Abstract
A genetically distinct strain of avian hepatitis E virus (avian HEV-VA strain) was isolated from a healthy chicken in Virginia, and thus it is important to characterize and compare its pathogenicity with the prototype strain (avian HEV-prototype) isolated from a diseased chicken. Here we first constructed an infectious clone of the avian HEV-VA strain. Capped RNA transcripts from the avian HEV-VA clone were replication-competent after transfection of LMH chicken liver cells. Chickens inoculated intrahepatically with RNA transcripts of avian HEV-VA clone developed active infection as evidenced by fecal virus shedding, viremia, and seroconversion. To characterize the pathogenicity, RNA transcripts of both avian HEV-VA and avian HEV-prototype clones were intrahepatically inoculated into the livers of chickens. Avian HEV RNA was detected in feces, serum and bile samples from 10/10 avian HEV-VA-inoculated and 9/9 avian HEV-prototype-inoculated chickens although seroconversion occurred only some chickens during the experimental period. The histopathological lesion scores were lower for avian HEV-VA group than avian HEV-prototype group in the liver at 3 and 5 weeks post-inoculation (wpi) and in the spleen at 3 wpi, although the differences were not statistically significant. The liver/body weight ratio, indicative of liver enlargement, of both avian HEV-VA and avian HEV-prototype groups were significantly higher than that of the control group at 5 wpi. Overall, the avian HEV-VA strain still induces histological liver lesions even though it was isolated from a healthy chicken. The results also showed that intrahepatic inoculation of chickens with RNA transcripts of avian HEV infectious clone may serve as an alternative for live virus in animal pathogenicity studies.
1. Introduction
Hepatitis E virus (HEV) causes both endemic and epidemic forms of hepatitis E in many developing countries worldwide, and is responsible for sporadic cases of hepatitis E in some industrialized countries as well (Arankalle et al., 1994; Emerson & Purcell, 2003; Meng et al., 2010b). HEV is primarily transmitted through fecal-oral route and the mortality rate associated with HEV infection can reach up to 20% in infected pregnant women (Emerson & Purcell, 2003; Chandra et al., 2008; Meng et al., 2010b). HEV is classified in the genus Hepevirus of the family Hepeviridae (Emerson et al., 2004). It is a nonenveloped RNA virus which contains a positive-sense, single-stranded genome of approximately 7.2 kb in length (Okamoto, 2007). The viral genome consists of three open reading frames (ORFs) (Okamoto, 2007; Chandra et al., 2008): ORF1 encodes a non-structural polyprotein, ORF2 encodes an immunogenic capsid protein, (Graff et al., 2008; Kalia et al., 2010; Riddell et al., 2000) and ORF 3 encodes a multi-functional phosphoprotein (Chandra et al., 2008, 2010; Graff et al., 2005, 2006; Yamada et al., 2009).
Swine HEV, the first animal strain of HEV, was isolated and characterized in 1997 from a pig in the United States (Meng et al., 1997). Strains of swine HEV identified thus far belong to either genotype 3 or 4, and are genetically closely-related or indistinguishable from genotypes 3 and 4 strains of human HEV (Meng, 2010a; Nishizawa et al., 2003; Wang et al., 1999, 2000, 2001). Cross-species infection has been documented: genotypes 3 and 4 swine HEV infected non-human primates and conversely, genotypes 3 and 4 human HEV infected pigs (Arankalle et al., 2006; Feagins et al., 2008; Halbur et al., 2001; Meng et al., 1998; Meng, 2010a). Increasing evidence indicated that hepatitis E is a zoonotic disease (Meng, 2010a, 2010b; Takahashi et al., 2004; Tei et al., 2003).
A HEV-related virus, designated big liver and spleen disease virus (BLSV), was first detected from diseased chickens in Australia (Payne et al., 1999). The prototype strain of avian HEV was identified and characterized in 2001 from the bile of chickens with Hepatitis-Splenomegaly (HS) syndrome in California (Haqshenas et al., 2001, 2002). Sequence analyses showed that avian HEV and BLSV are variants of the same virus. Avian HEV has now been detected in several countries (Agunos et al., 2006; Bilic et al., 2009; Huang et al., 2002; Marek et al., 2010; Morrow et al., 2008; Sun et al., 2004), and at least three distinct genotypes of avian HEV have now been reported (Bilic et al., 2009; Marek et al., 2010). Avian HEV is genetically distinct from the known mammalian HEV strains sharing only about 50–60% nucleotide sequence identity and forming a distinct clade in the phylogenetic tree (Bilic et al., 2009; Huang et al., 2002, 2004). The putative functional domains and motifs in ORF1 between avian HEV and mammalian HEV are conserved (Billam et al., 2007; Guo et al., 2006; Haqshenas et al., 2002; Huang et al., 2004). In addition, avian HEV also shares common antigenic epitopes with that of mammalian HEVs (Guo et al., 2006; Haqshenas et al., 2002). Thus, avian HEV is being used as a model to study HEV pathogenesis and replication (Billam et al., 2005; Huang FF et al., 2005).
Under experimental conditions, specific-pathogen-free (SPF) chickens experimentally infected with the prototype strain of avian HEV developed microscopic liver lesions including lymphocytic phlebitis and periphlebitis, and a small proportion of the infected chickens also had hepatomegaly and subcapsular hemorrhages in the liver (Billam et al., 2005). Under field conditions, only a very small number of chickens infected by avian HEV actually developed HS syndrome (Bilic et al., 2009; Haqshenas et al., 2001; Huang et al., 2002; Morrow et al., 2008). The majority of chickens naturally infected by avian HEV are seropositive for anti-HEV antibodies but remain clinically healthy (Huang et al., 2002; Sun et al., 2004), indicating widespread subclinical infection of avian HEV in the United States. A genetically unique strain of avian HEV, designated avian HEV-VA, was recently recovered from a healthy chicken without any apparent clinical disease in a chicken flock in Virginia (Billam et al., 2007; Sun et al., 2004). The avian HEV-VA strain shares only about 90% nucleotide sequence identity with the prototype strain of avian HEV recovered from a chicken with HS syndrome in California (Billam et al., 2007). Compared to the prototype strain of avian HEV, the ORF1 of the avian HEV-VA strain had numerous non-silent mutations and deletions with 89.1% nucleotide sequence identity. The ORF2 capsid gene and ORF3 showed 90.7% sequence identity with six non-silent mutations and 97% sequence identity with four non-silent mutations, respectively, with the prototype strain (Billam et al., 2007). Due to the lack of a high titer infectious stock of avian HEV-VA, a pathogenicity study of the avian HEV-VA strain is limited and the results are somewhat inconclusive since the chickens were inoculated with 5 × 102.5 50% chicken infectious dose of the virus, the highest titer we could generate for the HEV-VA strain (Billam et al., 2009).
Recent successful constructions of infectious cDNA clones of human HEV (Emerson et al., 2001; Huang YW et al., 2005; Panda et al., 2000; Yamada et al., 2009) and the prototype avian HEV (Huang FF et al., 2005) afforded the opportunities to explore the structural and functional relationship of HEV genes. Due to the lack of an efficient cell culture system, direct genetic manipulation using infectious cDNA clones has been an indispensable tool for studying HEV replication and pathogenesis (Emerson et al., 2004; Huang FF et al., 2005; Hwang et al., 2007; Pudupakam et al., 2009). Therefore, the main objectives of this study are (1) to develop an infectious cDNA clone of the avian HEV-VA strain, and (2) to characterize the pathogenicity of the avian HEV-VA strain in SPF chickens via the direct intrahepatic inoculation of RNA transcripts of the avian HEV-VA infectious cDNA clone into chickens, a procedure that bypasses the in vitro cell culture steps for studying HEV infection in chickens (Huang FF et al., 2005).
2. Materials and methods
2.1. Virus and cells
The virus used for the construction of the infectious cDNA clone of avian HEV-VA in the study was an infectious stock of avian HEV-VA strain recovered from a clinically healthy chicken on a commercial layer chicken farm in Virginia. The infectious titer of the virus stock was 5 × 102.5 50% chicken infectious dose (CID50) per ml (Sun et al., 2004; Billam et al., 2009). The LMH chicken liver cell line (CRL-2117) was purchased from ATCC, and the cells were incubated at 37°C with 5% CO2 in Waymouth’s MB752/1 medium (Invitrogen) with 10% fetal bovine serum, as previously described (Huang FF et al., 2005).
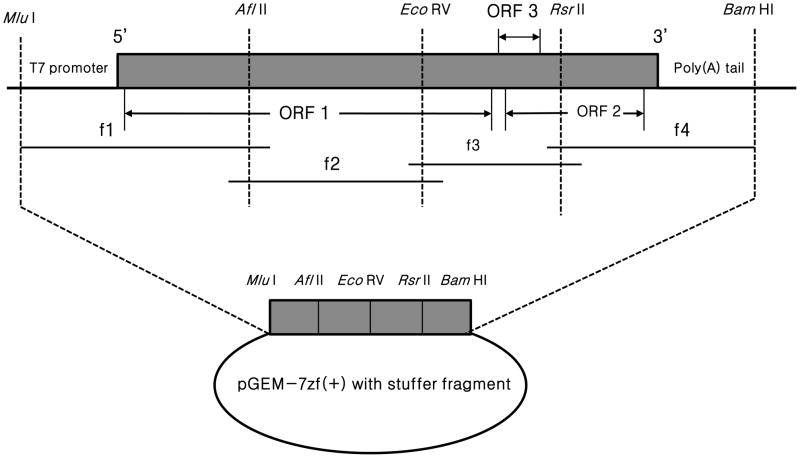
2.2. RNA extraction and RT-PCR
Viral RNA was extracted from the infectious stock of avian HEV-VA strain using TRI Reagent (MRC) and used for cDNA synthesis with SuperScript III reverse transcriptase (Invitrogen) and avian HEV-VA-specific reverse primers (Table 1). To construct the infectious cDNA clone, four overlapping fragments (Fig. 1) covering the full-length viral genome flanked by unique restriction enzyme sites were amplified by PCR with pfuUltra® II Hotstart PCR Master Mix (Stratagene) using four sets of primers which were designed based on the genomic sequence of avian HEV-VA strain (Table 1, Billam et al, 2007). Fragment 1 representing the 5′ end of the viral genome was amplified with primers MluIT7F1 and AflII1690R1. The sense primer MluIT7F1 contained, in the 5′ to 3′ direction, an engineered MluI site, the T7 core promoter sequence, and the 5′ end of avian HEV-VA sequence (Table 1, fragment 1). The antisense primer (T18BamHIR4) that was used to amplify fragment 4 representing the 3′ end of the viral genome contained 18 adenosine nucleotides and a BamHI restriction site at the end of the avian HEV-VA genome. A stuffer fragment containing all the unique restriction sites (MluI, AflII, EcoRV, RsrII, and BamHI) was prepared by PCR with two overlapping synthetic oligonuclotides. All RT-PCR-amplified fragments were purified from agarose gels, and cloned in the StrataClone blunt PCR cloning vector (Stratagene). Individual cDNA clones were sequenced, and the clones containing the consensus sequence of avian HEV-VA strain were used for the construction and assembly of the full-length cDNA clone.
Table 1.
Oligonucleotide primers used for the construction of infectious cDNA clone of the avian HEV-VA strain recovered from a clinically healthy chicken.
| Primer ID | Primer sequences (5′ to 3′)a |
|---|---|
| Fragment 1 (f1) | |
| MluIT7F1 | cacgcgttaatacgactcactataGCATGACCCCATGCCAGGGT (Mlu I) |
| AflII1690R1 | GCCGGTGATACGCTGCTGAC |
| Fragment 2 (f2) | |
| AflII1542F2 | TGACAGCACGGAGGATTTGA |
| EcoRV3826R2 | TAATAATAACCCGGGGGCAG |
| Fragment 3 (f3) | |
| EcoRV3727F3 | CATGGTAAAGTGGGACAGGG |
| RsrII5349R3 | ATGAGCATGCCAGACGTAGC |
| Fragment 4 (f4) | |
| RsrII5279F4 | GACCATACCACACGAGCGTT |
| T18BamHIR4 | gcggatccttttttttttttttttttACTATGCCCGAGATG (Bam HI) |
Lower-case letters indicate non-viral sequences. The T7 core promoter sequence in primer MluIT7F1 is underlined. The restriction enzyme sites introduced by PCR are shown in italics and specified in parentheses. Fragments 1–4 correspond to the fragment numbers in Fig. 1.
Fig. 1.
A schematic diagram of the strategies used to assemble the full-length cDNA clone of avian HEV-VA strain originally isolated from a healthy chicken in Virginia. The genome organization of the avian HEV-VA and the positions of the unique restriction enzyme sites used for cloning purposes are indicated. The complete genome of avian HEV-VA is amplified by four overlapping fragments (f 1 to 4) flanked by unique restriction enzyme sites. An Mlu I site and a T7 core promoter sequence were engineered at the 5′ end of the f1 fragment. A stretch of 18 adenosines and Bam HI sites were introduced at the 3′ end of the viral genome (f 4 fragment). The 4 overlapping fragments were individually cloned into the pGEM-7z(+) vector that contains an engineered stuffer fragment with unique restriction enzyme sites.
2.3. Construction of full-length cDNA clone of avian HEV-VA strain
The plasmid pGEM-7z(+) vector was first modified by replacing the fragment between the MluI and BamHI sites with the synthetic stuffer fragment generated in this study as described above. Subsequently, each of the independent cDNA fragments (Fig. 1, f1 through f4) was excised from the recombinant vectors containing each insert, gel-purified, and ligated into pGEM-7z(+) vector after digestion of pGEM-7z(+) vector with the same restriction enzymes. After each ligation step, each recombinant pGEM-7z(+) plasmid was transformed into Escherichia coli DH5 (Invitrogen) and grown overnight at 37°C in the presence of ampicillin. The assembled final full-length cDNA clone was designated pT7-aHEV-VA.
2.4. In vitro transcription and transfection
The full-length cDNA clone pT7-aHEV-VA was linearized by digestion with BamHI. The linearized plasmid DNA was subsequently purified by phenol/chloroform extraction and ethanol precipitation. Capped RNA transcripts from the linearized plasmid DNA were transcribed with T7 polymerase using the mMESSAGE mMACHINE T7 kit (Ambion). Briefly, each reaction was performed in a 20 μl reaction mixture containing 1 μg linearized cDNA template, 2 μl 10× reaction buffer, 10 μl 2× NTP/Cap, 2 μl enzyme mix and an additional 1 μl 30 mM GTP stock. The mixtures were incubated at 37°C for 2 h and 0.5 μl of reaction mixture was run on a 1.0% agarose gel to check the quality of the in vitro-transcribed RNA. RNA transcripts from the full-length cDNA clone of avian HEV-VA strain were directly used for in vitro transfection of LMH chicken liver cells. As a positive control, capped RNA transcripts were also generated from the infectious cDNA clone (pT7-aHEV-5) of the prototype strain of avian HEV recovered from a chicken with HS syndrome (Huang FF et al., 2005).
To test the infectivity of the pT7-aHEV-VA clone, LMH chicken liver cells were transfected with the capped RNA transcripts (approximately 4 μg) of the pT7-aHEV-VA clone. Briefly, LMH cells growing on a 12-well plate were washed with Waymouth’s MB752/1 medium. Capped RNA transcripts from pT7-aHEV-VA clone were mixed with 4 ml Plus Reagent (Invitrogen) in 25 ml Waymouth’s MB752/1 medium. After a 15-min incubation, the mixtures were combined with 1 ml Lipofectamine (Invitrogen) diluted in 25 ml Waymouth’s MB 752/1 medium and incubated for 15 min. The RNA transcripts, Plus Reagent and Lipofectamine mixtures were then added to washed LMH cells covered with 200 ml Waymouth’s MB752/1 medium. After incubation at 37°C for 3 h, 600 μl fresh culture medium was added and the transfected cells were incubated at 37°C. On day 5 post-transfection, the cells were fixed and stained by an immunofluoresence assay (IFA) as described previously (Huang FF et al. 2005). Briefly, cells on slides were fixed with a solution containing 80% acetone and 20% methanol, and allowed for air-dry. A 1:100-diluted avian anti-HEV convalescent serum from a SPF chicken experimentally infected with avian HEV (Huang FF et al., 2005; Pudupakam et al., 2009) was added to the fixed cells and incubated for 25 min at 37°C. After washing with cold PBS, 1:100-diluted fluorescein-labeled goat anti-chicken IgG (KPL) was added and incubated at 37°C for 45 min. The plates were then washed with PBS containing 0.05% Tween 20, covered with fluoromount-G, and viewed under a fluorescence microscope.
To generate the capped RNA transcripts for the in vivo infection study in chickens, each in vitro transcription reaction was performed in a 100 μl reaction as described above to produce capped RNA transcripts from 5 μg pT7-aHEV-VA cDNA clone linearized with BamHI and purified by phenol-chloroform extraction. For the positive control, the prototype avian HEV pT7-aHEV-5 infectious clone linearized with XhoI was used for the in vitro transcription to produce capped RNA transcripts. The 100 μl RNA transcripts were diluted 1:4 with cold (stored at 4°C) RNase-, DNase- and proteinase-free PBS buffer (pH 7.4), frozen on dry ice, and used for inoculation of each chicken the next day.
2.5. Intrahepatic inoculation of capped RNA transcripts from avian HEV infectious cDNA clones into the livers of SPF chickens
The animal experiment in this study was approved by the Virginia Tech Institutional Animal Care and Use Committee. Thirty, 8-week-old, SPF chickens negative for avian HEV RNA and antibodies were purchased from a commercial SPF flock (Charles River SPAFAS Inc), and randomly divided into three groups of 10 chickens each. Intrahepatic inoculation of capped RNA transcripts was performed surgically using a right parasternal approach by way of a 1 cm, paramedian, intercostal incisions. Following visualization of the liver, the RNA transcripts were directly injected into two different sites of the right lobe of the liver. Ten chickens in the pT7-aHEV-VA group were each inoculated intrahepatically with 400 μl RNA transcripts from clone pT7-aHEV-VA (approximately 75 μg) of the avian HEV-VA strain. As a positive control, 10 chickens in the pT7-aHEV-5 group were each injected intrehepatically with 400 μl RNA transcripts from clone pT7-aHEV-5 of the prototype avian HEV strain (approximately 75 μg). Ten chickens in the negative control group were each inoculated intrahepatically with 400 μl sterile PBS (pH 7.4).
Fecal samples obtained by swabbing the cloaca and colorectum, and sera were collected prior to inoculation and weekly thereafter from each chicken. Weekly serum samples were tested by an avian HEV-specific ELISA for anti-HEV antibodies as described previously (Huang et al., 2002; Sun et al., 2004). Weekly serum and faecal samples were tested for avian HEV RNA by RT-PCR (Billam et al., 2009). In this experiment, a one-step RT-PCR kit (Invitrogen) was used to detect avian HEV RNA. Five chickens randomly selected from each group were euthanized using carbon dioxide and necropsied at 3 weeks post-inoculation (wpi), and the remaining chickens from each group were euthanized using carbon dioxide and necropsied at 5 wpi. During each necropsy, each chicken and its liver were weighed and the liver/body weight ratio was calculated. Livers were evaluated for evidence of gross lesions such as subcapsular hemorrhage as previously described (Billam et al., 2005). Samples of liver, spleen, lung, kidney, and pancreas were collected at each necropsy and were fixed in 10% neutral buffered formalin for routine histopathological examination. Lesion severity was scored using a standardized scale previously described by Billam et al. (2005). Liver lesion scores range from 0 to 4 (0, no lesions; 1, <5 foci of inflammation; 2, 5–8 foci; 3, 9–15 foci; 4, >15 foci; the numbers of foci were counted as the average from 3 separate sections). Thymic lesions were given scores from 0 to 4 (0, no foci of necrosis or atrophy; 1, 1–5 foci; 2, 5–10 foci; 3, 10–20 foci; 4, >20 foci) and spleens were scored from 0 to 3 (0, normal; 1, minimal hyperplasia; 2, moderate, 3, severe). Bile was also collected from each chicken during each necropsy and tested for avian HEV RNA by RT-PCR (Billam et al., 2005, 2009).
2.6. Statistical analyses
Normal probability plots showed that liver weight to body weight ratios multiplied by 1000 followed an approximate Gaussian distribution while the liver and spleen lesion scores were skewed. Accordingly, the liver to body weight ratios were compared between the 3 treatment groups (separately at 3 and 5 weeks) using analysis of variance (ANOVA) followed by Tukey’s procedure for multiple comparisons. Residual plots for each of the ANOVA models were inspected to verify that the errors followed a normal distribution with constant variance. For the histologic lesion scores, the 3 treatments groups were compared (also separately at 3 and 5 weeks) using the exact Kruskal-Wallis test followed by Dunn’s procedure for multiple comparisons. Statistical significance was set to alpha = 0.05. All analyses were performed using SAS version 9.2 (Cary, NC, USA).
3. Results
3.1. Construction of full-length cDNA clone of avian HEV-VA strain and determination of its infectivity in LMH chicken liver cells
A full-length genomic cDNA clone of the avian HEV-VA strain (pT7-aHEV-VA) was constructed by assembling overlapping PCR fragments flanked by unique restriction sites using a stuffer fragment in pGEM-7z(+) vector (Fig. 1). This cDNA clone contains a MluI restriction site followed by the T7 core promoter sequence at the 5′ end of the viral genome, the full-length genome of avian HEV-VA strain, and 18 adenosines followed by a BamHI restriction site at the end of genome. The individual PCR fragments (labeled f1 through f4 in Fig. 1) used for the assembly of the infectious cDNA clone were verified by sequencing, and the final assembled full-length cDNA clone was sequenced again to confirm that no unwanted mutations were introduced during the PCR and cloning steps. After determining the complete sequences of the initial full-length cDNA clone and comparing it with the consensus sequence of avian HEV-VA (GenBank accession number EF206691) (Billam et al., 2007), two silent mutations (C1578A and C1905T) and one non-silent mutation (A6297C) were found. The non-silent mutation was subsequently corrected by replacing the f4 fragment containing the non-silent mutation with a newly-amplified f4 fragment with a consensus sequence. The assembled final full-length cDNA clone (pT7-aHEV-VA) was sequenced again and confirmed that there was no non-silent mutation in the entire genome. The two silent mutations (C1578A and C1905T) were not corrected and used as the genetic markers.
To test the infectivity of the pT7-aHEV-VA cDNA clone in vitro, capped RNAs were transcribed in vitro with T7 polymerase from the BamHI-linearized full-length cDNA clone, and the quality of the RNAs was verified in an agarose gel electrophoresis (data not shown). The capped RNAs were transfected into leghorn male hepatoma (LMH) chicken liver cells to determinate the infectivity. Avian HEV-specific antigens were detected in transfected LMH cells by an immunofluorescence assay (IFA) using anti-avian HEV convalescent serum, indicating that the transfected viral RNA was replication-competent in the LMH cells (Fig. 2). The positive fluorescent signals were mainly found in the cytoplasm of the LMH cells but not found in the mock-transfected cells.
Fig. 2.
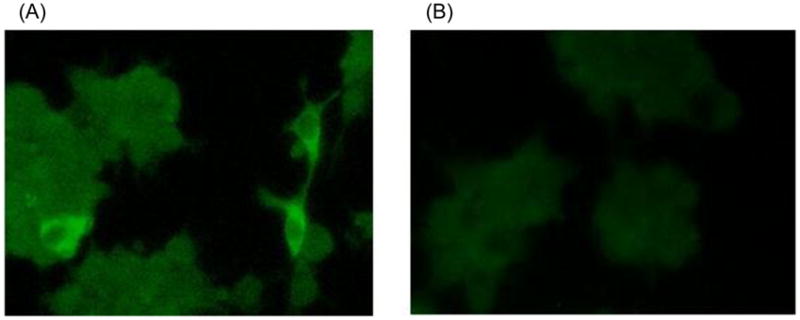
Immunofluorescent staining of LMH chicken liver cells transfected with RNA transcripts from the avian HEV cDNA clone of pT7-aHEV-VA (A), and non-transfected cells as a negative control (B).
3.2. Capped RNA transcripts of the avian HEV-VA cDNA clone were infectious when injected intrahepatically into the livers of SPF chickens
Chickens inoculated intrahepatically with the capped RNA transcripts of the avian HEV-VA cDNA clone were tested for evidence of avian HEV infection. Avian HEV-VA RNA was detected variably in fecal material, and viremia was also detected variably in serum samples (Table 2). Viral RNAs in feces and serum were detected mostly during the first 3 weeks post-inoculation (wpi). Viremia lasted from 1 to 3 wpi, and avian HEV-VA was shed in the feces from 1 to 5 wpi. Avian HEV-VA RNA was also detected in bile samples collected from necropsied chickens at 3 and 5 wpi. Although all the 10 chickens intrahepatically-inoculated with RNA transcripts of avian HEV-VA became infected as evidenced by the detection of avian HEV RNA in feces, serum or bile samples at some points from all 10 inoculated chickens (Table 2), only 3 chickens seroconverted to IgG anti-HEV (Table 3).
Table 2.
Detection of avian HEV RNA in specific-pathogen-free chickens experimentally inoculated with capped RNA transcripts from infectious cDNA clones of avian HEV-VA strain (pT7-aHEV-VA) recovered from a healthy chicken, and avian HEV-prototype strain (pT7-aHEV-5) recovered from a chicken with HS syndrome.
| Group | Samples | No. of positive samples/total no. tested at indicated wpi |
Overall no. of infected chickens in each groupb | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3a | 4 | 5a | |||
| Avian HEV-VA (pT7-aHEV-VA) | Feces | 0/10 | 6/10 | 4/10 | 2/10 | 1/5 | 1/5 | |
| Serum | 0/10 | 1/10 | 1/10 | 4/10 | 0/5 | 0/5 | 10/10 | |
| Bile | - | - | - | 4/5 | - | 1/5 | ||
| Avian HEV-prototype (pT7-aHEV-5) | Feces | 0/9c | 2/9 | 4/9 | 1/9 | 1/4 | 0/4 | |
| Serum | 0/9c | 2/9 | 0/9 | 1/9 | 0/4 | 0/4 | 9/9 | |
| Bile | - | - | - | 2/5 | - | 0/4 | ||
Five chickens were necropsied at 3 wpi and the remaining chickens were necropsied at 5 wpi. Samples from all chickens in the control group remained negative throughout the experiment and were not included in the table.
No. of chickens which had detectable avian HEV RNAs by RT-PCR in feces, sera, or bile at certain points during experiment.
One chicken died 1 day after inoculation.
Table 3.
Seroconversion in specific-pathogen-free chickens experimentally inoculated with capped RNA transcripts from infectious cDNA clones of avian HEV-VA strain (pT7-aHEV-VA) recovered from a healthy chicken, and avian HEV-prototype strain (pT7-aHEV-5) recovered from a chicken with HS syndrome.
| Group | Number of seropositive chickens/total number tested at indicated wpi |
Overall no. of seroconverted chickens in each group | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3a | 4 | 5a | ||
| Avian HEV-VA (pT7-aHEV-VA) | 0/10 | 0/10 | 0/10 | 2/10 | 3/5 | 2/5 | 3/10 |
| Avian HEV-prototype (pT7-aHEV-5) | 0/9b | 0/9 | 0/9 | 4/9 | 2/4 | 2/4 | 5/9 |
| PBS (Negative control) | 0/10 | 0/10 | 0/10 | 0/10 | 0/5 | 0/5 | 0/10 |
Five chickens were necropsied at 3 wpi and the remaining chickens were necropsied at 5 wpi.
One chicken died 1 day after inoculation.
In the positive control group where chickens were inoculated intrahepatically with the capped RNA transcripts from the prototype strain of avian HEV, evidence of avian HEV infection including viremia, fecal virus shedding and seroconversion was also detected. Again, although all the 9 chickens intrahepatically inoculated with the RNA transcripts from avian HEV-prototype became infected as evidenced by the detection of avian HEV RNA in all 9 inoculated chickens at some points in feces, serum or bile samples (Table 2), only 5/9 chickens seroconverted to IgG anti-HEV (Table 3). None of the negative control chickens inoculated intrahapetically with PBS had any evidence of avian HEV infection.
3.3. Characterization of the pathogenicity of the avian HEV-VA recovered from a clinically healthy chicken and comparison with that of the prototype avian HEV strain recovered from a chicken with HS syndrome
Since avian HEV cannot be propagated in vitro, infectivity or pathogenicity studies with live infectious virus are limited and sometimes results are inconclusive due to the low titer of available infectious virus stock (Billam et al., 2009). With the successful construction of an infectious cDNA clone of the avian HEV-VA, we can now bypass the cell culture virus propagation steps and directly infect chickens through a unique intrahepatic inoculation procedure (Huang FF et al., 2005) with large amounts of capped RNA transcripts of the avian HEV-VA infectious cDNA clone. This intrahepatic inoculation procedure (also known as in vivo transfection) has been successfully used for pathogenicity studies of swine and human HEV (Emerson et al., 2001; Huang YW et al., 2005, 2007; Pudupakam et al., 2009).
Gross lesions were limited to minimal to moderate focal capsular fibrosis and hepatic scarring at the sites of inoculation in the liver. Some chickens from all three groups (including controls) had focal adherence of the capsular surface to the coelomic wall. Microscopically, lesions consisted of mild to severe lymphocytic periphlebitis, and lymphocytic and heterophilic phlebitis with fibrinoid necrosis of the vessel wall (Fig. 3). Amyloidosis and hepatic necrosis were not seen in any of the three groups. Foci of lymphoid hyperplasia were seen in the spleen, and minimal cortical hyperplasia was seen in the thymus.
Fig. 3.
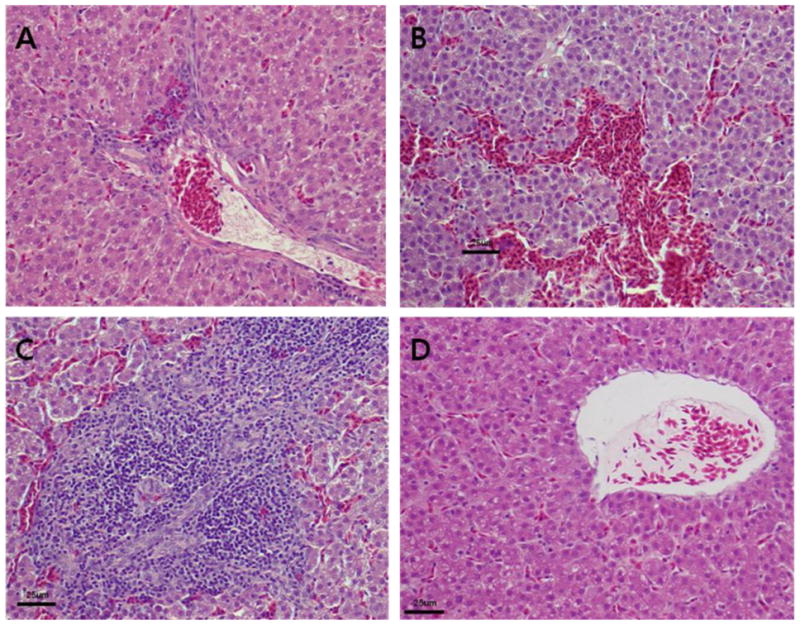
Microscopic lesions in the liver. (A) Section of a liver from a chicken inoculated with RNA transcripts from the avian HEV-VA cDNA clone (pT7-aHEV-VA) showing mild periphlebitis. (B and C) Sections of a liver from a chicken inoculated with RNA transcripts from the avian HEV-prototype cDNA clone (pT7-aHEV-5) showing hemorrhages (B) and severe periphlebitis (C). (D) Section of a liver from a negative control chicken inoculated with PBS buffer. The tissues were stained with hematoxylin and eosin.
The mean microscopic hepatic lesion scores for the pT7-aHEV-VA-infected group were lower than the scores for the pT7-aHEV-5 group at both 3 and 5 wpi but the differences were not statistically significant (p>0.05) (Table 4). The mean histologic spleen lesion scores for the pT7-aHEV-VA-infected group were lower than that of the pT7-aHEV-5 group at 3 wpi but were similar at 5 wpi (Table 4). Liver/body weight ratios, an indicator of liver enlargement, were higher for the pT7-aHEV-VA-infected group and pT7-aHEV-5 group than that of the negative control group at 3 and 5 wpi, and the difference at 5 wpi between infected groups and negative group was significant (p<0.05) (Table 4). There is no correlation between the lesion score or the liver/body ratio and the detection of viral RNA in the individual animals at the time point of analysis.
Table 4.
Pathogenicity in specific-pathogen-free chickens experimentally inoculated with capped RNA transcripts from infectious cDNA clones of avian HEV-VA strain (pT7-aHEV-VA) recovered from a healthy chicken, and avian HEV-prototype strain (pT7-aHEV-5) recovered from a chicken with HS syndrome.
| Group | Lesion scorea (Mean±SD) |
Liver/Body weight ratiob (Mean±SD) | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | |||||
| 3 wpi | 5 wpi | 3 wpi | 5 wpi | 3 wpi | 5 wpi | |
| Avian HEV-VA (pT7-aHEV-VA) | 2.2 ± 0.84ac | 2.4 ± 0.89a | 1.8 ± 0.4a | 1.6 ± 0.55a | 25.18 ± 2.22a | 26.20 ± 2.63b |
| Avian HEV-prototype(pT7-aHEV-5) | 2.6 ± 1.52a | 3.0 ± 0.82a | 2.2 ± 0.84a | 1.5 ± 0.58a | 26.56 ± 1.53a | 27.25 ± 1.42b |
| PBS (Negative control) | 2.6 ± 0.89a | 2.0 ± 1.58a | 1.4 ± 0.55a | 1.0±1.0a | 24.21 ± 3.09a | 21.04 ± 1.84a |
Liver lesion scores range from 0 to 4 (0, no lesions; 1, <5 foci; 2, 5–8 foci; 3, 9–15 foci; 4, >15 foci; the numbers of foci were bond on a single shade). Spleens were scored from 0 to 3 (0, normal; 1, minimal; 2, moderate; 3, severe).
Liver to body weight ratio was calculated by (liver weight)/(body weight) ×1000 and presented as the mean ± SD from each group.
Values followed by different lowercase letters are significantly different (p < 0.05).
Avian HEV RNA was detected in fecal material and sera by RT-PCR with primers specific each for avian HEV-VA strain and avian HEV-prototype strain, respectively (Table 2). Fecal virus shedding and viremia were detected from 1 wpi in both experimental groups but the detection rates were variable. In the pT7-aHEV-VA group, overall 4/5 chickens necropsied at week 3 had detective viral RNA in feces during the first 3 weeks and 5/5 chickens necropsied at week 5 had detective viral RNA in feces during the course of the 5 week study. In the pT7-aHEV-5 group, overall 3/5 chickens necropsied at week 3 had detective viral RNA in feces during the first 3 weeks and 4/4 chickens necropsied at week 5 had detective viral RNA in feces during the course of 5 week study. In the pT7-aHEV-VA group, overall 2/5 chickens necropsied at week 3 had detectable viral RNA in sera during the first 3 weeks and 3/5 chickens necropsied at week 5 had detectable viral RNA in sera during the 5 weeks. Viremia was observed in 2/9 chickens at 1 wpi, 1/9 at 3 wpi in the group inoculated with the pT7-aHEV-5. In the pT7-aHEV-5 group, overall 0/5 chickens necropsied at week 3 had detectable viral RNA in sera during the 3 weeks and 2/4 chickens necropsied at week 5 had detectable viral RNA in sera during the 5 weeks. Bile samples were positive for avian HEV RNA by RT-PCR for pT7-aHEV-VA group in 4/5 chickens at 3 wpi and 1/5 chickens 5 wpi, and positive for avian HEV RNA by RT-PCR for pT7-aHEV-5 groups in 2/5 chickens at 3 wpi. Altogether the results showed that 10/10 chickens in avian HEV-VA-inoculated group and 9/9 chickens in avian HEV-prototype-inoculated group were infected since avian HEV RNA was detected in feces, serum or bile samples at some points from all inoculated chickens in the two groups.
All chickens were seronegative for avian HEV antibodies prior to inoculation and chickens in negative control were seronegative throughout the entire course of the experiment. Avian HEV seroconversion occurred at 3 wpi in both avian HEV-VA (pT7-aHEV-VA) group and avian HEV-prototype (pT7-aHEV-5) group (Table 3). Overall IgG anti-avian HEV antibodies were detected in 0/5 and 3/5 chickens necropsied at week 3, and in 3/5 and 2/5 chickens necropsied at week 5 in the pT7-aHEV-VA and pT7-aHEV-5 group, respectively. Although all 10 chickens in avian HEV-VA group and all 9 chickens in avian HEV-prototype group are infected, only 3/10 avian HEV-VA-inoculated chickens and 5/9 avian HEV-prototype-inoculated chickens had seroconverted to IgG avian HEV antibodies.
To confirm the identity of the viruses recovered from infected chickens, the PCR products amplified from feces, sera and bile of selected chickens were sequenced. Sequence analyses confirmed that the PCR products amplified from the infected chickens originated from their respective original full-length cDNA clones. The two introduced genetic markers (2 silent mutations in ORF1) in pT7-aHEV-VA were present in the recovered viruses.
4. Discussion
The avian HEV-VA strain is unique in that it was recovered from a clinically healthy chicken in a commercial chicken farm in Virginia (Sun et al., 2004) and differed by 10% nucleotide sequence across the entire genome when compared to the genome of the prototype avian HEV recovered from a chicken with HS syndrome in California (Billam et al., 2007). Since HEV cannot be grown in cell cultures, it is difficult to obtain infectious virus stock with high titer for animal studies (Chandra et al., 2008). The pathogenicity of the avian HEV-VA strain remains inconclusive due to the lack of a high titer infectious stock of avian HEV-VA strain (Billam et al., 2009), since each chicken was inoculated with only 5 × 102.5 50% chicken infectious dose of the virus, the highest titer we could generate for the HEV-VA strain in the previous study (Billam et al., 2009). With the development of a unique in vivo transfection procedure, the pathogenicity of several single-strand positive-sense RNA viruses including HEV can now be studied by directly inoculating animals with capped RNA transcripts (Emerson et al., 2001; Huang et al., 2007; Pudupakam et al., 2009). Infectious cDNA clones of genotypes 1, 3 and 4 human and swine HEV have recently been constructed to study the mechanisms of HEV pathogenesis and replication and to investigate viral virulence determinants (Emerson et al., 2001; Huang YW et al., 2005, 2007; Panda et al., 2000; Yamada et al., 2009). Therefore, the availability of an infectious clone of the avian HEV-VA strain will facilitate the understanding of avian HEV pathogenesis. In the present study, an infectious cDNA clone of avian HEV-VA strain was first constructed and its pathogenicity was subsequently characterized in SPF chickens by direct intrahepatic inoculation of capped RNA transcripts of the infectious clone. The pathogenicity of avian HEV-VA strain was also compared in SPF chickens to that of the prototype strain of avian HEV.
Capped RNA transcript from the full-length cDNA clone of avian HEV-VA strain was infectious both upon transfection into LMH chicken liver cells in vitro and after intrahepatic inoculation in SPF chickens. The pGEM-7z(+) vector with a synthetic stuffer fragment containing the unique restriction enzyme sites was used for the construction of full-length cDNA clone of aHEV-VA strain in this study. Using a stuffer fragment greatly facilitated the construction of cDNA clone and the genomic regions containing introduced mutations could easily be replaced with the correct sequence. The availability of the infectious cDNA clones of both the avian HEV-VA strain and the avian HEV-prototype strain afforded us an opportunity to characterize the pathogenicity of the respective virus using capped RNA transcripts synthesized from infectious cDNA clones, which can be produced in large quantity by in vitro transcription.
To characterize the pathogenicity of the avian HEV-VA strain and compare it to that of the avian HEV-prototype strain, large quantities of capped RNA transcripts from each infectious clone were transcribed and inoculated directly into the livers of SPF chickens via the unique intrahepatic inoculation procedure (Huang FF et al., 2005). The results demonstrated that all 10 chickens intrahepatically-inoculated with avian HEV-VA and all 9 chickens intrahepatically-inoculated with avian HEV-prototype became infected as evidenced by the detection of avian HEV RNA in feces, serum or bile samples at some points during the study from all inoculated chickens. There was no significant difference in the time points for the virus shedding, viremia and seroconversion in chickens inoculated with avian HEV-VA strain and avian HEV-prototype strain although the percentage of positive chickens was different for the two groups. Overall, the detection rate of avian HEV RNA of pT7-aHEV-VA group was higher than that of the pT7-aHEV-5 group in fecal, serum and bile samples. Transient fecal shedding and viremia or absence of them have been reported previously in chickens and pigs experimentally inoculated with live infectious HEV or with capped RNA transcripts of other HEV strains (Billam et al., 2005, 2009; Huang YW et al., 2005, 2007; Huang FF et al., 2005; Meng et al., 1998), and the results of fecal shedding and viremia in this study are consistent with previous studies.
Seroconversion has been used as a reliable indicator of active HEV infection in animals inoculated with live infectious viruses or with capped RNA transcripts from infectious HEV cDNA clones (Billam et al., 2005, 2009; Huang FF et al., 2005). Although all 19 chickens in the two experimental groups had become infected, only 3/10 chickens inoculated with avian HEV-VA seroconverted to IgG anti-HEV compared to 5/9 chickens in avian HEV-prototype group. In the case of pT7-aHEV-VA group, the five chickens necropsied at 3 wpi had not seroconverted but 3 of the remaining 5 chickens seroconverted by necropsy at 5 wpi. In the pT7-aHEV-5 group, 3/5 chickens necropsied at 3 wpi seroconverted to avian HEV, and 2/4 chickens necropsied at 5 wpi had seroconverted. In general, it takes at least 3 weeks for seroconversion to occur in experimentally-infected chickens (Huang FF et al., 2005; Pudupakam et al., 2009). Therefore, the seronegative chickens necropsied at 3 wpi may have seroconverted at a later time point had they not been euthanized. It appears that seroconversion occurred a little earlier in more chickens (5/9 chickens) for the pT7-aHEV-5 avian HEV-prototype strain than the avian HEV-VA strain (3/10 chickens).
There were no noticeable significant gross lesions except for those associated with the sites of inoculation in livers of necropsied chickens, which is consistent with previous finding (Billam et al., 2009; Huang FF et al., 2005). The doses of virus, diets and ages have all been implicated as potential co-factors for the manifestation of clinical disease and several pathological lesions associated with avian HEV infection (Agunos et al., 2006; Meng, 2010b). Since the vast majority of chickens in the United States are infected by avian HEV but have no sign of clinical diseases (Huang et al., 2002), future work is warranted to identify cofactors that may potentiate or synergize avian HEV-induced diseases in chickens (Agunos et al., 2006). Although gross lesions are lacking, microscopic hepatic lesions such as phlebitis and periphlebitis, which are the distinct features of avian HEV infections (Billam et al, 2005, 2009), were observed in chickens inoculated with both avian HEV-VA strain and avian HEV-prototype pT7-aHEV-5 strain. Overall, the microscopic hepatic lesion scores of the pT7-aHEV-VA-infected chickens were lower than that of pT7-aHEV-5-infected chickens, and the microscopic splenic lesion scores of pT7-aHEV-VA group at 3 wpi were also lower than that of pT7-aHEV-5 group. Liver/body weight ratios of pT7-aHEV-VA group were also lower than that of avian HEV-prototype pT7-aHEV-5 group. The liver/body weight ratios of both avian HEV-VA and avian HEV-prototype groups at 5 wpi were significantly higher than that of the negative control group, indicating the enlargement of livers in chickens inoculated with both avian HEV-VA and avian HEV-prototype strains.
Based on the results of this study, the avian HEV-VA strain still maintained the ability to induce characteristic microscopic liver lesions even though it was originally isolated from a healthy chicken. However, it appears that avian HEV-VA strain is slightly attenuated when compared to the avian HEV-prototype strain. The results further confirm the subclinical nature of avian HEV infection in the field, and suggest that the full-spectrum of HS syndrome observed in some avian HEV-infected chickens under field conditions is likely caused by avian HEV and an unknown co-factor(s). We also demonstrated that the results from this study, where large amounts of capped avian HEV RNA transcripts (approximately 75 μg per chicken) were directly injected into the liver of chickens, were comparable to those in chickens experimentally infected with an infectious virus stock (Billam et al., 2009). Therefore, capped RNA transcripts synthesized from full-length infectious cDNA clones can be used to replace live infectious virus for studying virus pathogenesis and replication in animals for viruses such as HEV that cannot be propagated in cell culture. By using this approach, we can now easily manipulate the genomes of HEV in vitro using the infectious cDNA clones and then directly test for the effect of various genetic manipulations (such as mutations or deletions) on virus virulence, pathogenicity and replication in animals. By using swine HEV infectious cDNA clones and a similar approach in swine, we have previously identified 3 amino acid residues in the capsid protein that may be involved in virus attenuation (Huang YW et al., 2005, 2007). Therefore, with the availability of an infectious cDNA clone of the avian HEV-VA strain that is genetically distinct from the avian HEV-prototype strain, we can now begin to investigate some of the critical amino acids in avian HEV genome that may be important for virus virulence or replication in chickens.
In conclusion, we have constructed an infectious cDNA clone of an avian HEV-VA strain recovered from a healthy chicken and demonstrated the infectivity of its RNA transcripts both in LMH cells and in chickens. More importantly, we characterized the pathogenicity of the avian HEV-VA strain and demonstrated that it still produces histopathological lesions characteristic of avian HEV infection in chickens even though the avian HEV-VA strain was isolated from a healthy chicken. Furthermore, we showed that direct intrahepatic inoculation of capped RNA transcripts from avian HEV-VA infectious clone is comparable to the experimental inoculation of chickens with live infectious virus for HEV pathogenicity studies in chickens (Billam et al., 2009).
Acknowledgments
The authors would like to thank Dr Stephen Were for his assistance with the statistical analysis. This study was supported by grants from the U.S. National Institutes of Health (AI074667, and AI050611) and by a research grant from Kangwon National University, Republic of Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agunos AC, Yoo D, Youssef SA, Ran D, Binnington B, Hunter DB. Avian hepatitis E virus in an outbreak of hepatitis--splenomegaly syndrome and fatty liver haemorrhage syndrome in two flaxseed-fed layer flocks in Ontario. Avian Pathol. 2006;35:404–412. doi: 10.1080/03079450600920976. [DOI] [PubMed] [Google Scholar]
- Arankalle VA, Chadha MS, Tsarev SA, Emerson SU, Risbud AR, Banerjee K, Purcell RH. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc Natl Acad Sci USA. 1994;91:3428–3432. doi: 10.1073/pnas.91.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle VA, Chobe LP, Chadha MS. Type-IV Indian swine HEV infects rhesus monkeys. J Viral Hepat. 2006;13:742–745. doi: 10.1111/j.1365-2893.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- Billam P, Huang FF, Sun ZF, Pierson FW, Duncan RB, Elvinger F, Guenette DK, Toth TE, Meng XJ. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J Virol. 2005;79:3429–3437. doi: 10.1128/JVI.79.6.3429-3437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billam P, Sun ZF, Meng XJ. Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus (avian HEV) identified major genetic differences compared with the prototype pathogenic strain of avian HEV. J Gen Virol. 2007;88:1538–1544. doi: 10.1099/vir.0.82754-0. [DOI] [PubMed] [Google Scholar]
- Billam P, LeRoith T, Pudupakam RS, Pierson FW, Duncan RB, Meng XJ. Comparative pathogenesis in specific-pathogen-free chickens of two strains of avian hepatitis E virus recovered from a chicken with Hepatitis-Splenomegaly syndrome and from a clinically healthy chicken. Vet Microbiol. 2009;139:253–261. doi: 10.1016/j.vetmic.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic I, Jaskulska B, Basic A, Morrow CJ, Hess M. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J Gen Virol. 2009;90:863–873. doi: 10.1099/vir.0.007179-0. [DOI] [PubMed] [Google Scholar]
- Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci. 2008;33:451–464. doi: 10.1007/s12038-008-0064-1. [DOI] [PubMed] [Google Scholar]
- Chandra V, Kar-Roy A, Kumari S, Mayor S, Jameel S. The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response. J Virol. 2008;82:7100–7110. doi: 10.1128/JVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra V, Kalia M, Hajela K, Jameel S. The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex. J Virol. 2010;84:3857–3867. doi: 10.1128/JVI.01994-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Anderson D, Arankalle A, Meng XJ, Purdy M, Schlauder GG, Tsarev SA. Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy. Eigth Report of the International Committee on Taxonomy of Viruses. London: Elsevier/Academic Press; 2004. pp. 853–857. [Google Scholar]
- Emerson SU, Zhang M, Meng XJ, Nguyen H, St Claire M, Govindarajan S, Huang YK, Purcell RH. Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc Natl Acad Sci USA. 2001;98:15270–15275. doi: 10.1073/pnas.251555098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Huang YW, Halbur PG, Meng XJ. Cross-species infection of specific-pathogen-free pigs by a genotype 4 strain of human hepatitis E virus. J Med Virol. 2008;80:1379–1386. doi: 10.1002/jmv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Nguyen H, Yu C, Elkins WR, St Claire M, Purcell RH, Emerson SU. The open reading frame 3 gene of hepatitis E virus contains a cis-reactive element and encodes a protein required for infection of macaques. J Virol. 2005;79:6680–6689. doi: 10.1128/JVI.79.11.6680-6689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Torian U, Nguyen H, Emerson SU. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J Virol. 2006;80:5919–5926. doi: 10.1128/JVI.00046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Zhou YH, Torian U, Nguyen H, St Claire M, Yu C, Purcell RH, Emerson SU. Mutations within potential glycosylation sites in the capsid protein of hepatitis E virus prevent the formation of infectious virus particles. J Virol. 2008;82:1185–1194. doi: 10.1128/JVI.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zho EM, Sun ZF, Meng XJ, Halbur PG. Identification of B-cell epitopes in the capsid protein of avian hepatitis E virus (avian HEV) that are common to human and swine HEVs or unique to avian HEV. J Gen Virol. 2006;87:217–223. doi: 10.1099/vir.0.81393-0. [DOI] [PubMed] [Google Scholar]
- Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis splenomegaly syndrome in the United States. J Gen Virol. 2001;82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- Haqshenas G, Huang FF, Fenaux M, Guenette DK, Pierson FW, Larsen CT, Shivaprasad HL, Toth TE, Meng XJ. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J Gen Virol. 2002;83:2201–2209. doi: 10.1099/0022-1317-83-9-2201. [DOI] [PubMed] [Google Scholar]
- Huang FF, Haqshenas G, Shivaprasad HL, Guenette DK, Woolcock PR, Larsen CT, Pierson FW, Elvinger F, Toth TE, Meng XJ. Heterogeneity and seroprevalence of a newly identified avian hepatitis e virus from chickens in the United States. J Clin Microbiol. 2002;40:4197–4202. doi: 10.1128/JCM.40.11.4197-4202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol. 2004;85:1609–1618. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- Huang FF, Pierson FW, Toth TE, Meng XJ. Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. J Gen Virol. 2005;86:2585–2593. doi: 10.1099/vir.0.81070-0. [DOI] [PubMed] [Google Scholar]
- Huang YW, Haqshenas G, Kasorndorkbua C, Halbur PG, Emerson SU, Meng XJ. Capped RNA transcripts of full-length cDNA clones of swine hepatitis E virus are replication competent when transfected into Huh7 cells and infectious when intrahepatically inoculated into pigs. J Virol. 2005;79:1552–1558. doi: 10.1128/JVI.79.3.1552-1558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, Opriessnig T, Halbur PG, Meng XJ. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J Virol. 2007;81:3018–26. doi: 10.1128/JVI.02259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol. 2010;91:750–758. doi: 10.1099/vir.0.016584-0. [DOI] [PubMed] [Google Scholar]
- Kalia M, Chandra V, Rahman SA, Sehgal D, Jameel S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J Virol. 2009;83:12714–12724. doi: 10.1128/JVI.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek A, Bilic I, Prokofieva I, Hess M. Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Vet Microbiol. 2010 Mar 17; doi: 10.1016/j.vetmic.2010.03.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010a;140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ. Recent advances in hepatitis E virus. J Viral Hepatitis. 2010b;17:153–161. doi: 10.1111/j.1365-2893.2009.01257.x. [DOI] [PubMed] [Google Scholar]
- Morrow CJ, Samu G, Mátrai E, Klausz A, Wood AM, Richter S, Jaskulska B, Hess M. Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathol. 2008;37:527–35. doi: 10.1080/03079450802356946. [DOI] [PubMed] [Google Scholar]
- Nishizawa T, Takahashi M, Mizuo H, Miyajima H, Gotanda Y, Okamoto H. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99 % identity over the entire genome. J Gen Virol. 2003;84:1245–1251. doi: 10.1099/vir.0.19052-0. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007;127:216–228. doi: 10.1016/j.virusres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Panda SK, Ansari IH, Durgapal H, Agrawal S, Jameel S. The in vitro-synthesized RNA from a cDNA clone of hepatitis E virus is infectious. J Virol. 2000;74:2430–2437. doi: 10.1128/jvi.74.5.2430-2437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119–125. doi: 10.1016/s0378-1135(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Pudupakam RS, Huang YW, Opriessnig T, Halbur PG, Pierson FW, Meng XJ. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: evidence for attenuation of HVR deletion mutants in vivo. J Virol. 2009;83:384–395. doi: 10.1128/JVI.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell MA, Li F, Anderson DA. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J Virol. 2000;74:8011–8017. doi: 10.1128/jvi.74.17.8011-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Dunlop A, Huang FF, Pierson FW, Toth TE, Meng XJ. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J Gen Virol. 2004;85:693–700. doi: 10.1099/vir.0.19582-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–505. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ling R, Erker JC, Zhang H, Li H, Desai S, Mushahwar IK, Harrison TJ. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J Gen Virol. 1999;80:169–177. doi: 10.1099/0022-1317-80-1-169. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Ling R, Li H, Harrison TJ. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J Gen Virol. 2000;81:1675–1686. doi: 10.1099/0022-1317-81-7-1675. [DOI] [PubMed] [Google Scholar]
- Wang Y, Levine DF, Bendall RP, Teo CG, Harrison TJ. Partial sequence analysis of indigenous hepatitis E virus isolated in the United Kingdom. J Med Virol. 2001;65:706–709. doi: 10.1002/jmv.2094. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takahashi M, Hoshino Y, Takahashi H, Ichiyama K, Tanaka T, Okamoto H. Construction of an infectious cDNA clone of hepatitis E virus strain JE03-1760F that can propagate efficiently in cultured cells. J Gen Virol. 2009;90:457–462. doi: 10.1099/vir.0.007559-0. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takahashi M, Hoshino Y, Takahashi H, Ichiyama K, Nagashima S, Tanaka T, Okamoto H. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J Gen Virol. 2009;90:1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]