Abstract
Given the association with autoimmune disease, there is great interest in defining cellular factors that limit overactive or misdirected Th17-type inflammation. Using in vivo and in vitro models, we investigated the molecular mechanisms for cytokine-mediated inhibition of Th17 responses, focusing on the role of STAT1 and T-bet in this process. These studies demonstrate that, during systemic inflammation, STAT1- and T-bet-deficient T cells each exhibit a hyper-Th17 phenotype relative to WT controls. However, IL-17 production was higher in the absence of T-bet and, when both STAT1 and T-bet were deleted, there was no further increase, with the double-deficient cells instead behaving more like STAT1-deficient counterparts. Similar trends were observed during in vitro priming, with production of Th17-type cytokines higher in T-bet−/− T cells than in either STAT1−/− or STAT1−/− T-bet−/− counterparts. The ability of IFN-γ and IL-27 to suppress Th17 responses was reduced in T-bet-deficient cells and, most importantly, ectopic T-bet could suppress signature Th17 gene products, including IL-17A, IL-17F, IL-22 and RORγT, even in STAT1-deficient T cells. Taken together, these studies formally establish that, downstream of IFN-γ, IL-27 and likely all STAT1-activating cytokines, there are both STAT1 and T-bet-dependent pathways capable of suppressing Th17 responses.
Keywords: T cells, autoimmunity, STAT1, T-bet, IFN-γ, IL-27, IL-17, Th1, Th17, RORγT
Introduction
During an adaptive immune response, CD4+ helper T cells differentiate into multiple effector subsets, each characterized by their transcriptional profile and cytokine output. Among these, the recently identified Th17 subset has been increasingly linked to autoimmunity with its signature products, IL-17A and IL-17F, implicated in the pathogenesis of numerous inflammatory disorders including arthritis, multiple sclerosis, graft-versus-host disease and psoriasis (1). Though multiple factors are known to influence Th17 responses, cytokines have emerged as the key negative regulators, with IFN-γ, the signature product of Th1 effector cells, considered one of the most potent inhibitors. Deletion of IFN-γ or its receptor leads to increased IL-17 production and severe clinical manifestations in many of the experimental models where Th17 cells are considered pathogenic, including arthritis, experimental autoimmune encephalitis (EAE) and experimental autoimmune uveitis (EAU)(2–6). IFN-γ deficiency also results in a hyper-Th17 phenotype during in vitro T cell differentiation and, consistent with a T cell-intrinsic mode of action, the addition of exogenous IFN-γ yields a converse hypo-Th17 phenotype (7–9). These findings imply an inverse relationship between the Th1 and Th17 subsets but it should also be noted that cells producing both IL-17A and IFN-γ have been reported in numerous inflammatory settings and that conversion of IL-17-producing Th17 cells into IFN-γ-producing Th1 cells have been shown to occur both in vivo and in vitro, which points towards developmental plasticity and, perhaps, a linear relationship (2, 10–14).
IFN-γ exerts its functions through a high affinity cell surface receptor which is composed of two chains (IFN-γR1/IFN-γR2) and is expressed on range of immune and non-immune cells. In CD4+ T cells, IFN-γ propagates a Jak/STAT signaling cascade leading to robust activation of STAT1 and, to a lesser extent, of STAT3 (15). Among its principal STAT1-dependent activities in CD4+ T cells is the induction of T-bet, a transcription factor (TF) that is both necessary and sufficient for Th1 differentiation. T-bet drives expression of several hallmark Th1 genes, including IL-12Rβ2, and through its ability to drive IFN-γ production, it establishes a positive feedback loop where IFN-γ induces T-bet which, in turn, triggers more IFN-γ (16–20). T-bet deficiency confers resistance in several models of T cell-dependent auto-immunity, like diabetes, EAE and colitis (21–24), but in some inflammatory settings, like allograft rejection and myocarditis, it leads to increased pathology, typically characterized by a profound a Th1 defect and a corresponding rise in Th17-type cytokines (13, 25–27). Thus, while generally considered pro-inflammatory, T-bet-driven Th1 responses can also have anti-inflammatory consequences, best exemplified by their ability to limit Th17-mediated disease.
Like IFN-γ, other STAT1-activating cytokines can affect Th17 responses. One pertinent example is IL-27 which mirrors IFN-γ in three key ways: 1) it is a potent STAT1-activator, 2) it induces expression of T-bet and 3) it inhibits key Th17 gene products, like IL-17A, IL-17F and RORγT (28). Consistent with this analogy, IL-27 and IL-27 receptor deficient mice, similar to IFN-γ −/− and IFN-γR−/− mice, exhibit severe pathology and increased Th17 responses in models of T cell-dependent autoimmunity, including Toxoplasmic encephalitis (TE) and EAE (9, 29, 30). In vitro studies have shown that IFN-γ and IL-27 cannot suppress IL-17 production in the absence of STAT1, which suggests a common mechanism, but it remains unclear whether this is due to direct effects (i.e. STAT1 binding to Th17-associated loci) and or indirect effects (i.e. STAT1 regulating other inductive/inhibitory factors)(9, 29–33). The role of T-bet in this process is also poorly understood. It is known that IFN-γ and IL-27 can each induce expression of T-bet, and that ectopic T-bet expression can suppress IL-17 production, but whether this is due to a cell-intrinsic mechanism or its ability to drive IFN-γ-mediated, STAT1-dependent inhibition has not been resolved (34, 35). Furthermore, though numerous studies have shown that IL-27 can limit Th17 responses in the absence of T-bet, thus demonstrating that it is not required for STAT1-mediated inhibition, the possibility remains that T-bet-dependent mechanisms are still working in parallel to or in concert with STAT1 (9, 30, 31). The data presented here address these latter issues, formally establishing that, downstream of STAT1-activating cytokines, there are two distinct anti-Th17 pathways: first, the previously described STAT1-dependent, T-bet-independent pathway and, second, a novel T-bet-dependent, STAT1-independent pathway.
Materials & Methods
Animals
Gene-deficient donor mice were generated by crossing DO11.10 TCR transgenics (Jackson Laboratories, Bar Harbor, ME) with the following Balb/c strains: IFN-γ −/− (Jackson), T-bet−/− (from L. Glimcher, Harvard University)(36), IL-17A−/− (from Y. Iwakura, University of Tokyo)(37) and IL-4Rα −/− (Taconic, Germantown, NY)(38). STAT1−/− mice from Taconic were used in compliance with their Research Cross-breeding Agreement. These were backcrossed (>8 generations) onto the Balb/c background and then bred with either WT or T-bet−/− DO11.10 mice. sOva-transgenic mice were generated as described (13) and bred onto WT or IFN-γ −/−Rag2-deficient backgrounds (sOva Rag2−/− or IFN-γ −/− sOva Rag2−/−). Genotyping was done by PCR. All animals were maintained in specific pathogen-free housing at the University of California, San Francisco and experiments carried out according to guidelines set by the Institutional Animal Care and Use Committee.
Adoptive Transfers
Lymph nodes were dissected from 4–6 week-old donor mice and stained directly ex vivo with fluorochrome-conjugated anti-CD4, anti-DO11.10, anti-CD44 and anti-CD25 antibodies (eBioscience, San Diego, CA). Naïve CD4+ DO11.10 TCR+, CD44low, CD25−cells were then purified by high-speed cell sorting (>99% purity) and intravenously injected into age/sex matched recipients (5 × 105 cells in 400 μl PBS per host). For some experiments, WT or gene-deficient donor mice were crossed onto a Rag2-deficient background (DO11.10 Rag2−/−) and CD4+ DO11.10+ cells purified by positive selection using magnetic beads (>96% purity -Dynal Beads: Invitrogen, Carlsbad, CA). No difference was observed between DO11.10 and DO11.10 Rag2−/− donor T cells in terms of proliferation or cytokine production (Data not shown).
Ex vivo T cell monitoring
Lymphocytes from recipient mice were re-stimulated over-night with bone marrow-derived dendritic cells (BM-DCs) that were pre-activated with LPS (1 μg/ml; Sigma; St. Louis, MO) and pre-loaded with Ova peptide (1 μg/ml - 5:1 lymphocyte to DC ratio). Cultures were then treated with Brefeldin A (BFA; 10 ug/ml) for 2 hours, fixed (4% paraformaldehyde), permiabilized (0.25% Saponin) and stained with anti-CD4 and anti-DO11.10 in combination with and anti-IFN-γ, anti-IL-17A, anti-IL-17F, anti-TNF-α, anti-IL-2, anti-IL-4 and or anti-IL-13 antibodies (eBioscience). 4-color flow cytometry was performed on a FACScalibur instrument and analyzed using CellQuest Pro Software (Becton Dickinson; Franklin Lakes, New Jersey, U.S.A.). Logarithmic scales used for all dot plots.
In vitro T cell differentiation
LNs and spleens were dissected from WT or gene-deficient mice, pooled and CD4+ cells purified by positive selection using magnetic beads. These were then stimulated with plate-bound anti- CD3ε (1 μg/ml, Clone: 17A2) and soluble anti-CD28 antibodies (1 μg/ml, Clone: 37.51) at a density of 1 × 106 cells/ml in flat-bottomed 24 or 96 well plates (Sigma/Costar). After 72 hours, they were re-stimulated with Phorbol 12-myristate 13-acetate (PMA 50 ng/ml) and ionomycin (500 ng/ml; 4 hours total with BFA for the final 2 hours) and stained for ICS as above. For Th17-polarizing conditions, cultures were supplemented with recombinant human TGF-β (2.5 ng/ml; R&D Systems, Minneapolis, MN), recombinant mouse IL-6 (10 ng/ml; eBioscience) and neutralizing anti-IL-4 mAb (10 μg/ml; Clone: 11B11; NIH/NCI BRB Preclinical Repository). Where indicated, recombinant mouse IFN-γ or IL-27 was also added (25 ng/ml; eBioscience). All studies were performed in supplemented tissue culture media (RPMI-1640 with 10% Fetal Calf Serum, 1% sodium pyruvate, 1% nonessential amino acids, 0.1% β-Mercaptoethanol, 100 U/ml penicillin, 100 μg/ml/streptomycin; Gibco/Invitrogen).
Real-time PCR
For ex vivo studies, CD4+ DO11+ CD44high cells from recipient mice, along with naïve controls (CD4+ DO11+ CD25− CD44low cells from DO11.10 mice), were purified by high-speed cell sorting (2–10 × 104 per group). For in vitro studies, T cells were cultured for 48 hours and then collected (no re-stimulation). For retroviral experiments, T cells expressing more than one transgene (GFP+ Thy1.1+) were purified by high speed cell sorting (1–2 × 104 per group; Supplemental Figure 4). For all PCR studies, mRNA was extracted from purified T cells and converted to cDNA using oligo-dT priming and SuperScript III reverse transcriptase (100–250 ng RNA per reaction; Invitrogen). PCR Amplification was performed with SYBR green master mix (10–50 ng cDNA per reaction; Applied Biosystems, Foster City, California, U.S.A.) using an iQ5 Real-Time PCR thermal cycler (BioRad, Hercules, California, U.S.A.). Primer sequences and relevant information are provided in Supplemental Table 1. Reactions were performed in duplicate, Ct values normalized to β-actin levels and fold induction (n>1) or reduction (n<1) of each gene calculated (ΔΔCt) with respect to the indicated controls (n=1).
Retroviral Gene Transduction
RORγT cDNA was PCR amplified from Th17-polarized Balb/c CD4+ T cells using a high fidelity polymerase (Easy A; Stratagene, Cedar Creek, TX). PCR products were then digested, ligated into a modified MIG-R1 vector (directly upstream of the IRES and Thy1.1 marker;) and recombinant clones with ~100% sequence homology to the NCBI GenBank mRNA entry (Accession: AJ132394) were selected for further amplification (restriction enzymes and T4 ligase from NEB, Ipswich, MA; PCR sequencing by Sequentech, Mountain View, CA; Mini- and Maxi-prep kits from Quiagen). MIG-R1 expressing T-bet followed by an IRES and GFP was generated as described and kindly provided by S. Reiner (University of Pennsylvania, Philadelphia, PA)(19). RORγT and T-bet vectors, along with corresponding ‘empty’ controls, were transfected into Phoenix packaging cells (together with pCL-Eco helper plasmid) and the resulting culture supernatants used to infect T-bet−/− or STAT1−/− T cells. These were cultured under non-polarizing conditions (+/−anti-IFN-γ) for 36 hours, exposed to viral supernatant for 1 hour (@2200 RPM, 19°C) and then cultured for an additional 36 hours.
Statistics
Paired Student’s t test (one-tailed) was used to measure statistical deviation between experimental groups. In all figures, a star represents significant differences (p < 0.05–0.08) or, when this symbol is not used, p values are given.
Results
Kinetics of Th1 and Th17 responses during lymphopenia-induced autoimmune disease
To investigate mechanisms of T cell-mediated autoimmunity, we have developed a mouse model where naïve, TCR-transgenic T cells (DO11.10) are adoptively transferred into lymphopenic hosts which express their cognate antigen, chicken ovalbumin (Ova), as a soluble protein in the bloodstream (sOva Rag2−/−). This highly immunogenic environment results in an aggressive T cell response characterized by distinct waves of effector subsets, with a rapid expansion of Th17 cells followed by a more prolonged Th1-type response. Previous work has shown that, in this setting, IFN-γ and T-bet-deficient T cells exhibit a hyper-Th17 phenotype, thereby illustrating the anti-Th17 capacity of the IFN-γ/T-bet axis and validating this as a robust in vivo model for studying the regulation of Th17 responses (13).
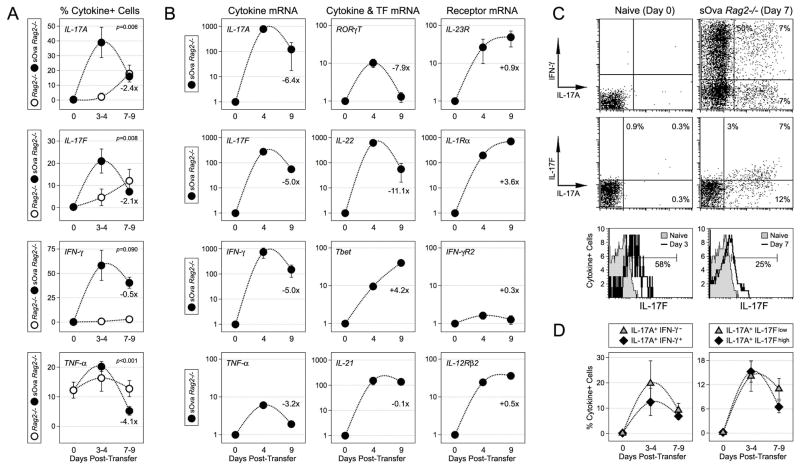
For our basic experimental set-up, naive CD4+ T cells were purified from DO11.10 Rag2−/−mice and adoptively transferred into sOva Rag2−/− hosts or, as controls, into lymphopenic Rag2−/− hosts that lack the sOva transgene. From 3 to 10 days later, LNs were dissected from recipient animals, re-stimulated overnight and cytokine production measured by intracellular flow cytometry (IFC). We found that, soon after transfer (days 3–4), there was a massive accumulation of Th17 cells in sOva Rag2−/− hosts, with about half of the donor cells producing IL-17A and a quarter producing high levels of IL-17F (Fig. 1A). At later time points (days 7–9), this Th17 response was depressed, as evidenced by the >2-fold reductions in IL-17A and IL-17F, while a Th1-type response dominated, as evidenced by sustained production of IFN-γ. In control Rag2−/−hosts, there were few IL-17A or IL-17F-positive donor T cells at the early time point but, surprisingly, these were readily detected at the later time point, which suggest that, even in the absence of a high affinity antigen, lymphopenia itself is permissive for Th17 differentiation. However, it should also be noted that, in contrast to sOva Rag2−/− hosts, this ‘homeostatic’ Th17 response was slower, lesser in magnitude (in terms of total cytokine-producing cells) and, ultimately, did not elicit autoimmune disease, which suggest that antigen is still key for determining the kinetics and pathogenicity of donor T cell responses. In addition, few IFN-γ+ donor cells could be detected in Rag2−/− hosts (Fig. 1A). Given that T cells are known to produce IFN-γ under such lymphopenic conditions (39, 40), this finding reflects both the importance of antigen and the fact that, while day 7 is a late time point for moribund sOva Rag2−/− hosts, it is a relatively early time point compared to previous studies on ‘antigen-independent’ homeostatic Th1 responses.
Figure 1. Kinetics of Th1 and Th17 responses during systemic autoimmune disease.
(A) Naïve DO11.10 CD4+ T cells were transferred into Rag2−/− or sOva Rag2−/− hosts. 3–9 days later, lymphocytes from recipient mice were re-stimulated ex vivo and cytokine production measured by intracellular flow cytometry. Shown is the percentage of cytokine+ donor cells (CD4+ DO11.10+) at the indicated time points. Day 0 represents cytokine production in naïve controls. Data are compiled from 5 experiments (5–10 mice/group) and shown is the fold change for sOva Rag2−/− hosts comparing the early and late time points. (B) Adoptive transfers were performed as in (A). CD4+ DO11.10+ CD44high donor cells were purified by high-speed cell sorting and mRNA levels quantified by real-time PCR. Data are representative of 4 experiments and are presented as the fold increase (X>1) or decrease (X<1) relative to naive controls (X=1). Shown is the fold change for the indicated mRNAs comparing the early and late time points. (C) Adoptive transfers and re-stimulations performed as in (A). Dot plots indicate the percentage of donor T cells producing IL-17A and or IFN-γ/IL-17F in sOva Rag2−/− hosts. Lower panel: histograms denote the percentage of donor T cells expressing detectable levels of IL-17F (see Supplemental Figure 1 for additional IL-17F analysis). (D) IL-17A/IFN-γ and IL-17A/IL-17F co-expression data are compiled from 3–4 individual experiments.
To further investigate the inflammatory response in sOva Rag2−/− mice, we purified donor T cells and used real-time time PCR to measure Th17-associated cytokines, receptors and transcription factors. Consistent with our IFC measurements, we found the early time point was associated with prominent induction of IL-17A, IL-17F, IL-22 and RORγT, while the later time point was associated with a >2-fold decline in all of these transcripts (Fig. 1B). Two Th17-associted receptors, IL-1R1 and IL-23R, were also strongly induced but, in those cases, mRNA levels continued to escalate throughout the course of study. Th1-type transcripts IFN-γ, IFN-γR2, T-bet and IL-12Rβ2 were highly expressed at all time points, as was IL-21, which is associated with both Th1- and Th17-type inflammation (Fig. 1B). Together with out IFC studies, these data suggest a complex relationship between Th17 and Th1 responses; there appears to be some chronology, with the former preceding the latter, but there is also evidence for concurrent expression of both Th1 and Th17-type factors within the donor population, if not within individual donor cells.
To explore the relationship between Th17 and Th1 cells in sOva Rag2−/− hosts, we used IFC to measure co-expression of IL-17A and IFN-γ. Consistent with previous work (13), we found that a large fraction of donor T cells produced both cytokines, and that, over time, these double-positive cells declined with similar kinetics to IL-17A single-positive counterparts (Fig. 1C-D). We also found that, in both sOva Rag2−/− and Rag2−/− hosts, most IL-17A+ donor cells co-expressed IL-17F. However, there was a notable difference in the flow cytometry for IL-17A and IL-17F, with the former exhibiting a bimodal distribution and the latter exhibiting a more unimodal distribution and a whole-sale population shift rather than a clear demarcation between positive and negative events (Fig. 1C; lower panel). Whether this reflects the actual protein output or a technical issue (i.e. antibody affinity) remains unknown but, given this caveat, we performed a detailed analysis to better gauge IL-17F production. This examination revealed that, while cells expressing low levels IL-17F+ cells were better visualized using histograms than dot plots, all of the observed trends, including time time-dependent inhibition and co-expression with IL-17A, were similar between these two types of analysis (Supplemental Fig. 1). In addition, both show that, while most IL-17A+ cells (>60%) expressed detectable levels of IL-17F, there were some that did not, which is consistent with published reports on the heterogeneity of Th17 cells (10, 41, 42). Taken together, these studies demonstrate that, when primed under lymphopenic conditions, helper T cells can differentiate into ‘classic’ Th1 and Th17 effectors, as well as a hybrid subset that shares the defining characteristics of both. They also suggest a hierarchy, with IFN-γ-producing Th1 cells eventually dominating over IL-17-producing ‘Th17-like’ subsets.
T-bet and STAT1 limit Th17 responses during systemic autoimmune disease
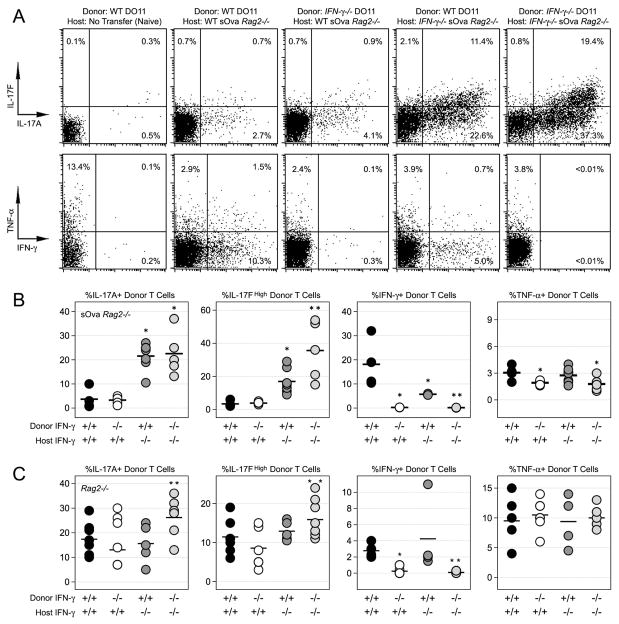
To investigate the role of IFN-γ in limiting Th17 responses, we crossed both our donor and recipient mouse strains onto an IFN-γ-deficient background and performed adoptive transfer experiments with each of the 4 possible donor/host combinations. We found that host-derived IFN-γ had the greatest impact, with production of IL-17A and IL-17F higher in IFN-γ-deficient hosts than in WT sOva Rag2−/− counterparts (Fig. 2A-B & Supplemental Figure 1). A similar trend was observed when donor T cells were transferred into IFN-γ-deficient Rag2−/− mice only, here, a combination host and donor-derived IFN-γ was required to limit ‘homeostatic’ Th17 responses (Fig. 2C & Supplemental Figure 1). Consistent with this result, and the idea that non-T cell-derived IFN-γ can play a major role, we noted that IFN-γ+ cells were readily detectable in the non-T cell fraction of our re-stimulation cultures and that, when WT T cells were transferred into IFN-γ −/− sOva Rag2−/− recipients, the percentage of IFN-γ+ donor cells was dramatically reduced (Fig 2A-B & Supplemental Fig. 2). In contrast, genetic ablation of IFN-γ had little effect on T cell TNF-α or IL-2 production whether in sOva Rag2−/− or Rag2−/− host (Fig. 2B & data not shown). Together with previous studies (13), these data indicate that both T cell- and non-T cell-derived IFN-γ can limit Th17-type inflammation in lymphopenic mice.
Figure 2. T cell and non-T cell-derived IFN-γ can limit Th17 responses during systemic autoimmune disease.
(A) Naïve CD4+ T cells from WT or IFN-γ −/− DO11.10 donors were adoptively transferred into either WT or IFN-γ −/− sOva Rag2−/− hosts. Shown is the percentage of cytokine+ donor cells after 7 days. (B) Data are compiled from 3 experiments (see Supplemental Figure 1 for additional IL-17F analysis). (C) Naïve CD4+ T cells from WT or IFN-γ −/− Rag2−/−DO11.10 donors were adoptively transferred into either WT or IFN-γ −/− Rag2−/− hosts (no antigen). Shown is the percentage of cytokine+ donor cells compiled from 3 experiments (n=3–6 mice/group). (B & C) One star denotes significant differences between the indicated group and the WT into WT group. Two stars denotes significant differences between the indicated group and the WT into IFN-γ −/− group (p<0.05).
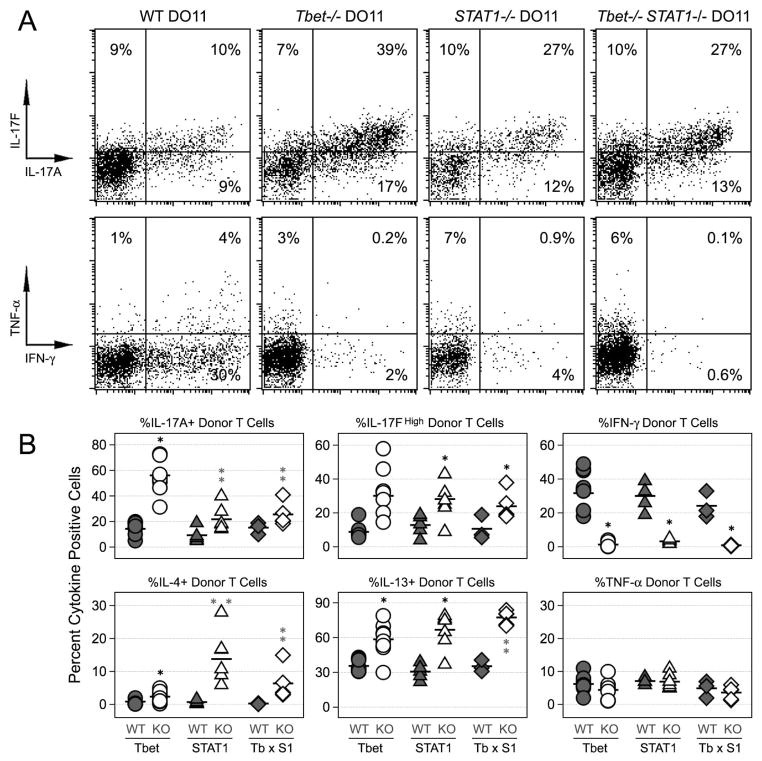
To better define the mechanism for IFN-γ-mediated suppression, we compared the ability of STAT1- and T-bet-deficient T cells to generate Th17 responses in sOva Rag2−/− hosts. We found that, while each exhibited a hyper-Th17 phenotype relative to WT controls, IL-17 production was higher in T-bet−/− donors than in STAT1−/− counterparts (Fig. 3 & Supplemental Figure 1). To ask whether they operate in the same inhibitory pathway, we generated donor mice lacking both T-bet and STAT1 (T-bet−/− × STAT1−/− DO11) and compared Th17 responses to those of T-bet- or STAT1-deficient donors. Surprisingly, when both T-bet and STAT1 were deleted, there was no additive effect, with the double-deficient cells instead behaving more like STAT1−/− cells (Fig. 3A-B). The loss of T-bet and or STAT1 led to similarly profound defects in IFN-γ production and had little impact on TNF-α or IL-2 (Fig. 3 & data not shown). Taken together, these data imply that hyper-Th17 phenotype of T-bet deficient donors cannot be fully explained by a lack of STAT1-dependent regulation. Furthermore, though STAT1 is known to be a potent inducer of T-bet (REF), they also argue that the milder hyper-Th17 phenotype of STAT1-deficient donors cannot be fully explained by a lack of T-bet.
Figure 3. T-bet and STAT1-deficient T cells exhibit a hyper-Th17 phenotype during systemic autoimmune disease.
(A) Naïve T cells were purified from WT, T-bet−/−, STAT1−/− or T-bet−/− × STAT1−/− DO11.10 mice and adoptively transferred into WT sOva Rag2−/− hosts. Shown is the percentage of cytokine+ donor cells (CD4+ DO11.10+) on day 7 post-transfer. (B) Data are compiled from 3–5 experiments. One star denotes statistically significant differences between the indicated group and WT donors. Two stars denotes significant differences between the indicated group and T-bet−/− donors (p<0.05). See Supplemental Figure 1 for additional IL-17F analysis.
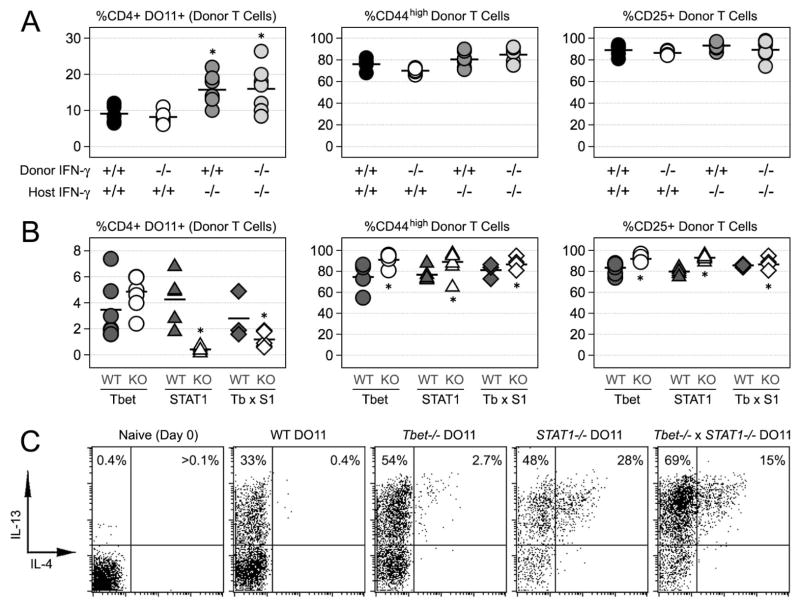
Aside from IL-17 production, there were other notable differences in the behavior of STAT1-and T-bet-deficient donors in sOva Ra2−/− hosts. Whether T-bet−/− or +/+, STAT1-deficient T cells did not proliferate to the same extent as either WT or T-bet−/− counterparts (Fig. 4B). They also produced more IL-4 and IL-13, two known Th17 inhibitors (Fig. 4C)(7, 8, 43). To determine the impact of this elevated Th2-type response, we compared IL-17 production from donors lacking STAT1 to that of donors lacking STAT1 and IL-4Rα, a shared receptor component for IL-4 and IL-13. Relative to STAT1-deficient counterparts, the compound loss STAT1 and IL-4Rα had little effect on IL-17 but did lead to significant reductions in IL-4 and IL-13 (data not shown).
Figure 4. Phenotypic differences between T-bet- and STAT1-deficient donor T cells in sOva Rag2−/− mice.
(A) Naïve T cells were purified from either WT or IFN-γ −/− DO11.10 mice and then adoptively transferred into either WT or IFN-γ −/− sOva Rag2−/− hosts. At 7 days post-transfer, lymphocytes were stained directly ex vivo for flow cytometry. Shown are the percentages of total and activated donor T cells (CD4+ DO11.10+; CD25+ or CD44high). (B) Naïve T cells were purified from WT, T-bet−/−, STAT1−/− or T-bet−/− × STAT1−/− DO11 mice and transferred into sOva Rag2−/− hosts. Shown are the percentages of total and activated donor T cells after 7 days (A & B) Data are compiled from 3 individual experiments. A star denotes statistically significant differences between the indicated group and WT controls (p<0.05). (C) Adoptive transfers were performed as in (B). At 7 days post-transfer, lymphocytes were re-stimulated and stained for intracellular flow cytometry. Shown are the percentages of IL-4+ and IL-13+ donor T cells (gated on CD4+ DO11.10 TCR+). Data are representative of 3 individual experiments.
Direct Evidence for both T-bet- and STAT1-dependent regulation of Th17 responses
To complement our in vivo studies, we used an in vitro model where Th17 differentiation could be monitored in the presence or absence of STAT1-activating cytokines. As expected, we found that WT T cells produced IFN-γ and almost no IL-17 in non-polarizing cultures. The inverse was true in Th17-polarizing cultures, where they produced little IFN-γ and significantly more IL-17 (Fig. 5A). Recombinant IFN-γ had little impact on WT T cells while IL-27 had dramatic effects, inducing IFN-γ production and suppressing Th17-type cytokines (Fig. 5A-B). IFN-γ-deficient T cells produced almost twice as much IL-17 as wild type counterparts under Th17 conditions and, in this case, both IFN-γ and IL-27 could suppress the Th17 response (Fig. 5A–B). Given that IFN-γ could only suppress IL-17 production in an IFN-γ-deficient setting, these data imply that IFN-γ responsiveness is rapidly saturated by autocrine IFN-γ production in WT cells. On the other hand, since IL-27 could suppress in either WT or IFN-γ −/− cultures, they also suggest that its anti-Th17 capacity is independent of its pro-Th1 capacity and, perhaps, hint at a level of cooperation, with IL-27 retaining the ability to suppress IL-17 production when cells become insensitive to IFN-γ.
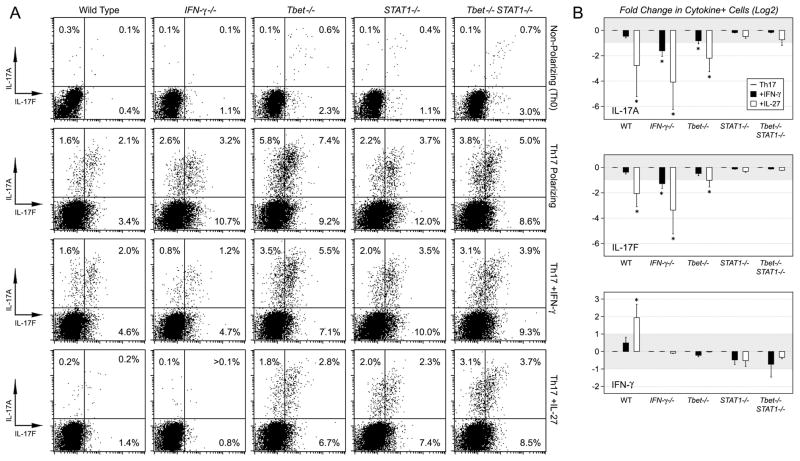
Figure 5. STAT1-activating cytokines fail to suppress Th17 responses in the absence of STAT1.
(A) CD4+ T cells were purified from WT, IFN-γ −/−, T-bet−/− and STAT1−/− mice. These were cultured under non-polarizing or Th17 conditions for 72 hours and shown is the percentage of cytokine+ cells (CD4+). (B) Data are compiled from 3–5 experiments (Th17 conditions) and are presented as the Log2 fold change in cytokine+ cells upon exposure to IFN-γ (Black Bars) or IL-27 (White Bars). Untreated groups have a relative value of 0 while cytokine-treated groups have values greater than or less than 0, depending on whether they have an enhancing (X>0) or inhibitory effect (X<0). Grey area denotes Log2 values that are greater than −1 but less than 1. Error bars represent the standard deviation between all fold changes for each group. Star denotes >2-fold, statistically significant differences between the indicated group and corresponding Th17 controls. (p<0.05).
As with the in vivo model, T-bet- and STAT1-deficient T cells exhibited a hypo-Th1, hyper-Th17 phenotype during in vitro differentiation. Under Th17 polarizing conditions, they each produced more IL-17 than WT counterparts but, again, T-bet deficiency led to a greater increase and, when both TFs were deleted, the double-deficient cells behaved like STAT1−/− counterparts (Fig. 5A–B). IFN-γ had a muted effect In T-bet-deficient cultures, prompting only small reductions in IL-17A and IL-17F. IL-27 was more potent, leading to >2-fold reductions in both, though it should be noted that the level of inhibition was less than what was observed in WT or IFN-γ −/− cultures. Neither IFN-γ nor IL-27 impacted IL-17 production in STAT1-deficient cultures, whether T-bet-deficient or sufficient, thereby illustrating the central role of STAT1 in this process (Fig. 5A–B).
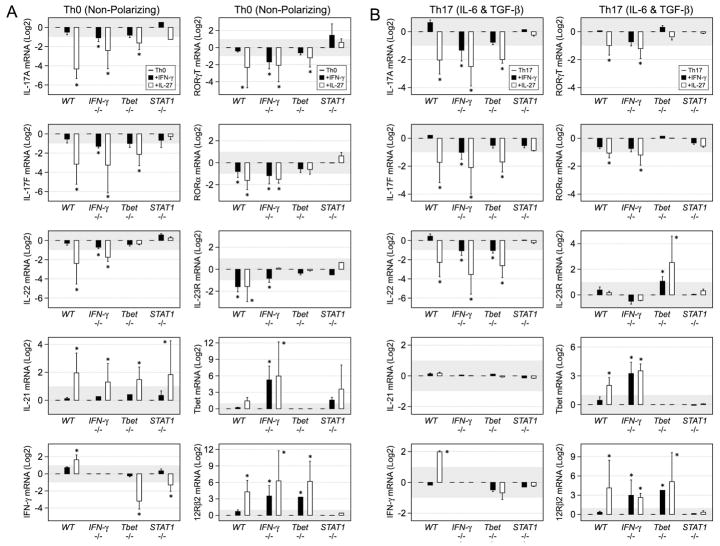
Consistent with our protein measurements, exogenous IFN-γ had little effect on IL-17A and IL-17F mRNA levels in WT cells but suppressed both transcripts in IFN-γ −/− T cells (Fig. 6). A similar trend was observed for IL-22, another Th17-associated cytokine, and for RORγT and RORα, two key Th17-associated TFs (Fig. 6). As before, IL-27 displayed potent activity in either WT or IFN-γ −/− cells, suppressing IL-17A, IL-17F and other Th17-associated transcripts while, at the same time, promoting Th1-associated transcripts. IFN-γ had little effect on Th17-associated mRNAs in T-bet-deficient cells and, though IL-27 could still prompt >2-fold reductions, its inhibitory capacity was less in T-bet−/− cells than in WT or IFN-γ −/− counterparts (Fig. 6). As above, neither IFN-γ nor IL-27 affected Th17-associated transcripts in STAT1-deficient cells (Fig. 4). Also noteworthy, and consistent with a recent report (44), IL-27 was a powerful STAT1 and T-bet-independent inducer of IL-21 mRNA (Fig. 6). Together with our flow cytometry studies, these data confirm that STAT1-dependent pathways are critical for limiting Th17 responses. In addition, given the reduced capacity of STAT1-activating cytokines to suppress in T-bet-deficient cells, they also suggest a T-bet-dependent, STAT1-independent pathway.
Figure 6. Transcription of Th17-associated genes is influenced by both T-bet and STAT1-dependent pathways.
Naïve T cells were cultured under (A) non-polarizing or (B) Th17 conditions and PCR used to measure the indicated transcripts. Data are pooled from 3 experiments and presented as the Log2 fold change upon exposure to IFN-γ (Black Bars) or IL-27 (White Bars). Error bars represent the standard deviation between all fold changes and a star denotes >1.9-fold, statistically significant differences between the indicated group and Th17 controls (p≤0.08). See Supplemental Figure 3 for additional PCR analysis.
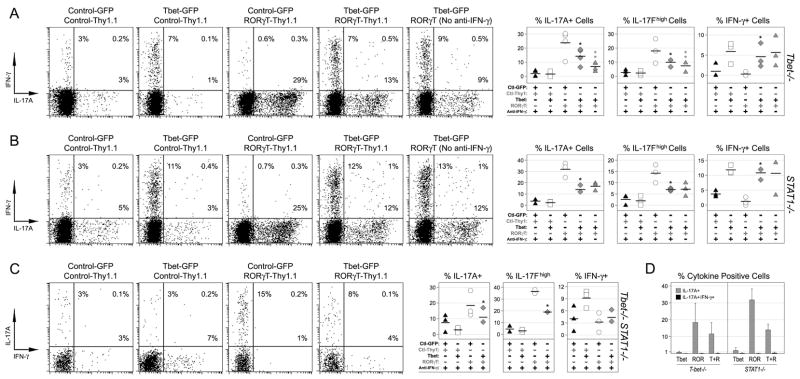
To directly test whether T-bet can suppress Th17 responses independently of STAT1, we used retroviral gene transduction to over-express T-bet and or RORγT in a STAT1-deficient setting (Supplemental Figure 2). As expected, we found that ectopic RORγT was a potent Th17 stimulus, prompting a dramatic rise in the percentage of IL-17+ cells whether in T-bet-, STAT1-or double-deficient T cells (Fig. 7A–C). However, when T-bet was also introduced, RORγT-driven IL-17 production was significantly decreased and, when these ‘double-infected’ cells were cultured without neutralizing anti-IFN-γ mAb, there was a further reduction in T-bet-deficient but not STAT1-deficient cells. These latter findings establish that T-bet can suppress Th17 responses independently of STAT1 and that, in WT cells, STAT1 and T-bet-mediated pathways may cooperate in achieving this shared function.
Figure 7. Direct evidence for T-bet-dependent regulation of Th17 responses.
T-bet (A), STAT1 (B) and Tbet/STAT1-deficient T cells (C) were cultured under non-polarizing conditions and transduced with retroviral vectors expressing T-bet (GFP), RORγT (Thy1.1), and or ‘empty’ controls (Supplemental Figure 4). Shown is the percentage of cytokine+ cells only for those infected with two vectors (CD4+ GFP+ Thy1.1+). One star denotes significant differences between the indicated group and the RORγT-only group. Two stars denotes significant differences in the absence of anti-IFN-γ (p<0.05). (D) Co-expression of IL-17A and IFN-γ is analyzed for cells expressing Tbet and or RORγT. (A-D) Data are compiled from 3 experiments.
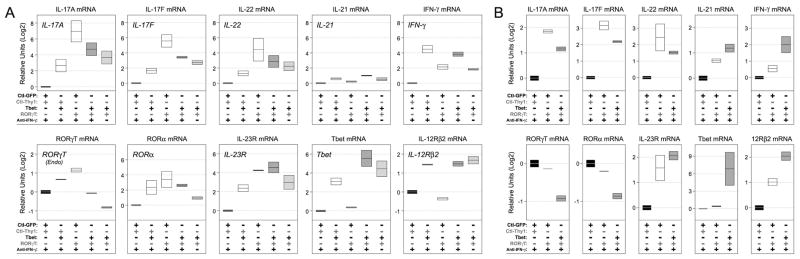
Consistent with our protein measurements, we also found that ectopic T-bet had significant impact on RORγT-driven Th17 responses at the mRNA level, prompting reduced expression of IL-17A, IL-17F and IL-22 in both T-bet−/− and STAT1−/− cells. Removing the anti- IFN-γ led to further reductions only in T-bet−/− cells which, again, hints at a degree of cooperation between T-bet and STAT1 in this process (Fig. 8A–B). We also noted that the ability of T-bet to suppress RORγT and RORa was modest by comparison and that it had little influence, positive or negative, on expression of IL-23R. Thus while T-bet clearly influences the output of Th17-type cytokines, it does not appear to do so by ‘locking down’ transcription of ROR family transcription factors.
Figure 8. T-bet limits transcription of key Th17-associated genes.
(A) T-bet or (B) STAT1-deficient T cells were cultured and transduced as in Figure 7. ‘Double-infected’ cells were purified by high-speed cell sorting and PCR used to measure the indicated mRNAs. Data are representative of 3 individual experiments and are presented as the Log2 fold change relative to the control group (Control-GFP/Control-Thy1.1) Area of each box plot denotes standard deviation within replicate measurements.
Discussion
In the preceding studies, we used in vivo and in vitro models of T cell differentiation to establish that, downstream of IFN-γ, IL-27 and likely all STAT1-activating cytokines, there are both STAT1- and T-bet-dependent mechanisms capable of suppressing Th17 responses. As evidence for STAT1-mediated inhibition, and consistent with published reports (8, 9, 29–31), we demonstrate that STAT1-deficient T cells exhibit a hyper-Th17 phenotype and are refractory to the anti-Th17 effects of IFN-γ and IL-27. As evidence for T-bet-mediated inhibition, we demonstrate that T-bet-deficient T cells also exhibit a hyper-Th17 phenotype, that T-bet-deficiency hinders the ability of STAT1-activating cytokines to suppress Th17 responses and, most importantly, that ectopic T-bet expression can suppress Th17 responses in the complete absence of STAT1. Previous studies have also suggested that STAT1 and T-bet might play independent roles in this process but, since T-bet is both upstream (an inducer) and downstream (induced by) of STAT1, they could not definitively exclude the possibility that T-bet may be arbitrating STAT1-dependent inhibition through its well known ability to drive IFN-γ production (26, 34). We overcame the ‘chicken-and-egg’ problem by using retroviral vectors to restore T-bet expression in either T-bet or STAT1-deficient T cells, finding that indeed there are two pathways at work, with STAT1 not required for T-bet-mediated inhibition and T-bet not required for STAT1-mediated inhibition.
Aside from those operating through STAT1, other cytokines have been implicated in the regulation of Th17 responses, including IL-2 and IL-4, which are known to act primarily through STAT5 and STAT6, respectively. Similar to STAT1-deficiency, genetic ablation of these STATs is associated with increased Th17 responses but whether this shared outcome is achieved through a common mechanism is yet to be resolved (8, 45). The most direct way that STATs could limit Th17 responses is by binding to promoter/enhancer regions of Th17-assoctaed genes and thereby obstructing the transcriptional machinery. There is some evidence for this, with STAT5 having been shown to bind the promoter of IL-17A, but whether this interaction is what determines the ability of STAT5 to suppress IL-17 production was not determined (45). Likewise, STAT1 has been shown to bind upstream of the RORα and RORc loci in human HELA cells but the nature of this interaction, be it stimulatory or inhibitory, and whether it happens in primary T cells, are questions that remain unanswered (46). Another, more indirect, way that STATs could impact Th17 responses is by inducing or promoting the function of auxiliary anti-Th17 factors. There is strong evidence for this since cytokines with anti-Th17 activity are already known to induce “Th17 inhibitors’, like T-bet, Ets1 and Gfi-1, and this is not likely to be an exhaustive list of indirect targets (47–49). STATs could also influence Th17 responses by interfering with pro-Th17 TFs or signaling pathways, as is the case with the ability of IL-27 to induce expression of SOCS3, which is known to curb STAT3-dependent Th17 responses (50), the ability of IFN-γ and IL-27 to suppress S1P, a receptor known to promote IL-6-driven Th17 responses (51), and the ability of several anti-Th17 cytokines to suppress RORγT, which is both necessary and sufficient for Th17 differentiation (29–31). Thus, while other regulatory pathways will likely emerge, it is already clear that cytokines limit Th17 responses through both direct and indirect STAT-driven pathways that, together, disable multiple steps in the Th17 differentiation program.
As with the STATs, T-bet could suppress Th17 responses in a variety of ways. A recent genome-wide mapping of T-bet binding sites did not reveal significant enrichment near the IL-17A, IL-17F, IL-22 or ROR loci, making a direct interaction between T-bet and relevant Th17-associated promoters seem unlikely (52). However, a direct protein-protein interaction between T-bet and pro-Th17 TFs remains a possibility, especially since T-bet is known to interact with and thereby limit the function of other TFs, including GATA-3 and RelA (53, 54). Consistent with this latter point, our studies demonstrate that T-bet can suppress IL-17 production even in the face of ectopic RORγT, which is driven by a retroviral promoter and, thus, impervious to transcriptional effects. These data suggest a physical interaction between T-bet and elements of the Th17 differentiation machinery, if not RORγT itself, though it should also be noted that T-bet might influence Th17 responses through more indirect means. Adding further complexity, T-bet has a functional homologue, Eomes, which is expressed in T cells and is known to exhibit anti-Th17 activity. Recent studies have shown that ectopic expression of Eomes can suppress IL-17 production but whether this is due to a cell-intrinsic effect or its ability to drive IFN-γ-mediated suppression was not resolved (35). It is also known that, unlike T-bet and STAT1, genetic ablation of both T-bet and Eomes results in a compound hyper-Th17 phenotype but, again, the increase in IL-17 production was mirrored by a corresponding reduction in Th1 responses, making it unclear whether the phenotype was due to direct effects or a lack of IFN-γ/STAT1-dependent inhibition (26). Based on the data presented here, we propose that both are true, that T-bet and Eomes can each limit Th17 responses through at least two shared mechanisms, one involving STAT1, with IFN-γ as an intermediary, and the other completely STAT1-independent.
Although our findings establish that STAT1 and T-bet influence Th17-type cytokines through genetically distinct pathways, we noted that T cells lacking both transcription factors did not exhibit an additive, or compound, phenotype. Instead, the hyper-Th17 phenotype of T-bet deficient cells was always more severe than that of STAT1- or double-deficient counterparts which, despite the well-known ability of STAT1 to drive T-bet expression, is also inconsistent with the notion that they operate within the same pathway. Taken together, these contradictory observations suggest epistasis, meaning that the loss of STAT1 affects cellular processes that, while not directly related to Th17 differentiation, impact the overall quality of T cell responses, thereby hindering production of Th17-type cytokines. We found that, beyond Th17 responses, T-bet and STAT1-deficient T cells behaved differently in vivo, with the latter exhibiting reduced proliferation and increased Th2-type cytokine production. We also found evidence for epistasis during in vitro differentiation. Those studies confirmed that STAT1 is required for IFN-γ and IL-27 to suppress IL-17 production but also showed that, compared to WT counterparts, expression of many Th17-type mRNAs was not grossly elevated in STAT1-deficient cells which, perhaps, indicates post-transcriptional effects (Supplemental Figure 3). Thus, while we can still conclude that STAT1 and T-bet influence Th17 responses through both divergent and convergent mechanisms, it must be noted that genetic dissection of these pathways was confounded by the wide-ranging effects of STAT1-deficiency.
The ability of signature Th1-type factors, like IFN-γ, STAT1 and T-bet, to inhibit signature Th17-type factors, like IL-17, IL-22 and RORγT, has led to the idea that there is an inverse relationship between the Th1 and Th17 subsets. However, despite this antagonism, T cells producing IFN-γ and IL-17 are known to occur in multiple inflammatory settings, which suggests a more nuanced relationship (2, 10–14). The current work illustrates both sides of this paradox. On one hand, we show that T cells produce either IFN-γ or IL-17 when primed in vitro and, on the other, that they can produce both when primed in vivo (in highly immunogenic sOva Rag2−/− mice). We also report that, when T-bet and RORγT were both highly expressed in the same cells, the result is dichotomous, with some cells expressing one cytokine or the other but rarely both (Fig 1D & Fig 7D). Based on this latter finding, and the fact that T-bet is known to promote long-term Th1 lineage commitment, we propose that ‘double-positive’ T cells represent a transitional phase in a linear progression from IL-17-producing Th17 cell to IFN-γ producing Th1 cell. Inherent to this hypothesis is the idea that Th17 cells can convert to other subsets, which has strong experimental support (10), and that such conversion is a part of normal immune responses, which is now supported by recent studies demonstrating that Th17-type cytokines are required for the development of Th1 responses during infection (55).
Although Th17 responses can have important, host-protective functions, it is also widely accepted that, when dysregulated, they can promote autoimmune disease. Given the current and prospective use of STAT1-activating cytokines as therapeutics for Th17-associated pathologies, best exemplified by the use of interferon-β to treat multiple sclerosis, it is critical to understand exactly how they suppress Th17-type inflammation. The studies presented here provide an important piece of mechanistic information, demonstrating that the ability of STAT1 to limit Th17-type responses is intimately linked to T-bet, a transcription factor that is at once a potent anti-Th17 effector and, through its ability to drive IFN-γ production, an essential STAT1 stimulus. Though we have focused on CD4+ T cells, which we interrogated in a select few model systems, this relationship between STAT1 and T-bet is likely to impact other IL-17-producing lineages, such as CD8+ T cells or NKT cells, and is likely to influence Th17 responses in a variety of immune and auto-immune settings, making these pathways acutely relevant in the context of inflammatory disease etiology and cytokine-based drug design.
Supplementary Material
Supplemental Figure 1. Analysis of donor T cell IL-17F production. (A) Naïve DO11.10 CD4+ T cells were transferred into Rag2−/− or sOva Rag2−/− hosts. 3–9 days later, lymphocytes from recipient mice were re-stimulated and IL-17F production measured by intracellular flow cytometry. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. Right panel: Data are compiled from 4 experiments (4–8 mice/group). Star indicates statistically significant differences between early (days 3–4) and late (days 7–9) time points (p<0.05). (B) Adoptive transfers and re-stimulations were performed as in (A). Shown is IL-17F production in either IL-17A− (grey histograms) or IL-17A+ (open histograms) donor T cells. (C) Adoptive transfers were performed as in Figure 2A. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. (D) IL-17F was measured in total and IL-17A+/− donor T cells from WT and IFN-γ −/− sOva Rag2−/− (left) or Rag2−/− hosts (right). Data are compiled from 3 experiments. (E) (C) Adoptive transfers were performed as in Figure 3. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. Lower panel: Data are compiled from 3 experiments. (D-E) One star denotes significant differences between the indicated group and WT controls.
Supplemental Figure 2. Host-derived IFN-γ production in sOva Rag2−/− mice. Naïve T cells were purified from either WT or IFN-γ −/− DO11.10 mice and then adoptively transferred into either WT or IFN-γ −/− sOva Rag2−/− hosts. At 7 days post-transfer, lymphocytes were re-stimulated and cytokine production measured as in Figure 1. Shown are the percentages of TNF-α+ and IFN-γ+ cells in the non-T cell fraction (CD4− DO11.10 TCR−). Data are representative of >5 individual experiments.
Supplemental Figure 3. PCR analysis of Th17-associated genes in T-bet and STAT1-deficient T cells. Naïve T cells were cultured under non-polarizing or Th17 conditions (+/− IFN-γ or IL-17) and PCR used to measure the indicated transcripts. Data are color (by genotype) and are presented as the fold increase (X>1) or decrease (X<1) relative to WT controls (X=1). Graphs are representative of 3 experiments and error bars represent the standard deviation within replicate measurements.
Supplemental Figure 4. Purifying retrovirally transduced T cells. T-bet-deficient T cells were cultured under non-polarizing conditions and transduced with retroviral vectors expressing T-bet (marked by GFP), RORγT (marked by Thy1.1), and or ‘empty’ GFP or Thy1.1 controls. 72 hours later, cells were stained for high-speed cell sorting and those infected with two viral vectors were purified (CD4+ GFP+ Thy1.1+; upper right quadrant). Shown are representative data from one of 3 independent experiments.
Acknowledgments
The authors would like to thank members of the Abbas, Anderson, Bluestone and Tang laboratories for helpful discussions and Drs. M. Ansel and S. Katzman for critical reading of this manuscript. We also thank S. Jiang for cell sorting and C. Benitez for animal husbandry.
Abbreviations
- TF
Transcription factor
- Ova
ovalbumin
- LN
lymph node
- ICS
intracellular cytokine staining
- RV
retrovirus
- EAE
experimental autoimmune encephalitis
- EAU
experimental autoimmune uveitis
- CNS
central nervous system
Footnotes
This work was supported by NIH grant RO1 AI64677 with a minority post-doctoral supplement to A.V.V. (PA-05-015)
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 5.Irmler IM, Gajda M, Brauer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 6.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 9.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 10.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 16.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 17.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 19.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 20.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 21.Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J Immunol. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 29.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 30.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 31.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 33.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol. 2008;181:8700–8710. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 37.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 38.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, Peebles RS., Jr A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182:5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, Euskirchen G, Bernier B, Varhol R, Delaney A, Thiessen N, Griffith OL, He A, Marra M, Snyder M, Jones S. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 47.Ichiyama K, Hashimoto M, Sekiya T, Nakagawa R, Wakabayashi Y, Sugiyama Y, Komai K, Saba I, Moroy T, Yoshimura A. Gfi1 negatively regulates T(h)17 differentiation by inhibiting RORgammat activity. Int Immunol. 2009;21:881–889. doi: 10.1093/intimm/dxp054. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, Guo L, Zhao K, Shevach EM, Paul WE. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206:329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 52.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 54.Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Analysis of donor T cell IL-17F production. (A) Naïve DO11.10 CD4+ T cells were transferred into Rag2−/− or sOva Rag2−/− hosts. 3–9 days later, lymphocytes from recipient mice were re-stimulated and IL-17F production measured by intracellular flow cytometry. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. Right panel: Data are compiled from 4 experiments (4–8 mice/group). Star indicates statistically significant differences between early (days 3–4) and late (days 7–9) time points (p<0.05). (B) Adoptive transfers and re-stimulations were performed as in (A). Shown is IL-17F production in either IL-17A− (grey histograms) or IL-17A+ (open histograms) donor T cells. (C) Adoptive transfers were performed as in Figure 2A. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. (D) IL-17F was measured in total and IL-17A+/− donor T cells from WT and IFN-γ −/− sOva Rag2−/− (left) or Rag2−/− hosts (right). Data are compiled from 3 experiments. (E) (C) Adoptive transfers were performed as in Figure 3. Open histograms denote IL-17F levels in donor cells (CD4+ DO11.10+) while grey histogram denote naïve controls. Lower panel: Data are compiled from 3 experiments. (D-E) One star denotes significant differences between the indicated group and WT controls.
Supplemental Figure 2. Host-derived IFN-γ production in sOva Rag2−/− mice. Naïve T cells were purified from either WT or IFN-γ −/− DO11.10 mice and then adoptively transferred into either WT or IFN-γ −/− sOva Rag2−/− hosts. At 7 days post-transfer, lymphocytes were re-stimulated and cytokine production measured as in Figure 1. Shown are the percentages of TNF-α+ and IFN-γ+ cells in the non-T cell fraction (CD4− DO11.10 TCR−). Data are representative of >5 individual experiments.
Supplemental Figure 3. PCR analysis of Th17-associated genes in T-bet and STAT1-deficient T cells. Naïve T cells were cultured under non-polarizing or Th17 conditions (+/− IFN-γ or IL-17) and PCR used to measure the indicated transcripts. Data are color (by genotype) and are presented as the fold increase (X>1) or decrease (X<1) relative to WT controls (X=1). Graphs are representative of 3 experiments and error bars represent the standard deviation within replicate measurements.
Supplemental Figure 4. Purifying retrovirally transduced T cells. T-bet-deficient T cells were cultured under non-polarizing conditions and transduced with retroviral vectors expressing T-bet (marked by GFP), RORγT (marked by Thy1.1), and or ‘empty’ GFP or Thy1.1 controls. 72 hours later, cells were stained for high-speed cell sorting and those infected with two viral vectors were purified (CD4+ GFP+ Thy1.1+; upper right quadrant). Shown are representative data from one of 3 independent experiments.