJ, Leke RG, Fouda G, Zhou A, Thuita L, Metenou S, Fogako J, Megnekou R, Taylor DW. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis. 2003;188:1074–1082. doi: 10.1086/378500. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 7.Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–1257. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009;30:313–319. doi: 10.1016/j.placenta.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, Molyneux ME. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun. 2003;71:267–270. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher GA, I, Clark A. The inhibition of Plasmodium falciparum growth in vitro by sera from mice infected with malaria or treated with TNF. Parasitology. 1990;101(Pt 3):321–326. doi: 10.1017/s0031182000060509. [DOI] [PubMed] [Google Scholar]
- 11.Arechavaleta-Velasco F, Mayon-Gonzalez J, Gonzalez-Jimenez M, Hernandez-Guerrero C, Vadillo-Ortega F. Association of type II apoptosis and 92-kDa type IV collagenase expression in human amniochorion in prematurely ruptured membranes with tumor necrosis factor receptor-1 expression. J Soc Gynecol Investig. 2002;9:60–67. doi: 10.1016/s1071-5576(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 12.Monzon-Bordonaba F, Vadillo-Ortega F, Feinberg RF. Modulation of trophoblast function by tumor necrosis factor-alpha: a role in pregnancy establishment and maintenance? Am J Obstet Gynecol. 2002;187:1574–1580. doi: 10.1067/mob.2002.128028. [DOI] [PubMed] [Google Scholar]
- 13.Wallach D, Engelmann H, Nophar Y, Aderka D, Kemper O, Hornik V, Holtmann H, Brakebusch C. Soluble and cell surface receptors for tumor necrosis factor. Agents Actions Suppl. 1991;35:51–57. [PubMed] [Google Scholar]
- 14.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–335. [PMC free article] [PubMed] [Google Scholar]
- 17.Yelavarthi KK, Hunt JS. Analysis of p60 and p80 tumor necrosis factor-alpha receptor messenger RNA and protein in human placentas. Am J Pathol. 1993;143:1131–1141. [PMC free article] [PubMed] [Google Scholar]
- 18.Austgulen R, Espevik T, Mecsei R, Scott H. Expression of receptors for tumor necrosis factor in human placenta at term. Acta Obstet Gynecol Scand. 1992;71:417–424. doi: 10.3109/00016349209021090. [DOI] [PubMed] [Google Scholar]
- 19.Fievet N, Moussa M, Tami G, Maubert B, Cot M, Deloron P, Chaouat G. Plasmodium falciparum induces a Th1/Th2 disequilibrium, favoring the Th1-type pathway, in the human placenta. J Infect Dis. 2001;183:1530–1534. doi: 10.1086/320201. [DOI] [PubMed] [Google Scholar]
- 20.Kern P, Hemmer CJ, Gallati H, Neifer S, Kremsner P, Dietrich M, Porzsolt F. Soluble tumor necrosis factor receptors correlate with parasitemia and disease severity in human malaria. J Infect Dis. 1992;166:930–934. doi: 10.1093/infdis/166.4.930. [DOI] [PubMed] [Google Scholar]
- 21.Akanmori BD, Kurtzhals JA, Goka BQ, Adabayeri V, Ofori MF, Nkrumah FK, Behr C, Hviid L. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur Cytokine Netw. 2000;11:113–118. [PubMed] [Google Scholar]
- 22.Molyneux ME, Engelmann H, Taylor TE, Wirima JJ, Aderka D, Wallach D, Grau GE. Circulating plasma receptors for tumour necrosis factor in Malawian children with severe falciparum malaria. Cytokine. 1993;5:604–609. doi: 10.1016/s1043-4666(05)80011-0. [DOI] [PubMed] [Google Scholar]
- 23.Deloron P, Roux Lombard P, Ringwald P, Wallon M, Niyongabo T, Aubry P, Dayer JM, Peyron F. Plasma levels of TNF-alpha soluble receptors correlate with outcome in human falciparum malaria. Eur Cytokine Netw. 1994;5:331–336. [PubMed] [Google Scholar]
- 24.Wenisch C, Varijanonta S, Looareesuwan S, Graninger W, Pichler R, Wernsdorfer W. Soluble intercellular adhesion molecule-1 (ICAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1), and tumor necrosis factor receptor (55 kDa TNF-R) in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1994;71:344–348. doi: 10.1006/clin.1994.1096. [DOI] [PubMed] [Google Scholar]
- 25.Hurt N, Thein M, Smith T, Bordmann G, Gallati H, Drees N, Tanner M, Weiss N. Immunological markers of childhood fevers in an area of intense and perennial malaria transmission. Clin Exp Immunol. 1995;100:59–66. doi: 10.1111/j.1365-2249.1995.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar J. 2008;7:26. doi: 10.1186/1475-2875-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davison BB, Kaack MB, Rogers LB, Rasmussen KK, Rasmussen TA, Henson EW, Henson MC, Parekh FK, Krogstad DJ. The role of soluble tumor necrosis factor receptor types I and II and tumor necrosis factor-alpha in malaria during pregnancy. J Infect Dis. 2006;194:123–132. doi: 10.1086/504694. [DOI] [PubMed] [Google Scholar]
- 28.McGuire W, D’Alessandro U, Stephens S, Olaleye BO, Langerock P, Greenwood BM, Kwiatkowski D. Levels of tumour necrosis factor and soluble TNF receptors during malaria fever episodes in the community. Trans R Soc Trop Med Hyg. 1998;92:50–53. doi: 10.1016/s0035-9203(98)90951-8. [DOI] [PubMed] [Google Scholar]
- 29.Manga L, VR, Mess J, Desfontaine M, Carnevale P. Le paludisme urbain a Yaoundé, Cameroon: l’étude entomologique dans deux quartiers centraux. Mem Soc R Belg Entomol. 1992;35:155–162. [Google Scholar]
- 30.Tako EA, Zhou A, Lohoue J, Leke R, Taylor DW, Leke RF. Risk factors for placental malaria and its effect on pregnancy outcome in Yaounde, Cameroon. Am J Trop Med Hyg. 2005;72:236–242. [PubMed] [Google Scholar]
- 31.Suguitan ALJ, Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenou S, Thuita L, Megnekou R, Fogako J, Leke RG, Taylor DW. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg. 2003;69:574–581. [PubMed] [Google Scholar]
- 32.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 33.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 34.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 35.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Arntzen KJ, Liabakk NB, Jacobsen G, Espevik T, Austgulen R. Soluble tumor necrosis factor receptor in serum and urine throughout normal pregnancy and at delivery. Am J Reprod Immunol. 1995;34:163–169. doi: 10.1111/j.1600-0897.1995.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 38.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 39.Desowitz RS, Alpers MP. Placental Plasmodium falciparum parasitaemia in East Sepik (Papua New Guinea) women of different parity: the apparent absence of acute effects on mother and foetus. Ann Trop Med Parasitol. 1992;86:95–102. doi: 10.1080/00034983.1992.11812638. [DOI] [PubMed] [Google Scholar]
- 40.Joyce DA, Gibbons DP, Green P, Steer JH, Feldmann M, Brennan FM. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol. 1994;24:2699–2705. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 41.Nubel T, Schmitt S, Kaina B, Fritz G. Lovastatin stimulates p75 TNF receptor (TNFR2) expression in primary human endothelial cells. Int J Mol Med. 2005;16:1139–1145. [PubMed] [Google Scholar]
- 42.Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Buurman WA, Moore MW, Dayer JM, Fiers W, Bluethmann H, Grau GE. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- 43.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol. 2008;180:2747–2751. doi: 10.4049/jimmunol.180.5.2747. [DOI] [PubMed] [Google Scholar]
- 44.Thellin O, Coumans B, Zorzi W, Igout A, Heinen E. Tolerance to the foeto-placental ‘graft’: ten ways to support a child for nine months. Curr Opin Immunol. 2000;12:731–737. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 45.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 46.Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, Scherjon SA. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structure requirement, and regulation of GPI activity. J Bio Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse ML, Heinrich M, Wickel M, Kronke M. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 51.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]