Abstract
Hypoxia followed by reoxygenation (HR) and ischemia-reperfusion (IR) cause cell death in neonatal rat ventricular myocytes (NRVM) primarily through the generation of oxidative stress. Extracellular catalase (CAT) has not been effective in reducing or eliminating IR or HR-induced cell death due both to extracellular degradation and poor cellular uptake.
Aims
1) to determine if a cell penetrating catalase derivative with enhanced peroxisome targeting efficiency (catalase-SKL) increases intracellular levels of the antioxidant enzyme in NVRM; and 2) to determine if catalase-SKL protects against both HR and IR injury.
Methods
NRVM were subjected to 3 or 6 hr of HR or 1 hr of IR. CAT concentration, activity, and subcellular distribution were determined using standard techniques. Reactive oxygen species (ROS) and related oxidative stress were visualized using 2’,7’-dichlorofluorescin diacetate. Cell death was measured using trypan blue exclusion or lactate dehydrogenase (LDH) release assays.
Results
CAT activity was higher in (catalase-SKL) transduced myocytes, was concentrated in a membranous cellular fraction, and potently inhibited oxidative stress. In contrast to non-transducible (unmodified) CAT, catalase-SKL-treated myocytes were protected against both HR and IR.
Conclusions
1) catalase-SKL increased myocyte CAT content and activity and dramatically increased resistance to hydrogen peroxide-induced oxidation; 2) catalase-SKL protects against both HR and IR; 3) catalase-SKL may represent a new therapeutic approach to protect hearts against myocardial HR or IR.
Keywords: myocyte, ischemia, cardioprotection, injury
Introduction
Myocardial ischemia
It is well known that sustained periods of ischemia (irreversible ischemia) causes many detrimental changes in the biochemical and structural composition of myocytes including a rapid decrease in high-energy phosphates (e.g. ATP), reduction of cellular pH, destabilization and related damage to the myocyte cytoskeleton, progressive mitochondrial damage, and calcium overload. As a result of the morbidity and mortality associated with IR in patients, considerable research has been directed at interventions that can limit or prevent myocyte death. One of the more controversial issues surrounding IR is determining how much cell death occurs as a result of the ischemic episode versus how much cell death occurs as a result of the reperfusion event. As a result of numerous experimental studies, it was learned that three major events are associated with reperfusion following ischemia that play an important role in determining the ultimate size of the myocardial infarct: the generation of reactive oxygen species (ROS) intracellular calcium levels, and acidification of the myocyte (including Na+/H+ exchange).
Role of ROS in IR injury
The role of ROS in IR injury has been studied intensely for the past 25 years by many investigators. The results of these studies have clearly demonstrated that at reperfusion, a burst of ROS can be measured (11, 35). It is less clear whether significant amounts of ROS are produced during the episode of ischemia. In the 1980’s it was thought that this burst of ROS was responsible for extensive damage to the myocardium over and above what occurred as the result of the ischemic episode; i.e. “lethal reperfusion injury”. Subsequent investigation revealed that ROS play a role in non-lethal or reversible forms of IR injury such as myocardial stunning (5) but the role of ROS in lethal reperfusion injury has remained controversial. A number of animal studies showed that antioxidant compounds or ROS scavengers such as superoxide dismutase (SOD) or catalase reduced cell death in experimental models of myocardial infarction (MI) (2, 7, 20). However, other studies showed no reduction in infarct size (10, 21, 22). Based upon these studies, clinical trials were initiated to determine whether free radical scavenger therapy, involving specifically SOD, would be beneficial to patients undergoing angioplasty. Unfortunately, the trials did not show a positive clinical benefit (12). However, although these specific trials were not positive, it is not necessarily true that free radical eradication therapy itself is not potentially protective. In these trials, drug delivery systems were not advanced and SOD was delivered in a bolus into the vascular space. It is possible that a more efficient cellular delivery of the therapeutic would provide significant protection. Indeed, recent studies have suggested that scavengers targeted to the intracellular space may be more protective than extracellular administration of the same drugs (1, 3, 26). In summary, it appears that localization and timing of administration may be more critical to achieving cardioprotection in IR than previously appreciated.
Catalase localization and activity
Catalase is localized in peroxisomes of all eukaryotic cells including cardiomyocytes (12, 31, 34). The normal function of catalase is to aid in the detoxification of ROS. These potentially damaging molecules are generated in a number of cellular locations including the peroxisome, during β-oxidation of specific lipids, and mitochondria, mostly from the reduction of oxygen with electrons that escape the (mitochondrial) respiratory chain. These latter reactions result in the formation of superoxide anion (O2-), which is converted into H2O2 by the action of superoxide dismutase. H2O2 can interact with reduced metal ions to form the highly toxic and exquisitely reactive species known as the hydroxyl radical (·OH). ·OH has an extremely short half-life and reacts quickly with many biologic molecules including lipids, proteins, and nucleic acids. Catalase functions to break down H2O2 into water and oxygen thereby favoring an oxidant-free environment devoid of the toxic ·OH.
Although catalase plays an important protective role, its activity in heart tissue is very low compared to other tissues. Some studies show that heart tissue has as little as 2% of the activity present in liver cells (9, 13). This has led to the hypothesis that heart cells are more susceptible to oxidative stress than other tissues in the body. Indeed, previous studies have shown that transgenic over-expression of catalase (up to 60-100 fold more catalase than normal) protects against IR injury in the heart, anoxia-reoxygenation-induced functional alterations, and even against doxorubicin-induced cardiotoxicity (6, 14, 18). Although transgenic mice are useful in the proof of principle that increased catalase is protective, it is not a therapy that can be easily adapted to human patients. An approach most likely to achieve clinical application would involve a method to acutely deliver catalase to the myocyte immediately prior to an anticipated oxidative stress. In essence, protein therapy employing a powerful antioxidant enzyme.
Role of catalase; past and present
Catalase is known to play an important role in cellular antioxidant defense and is largely concentrated in peroxisomes. Alterations in catalase expression, activity, stability, or localization have all been described and are associated with oxidative stress, accelerated aging, and human disease (15, 17, 28, 30). Recently, a novel way to increase intracellular catalase levels was developed taking advantage of an in-frame cell penetrating peptide (32). The molecule was also engineered to traffic to peroxisomes with enhanced efficiency by virtue of an altered carboxy-terminal peroxisomal targeting signal. This unique (United States patent 7601366 and related patents pending) recombinant enzyme, called catalase-SKL, rapidly enters cells, traffics to organelles (~peroxisomes), and efficiently metabolizes ROS (23, 32, 33). Catalase-SKL, transduced either with cell penetrating peptides or through retrovirus-mediated processes, restores enzyme levels in cells of hypocatalasemic individuals (30), neutralizes ROS and reduces expression on inflammatory cytokines in primary human keratinocytes (a model for psoriasis) (32), and delays appearance of aging markers in (human) fibroblasts (15). The purpose of the present study was to determine if this agent would increase myocyte catalase levels and whether the enzyme would be active (i.e. provide antioxidant activity) and appropriately localized. In addition, we determined if increasing myocyte catalase in peroxisomes would protect ventricular myocytes from HR or simulated IR injury.
Methods and Materials
Isolation of neonatal cardiomyocytes
For each isolate, the ventricular portion of 9–12 hearts from 1- to 2-day-old rats was pooled and gently agitated overnight at 4°C with trypsin (0.1 g in 100 ml) in Hanks’ balanced salt solution (HBSS). On the next day, the myocytes were digested further with serial incubations in collagenase (0.1 g in 100 ml of HBSS). The final cell isolate was centrifuged at 1,000 rpm at 4°C. The resulting supernatant was discarded, and the cells were resuspended in ice-cold Dulbecco’s modified Eagle Medium (DMEM), transferred to a 50-ml conical tube, and centrifuged again for 3 min at 1,000 rpm at 4°C. The resulting supernatant was discarded, and the cell yield was determined using a hemocytometer.
Cell culture
After isolation and purification, the myocytes were resuspended in DMEM [supplemented with 10% fetal bovine serum (FBS) and containing antibiotics (penicillin-streptomycin and gentamicin) to inhibit bacterial growth] and cultured on 100-mm plates for 1 hr to reduce fibroblast contamination. After they were preplated, the cells were cultured in standard 35-mm dishes (Corning, Corning, NY). After 24 hr of culture, the medium was changed to DMEM without FBS.
Protein therapy
Catalase-SKL was expressed and purified as described. Neonatal ventricular myocytes were treated with 0.5 uM catalase-SKL in serum-free media for the indicated times. The cells were washed 5 times in phosphate buffered saline (PBS) prior to cell lysis to remove any non-transduced catalase-SKL.
Western blot
Myocytes were harvested for protein analysis by standard Western blot techniques. Briefly, cells were lysed in 200 uL lysis buffer [50 mM Tris-HCl (pH 8·0), 150 mM NaCl, 1% Triton X-100 and 100 ul protease inhibitor cocktail (Sigma) per 1 mL lysis buffer]. Cellular debris was removed by centrifugation at 4°C. Protein concentration was determined in the supernatants using a BCA assay kit (Pierce, U.S.A.) and aliquots of 40 ug protein were separated in reducing/denaturing conditions by standard SDS gel electrophoresis on 10% gels. The separated proteins were then transferred onto nitrocellulose membranes. The membranes were then sequentially blocked for 1 hr in LiCor blocking buffer and incubated with rabbit primary antibodies to catalase at a dilution of 1:5000 followed by 4 × 5-min phosphate-buffered saline washes in between. Secondary antibodies used at a 1:10000 dilution were Alexa Fluor 680 goat antirabbit IgG (Molecular Probes, Eugene, OR, U.S.A.) and IRDye 800 antimouse IgG (Rockland Immunochemicals, Gilbertsville, PA, U.S.A.). Membranes were scanned and bands were quantified with the Odyssey infrared Imaging System LiCor. Three independent experiments were performed to confirm the results.
Catalase activity assay
Catalase activity was measured by its ability to degrade H2O2. The method employed was based upon that of Storrie and Madden (27). Relative enzyme activity was calculated based on the decline in the measured (405 nm) absorbance values as compared to standards. The concentration of catalase was then calculated from the activity values.
Cell fractionation
Briefly, ventricular myocyte cultures were washed with PBS and then immediately lysed in 25 μl of a protease inhibitor cocktail (Sigma) and 0.2 ml of ice-cold homogenization buffer [final concentration (in mM) 20 Tris-HCl (pH 7.5), 2 EDTA, 2 EGTA, 3-mercaptoethanol, 0.1 sodium vanadate, and 50 NaF]. The isolate was passed through a 26-gauge needle several times to enhance cell lysis and then centrifuged at 15,000 g for 10 min. The supernatant was removed (soluble fraction), and the pellet was resuspended in 0.1 ml of homogenization buffer containing 1% Triton X-100 (membrane fraction).
Cell injury models and measurements
Hypoxia assay
Hypoxia was achieved by using an anaerobic container, BD GasPak EZ Gas Generating Container Systems (BBL Microbiology Systems, Becton Dickinson and Co., Cockeysville, Md.) equipped with GasPak hydrogen and carbon dioxide-generating envelope and a methylene blue indicator to monitor oxygen depletion. Cultured neonatal rat myocytes were placed in glucose-free and serum-free DMEM and submitted to hypoxic conditions in the anaerobic container at 37°C for the time periods (3 hr/6 hr) indicated, and controls in glucose-free and serum-free DMEM were left at normoxic conditions at 37°C for the duration of the experiment (3 hr/6 hr).
Simulated IR injury (SI)
Cardiomyocytes were subjected to SI using our standard chemical IR protocol. To induce SI, the culture medium was removed and replaced with fresh PBS containing 3.0 mM iodoacetic acid to inhibit glycolysis and 3.0 mM amobarbital to inhibit mitochondrial respiration for up to 60 min. The ischemic buffer was exchanged with fresh hypotonic oxygenated culture medium containing glucose without the chemical inhibitors for 1 hr to simulate reperfusion. The resulting proportion of live and dead cells was determined by measurement of LDH release with a commercially available kit (Sigma, St. Louis, MO).
Trypan blue staining
Trypan blue (TB) (0.4%, Life Science Technologies) staining was used as an indication of cell death. Cells were trypsinized from the culture surface and neutralized with serum, and a small volume of 0.4 % TB was added to cells from each dish. Cells were counted immediately after addition of the dye to prevent counting of nonspecific stained cells, which occur over time. A cell was considered TB positive if the entire cytoplasm was diffusely stained with any shade of blue. The total numbers of viable cells and positive (dead) cells were counted with a Nikon TE300 inverted immunofluorescent microscope and the resulting data are presented as the percentage of cells that are TB positive.
LDH release assay
To determine the amount of cell injury induced by the SI protocol, release of LDH was measured. LDH is normally retained in the cytosol until the sarcolemmal membrane is ruptured, after which it is free to diffuse into the surrounding media. After completion of the SI protocol, the ischemic buffer in each well of the culture dish was assayed for LDH. The attached cells in each well were extracted, and the resulting extracts were analyzed for LDH. Total LDH was considered as the sum of the LDH released into the media during the SI protocol plus the residual LDH present in the attached cells. The percent LDH release was calculated by dividing the amount released into the media by the total LDH (released plus cellular content) for each experimental group. Cell culture medium (400 ul) was used to analyze the LDH activity by measuring the oxidation of NADH at 490 nm as described in the manufacturer’s protocol (Sigma Chemical Co.).
Measurement of oxidative stress
Cardiomyocytes were examined for H2O2 levels by using carboxy-H2-DCFDA (Invitrogen). Neonatal cardiomyocytes were transduced with catalase-SKL for 2 hr at 37°C. NRVC in 35-mm dishes were washed with 1X PBS and preincubated with 100 μM DCF-DA for 30 min at 37°C. Cells were again washed and H2O2 (diluted from a 30% stock solution, Sigma) was added to different aliquots to produce final concentrations of 100 uM-100 mM. DCF-DA is incorporated into lipid-rich regions of cells and hydrolysed to 2’,7’-dichlorofluorescein by cellular esterases. In the presence of peroxide, this is oxidized to fluorescein, which emits green fluorescence at 514 nm on excitation at 490 nm. Fluorescence images were captured using a Nikon TE300 inverted immunofluorescent microscope (10X objective). Quantification of the fluorescent signal was performed using Metamorph software (Molecular Devices). Data are presented as average intensities (in arbitrary units).
Statistics
Data are expressed as means ± SE. Statistically significant differences between groups were tested with a paired t-test analysis. A probability value of less than 0.05 was considered statistically significant.
Results
Effect of catalase-SKL on myocyte catalase levels (Figure 1)
Figure 1.
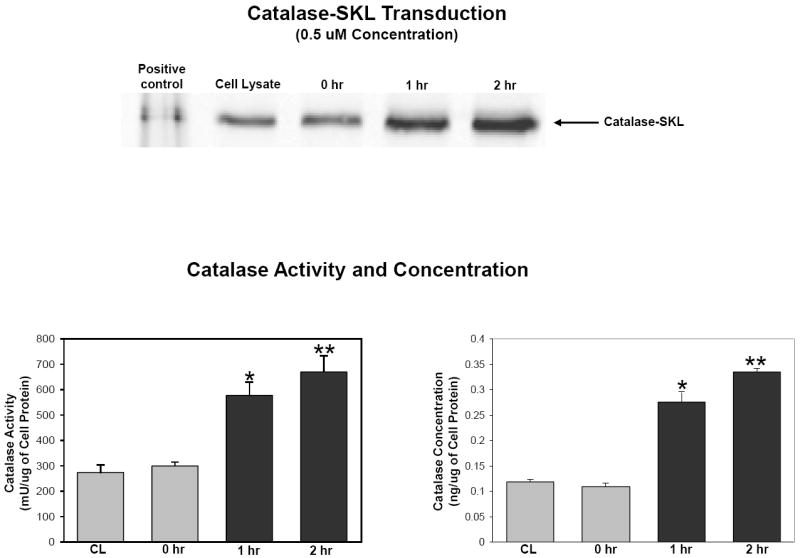
Effect of 0.5 uM catalase-SKL incubation for 2 hr on cellular catalase activity and concentration in cultured NRVM. Upper panel is a western blot of lysates of cells treated or not with 0.5 uM catalase-SKL for the times indicated. In the lane labeled positive control, pure catalase-SKL was loaded. Compared to control, myocytes incubated with catalase-SKL contained more catalase activity (lower left panel) and greater total amounts of the enzyme (lower right panel). CL = control cells. N=4 for all measurements. Significantly different from control; *p<0.05.
The first set of experiments was designed to determine if transducing catalase-SKL would increase intracellular catalase protein and activity levels in cultured NRVM. The data in Figure 1 are based on pilot data and show that both catalase activity and concentration were increased following 1 hr of incubation and remained elevated after 2 hr of incubation (669.2 ± 64.5 mU/ug vs. 275.5 ± 29.0 mU/ug and 0.340 ± 0.010 ng/ul vs. 0.112 ± 0.004 ng/ul, 2 hr vs. control, respectively; p<0.05 for both parameters).
Cellular localization of catalase (Figure 2)
Figure 2.
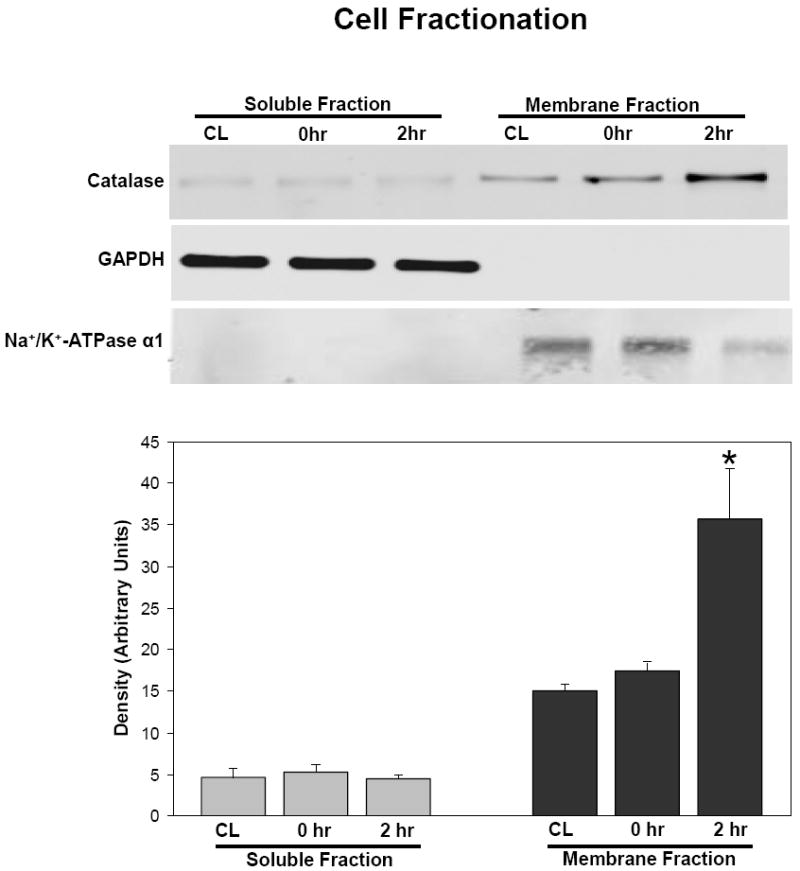
Subcellular localization of catalase after 2 hr incubation with 0.5 uM catalase-SKL. After incubation, myocytes were fractioned into standard soluble and membrane fractions and probed for catalase using SDS-PAGE and western blot techniques. Upper panel: GAPDH used a marker for the soluble fraction and Na+/K+ATPase used as a marker for the membrane fraction to ensure equal loading. Lower panel represents quantitation of the catalase western blot in the upper panel. At zero time, there were no significant differences between control (CL) and treated myocytes in terms of distribution. After 2 hr of incubation however, virtually all the catalase was localized in the membrane fraction in treated myocytes consistent with a peroxisomal localization of the transduced enzyme. Significantly different from control; *p<0.05. Similar results were seen in three experiments.
To determine where the increased catalase was localized in the myocyte, we analyzed subcellular fractions after 2 hr of transduction with catalase-SKL. The data in Figure 2 show that after 2 hr, nearly all of the catalase was localized in the membrane fraction. These fractions contain cell organelles and contractile proteins. Virtually none of the catalase was found in the soluble (cytosolic) fraction. These results confirm the expected outcome that the delivery agent targets catalase-SKL to subcellular organelles; presumably peroxisomes.
Effect of catalase-SKL on H2O2-induced oxidative stress (Figure 3)
Figure 3.
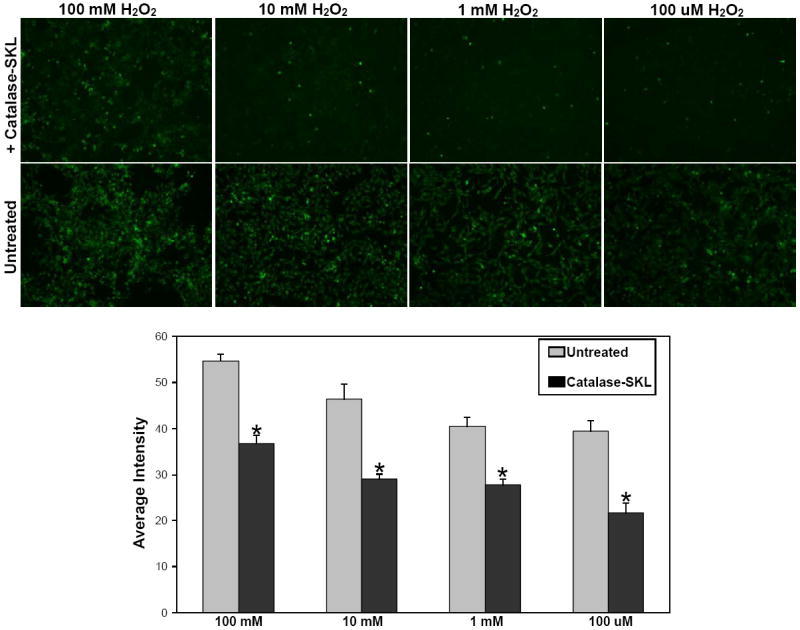
Effect of catalase-SKL on oxidative stress induced by H2O2 treatment. NRVM were treated for 2 hr with catalase-SKL and then exposed to increasing concentrations of H2O2 for 15 minutes. In untreated myocytes, increasing concentrations of H2O2 resulted in increasing amounts of oxidative stress as evidenced by the corresponding increase in fluorescence intensity. At any given dose of H2O2, myocytes pretreated with catalase-SKL exhibited significantly less oxidative stress (i.e. fluorescence) than untreated myocytes. Lower panel represents quantitation of pooled results from six experiments; upper panel is a representative image of data obtained. Significantly different from control; *p<0.05.
The results from figures 1 and 2 demonstrate the strategy employed was effective in increasing the level of cellular catalase. To determine if transduced catalase-SKL was capable of reducing oxidative stress, the well-known 2’,7’-dichlorofluorescin diacetate assay was used to monitor the generation of ROS. NRVM were transduced for 2 hr with catalase-SKL and then exposed to increasing concentrations of H2O2 for 15 minutes. Figure 3 shows that in untreated myocytes, increasing concentrations of H2O2 resulted in increasing amounts of fluorescence corresponding to elevated ROS. Myocytes transduced with catalase-SKL, at any given dose of H2O2 exhibited significantly less oxidative stress than untreated myocytes (p≤ 0.05 treated vs. untreated with each dose). These results confirm that the catalase that is delivered to the myocytes is active and effective in reducing ROS levels.
Effect of catalase-SKL on HR injury in NRVM (Figure 4A)
Figure 4.
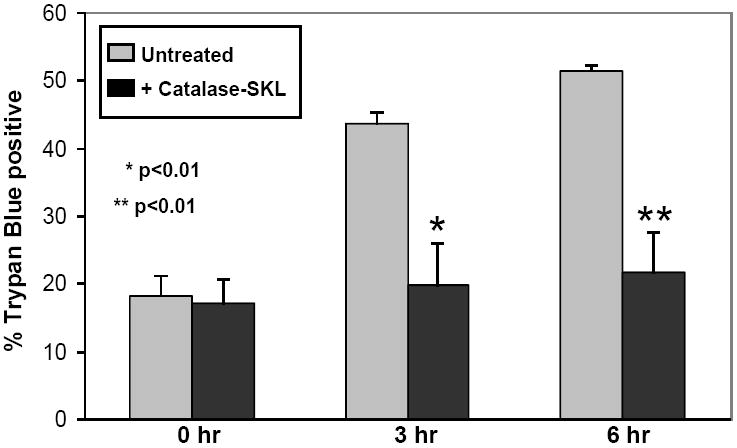
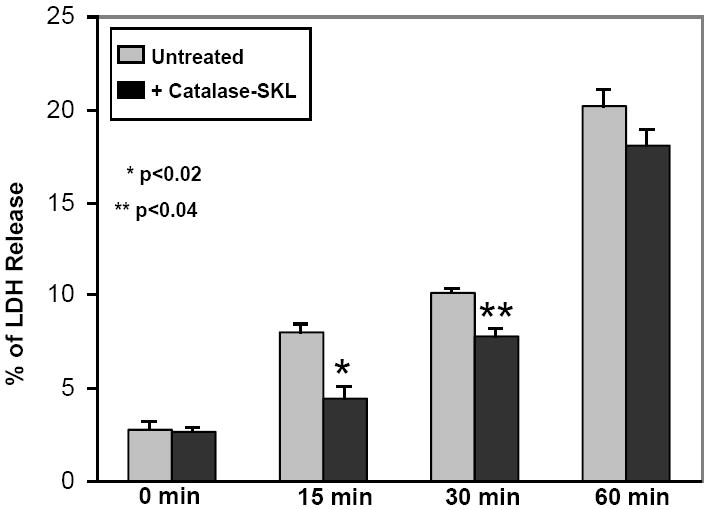
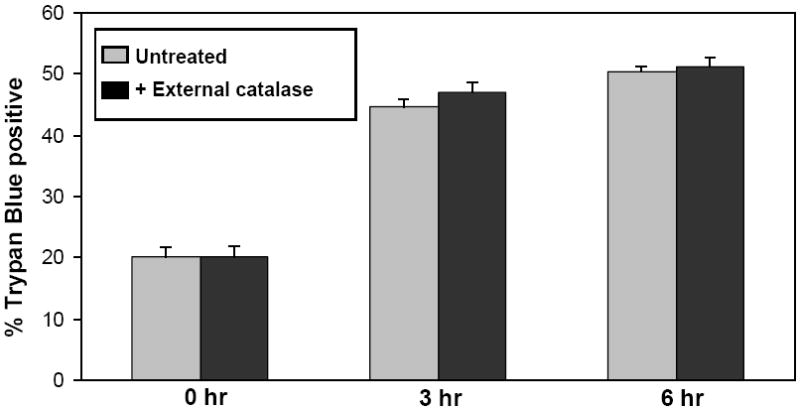
Effect of catalase-SKL on cell death. 4A. Control and catalase-treated (0.5 uM) NRVM were subjected to 3 or 6 hr of hypoxia followed by 1 hr of reoxygenation and cell death was assessed using trypan blue permeability. Compared to control myocytes, treated cells sustained substantially less death indicating a strong cardioprotective effect (* p≤ 0.01 vs. control; ** p< 0.01 vs. control. n=3 for each). 4B: Control and catalase-SKL-treated NRVM were subjected to 15, 30, or 60 min of simulated ischemia followed by 1 hr of reperfusion and cell death was assessed using LDH release into the culture media. At 15 and 30 min of MI/R, treated myocytes sustained substantially less cell death indicating a strong cardioprotective effect (*p≤ 0.01 vs. control; ** p< 0.01 vs. control. n=3 for each). However, after severe ischemia (60 min) the protective effect was largely lost (p=not significant; n=3). 4C: Control myocytes were compared to myoyctes treated with (0.5 uM) non-transducible (i.e. extracellular) catalase (labeled NT catalase). Myocytes were subjected to 3 or 6 hr of hypoxia followed by 1 hr of reoxygenation and cell death was assessed using trypan blue permeability. Extracellular catalase was present throughout the hypoxic incubation but failed to protect against cell death after either 3 or 6 hr of hypoxia/reoxygenation. (p=not significant; n=3 for each group).
The main purpose of the study was to determine if the catalase-SKL, designed to increase intracellular/peroxisomal catalase levels, would protect against cell death resulting from HR, a form of cell injury closely related to oxidative stress. Furthermore, we wanted to determine if catalase-SKL provided better protection than extracellular catalase alone. For these studies, NRVM were transduced for 2 hr with catalase-SKL prior to exposure to 3 or 6 hr of hypoxia followed by 1 hr of reoxygenation. Cell death was determined by assessing myocyte trypan blue permeability. Figure 4A shows that after 3 hr of HR, 43.7 ± 1.6% of the myocytes were dead (stained blue). Increasing the duration of HR to 6 hr resulted in a further increase in cell death to 51.5 ± 0.6%. At both time points, catalase-SKL treated myocytes suffered significantly less cell death (19.8 ± 6.2 and 21.7 ± 5.8 % 3 hr and 6 hr, respectively; p< 0.01 0 hr vs. 3 and 6 hr) indicating a protective effect against HR injury. Indeed, catalase-SKL almost completely eliminated HR injury in these cells.
Effect of catalase-SKL on SI injury in NRVM (Figure 4B)
In addition to determining if catalase-SKL protected against HR injury, we wanted to determine if it would protect against a more severe form of cell injury, namely IR. In these experiments, we utilized our chemical model of IR injury (SI). Similar to HR injury, all treated myocytes were transduced with catalase-SKL for 2 hr prior to initiation of SI. In these experiments, release of the intracellular enzyme LDH was used to assess the extent of cell death. Figure 4B shows that SI is a more severe form of injury in NRVM. After as little as 15 min of chemical ischemia followed by 1 hr of simulated reperfusion, significant cell death occurred (8.03 ± 0.34%) which increased after 30 min of incubation (10.19 ± 0.15% of LDH released). Compared to control (untreated) cells, myocytes treated with catalase-SKL exhibited significantly less cell death after 15 or 30 min of SI injury (4.56 ± 0.48% and 7.8 ± 0.33%, 15 and 30 min, respectively; p≤ 0.02 at 15 min; p≤0.04 at 30 min). However, indicative of the more severe stress associated with the SI model, by 60 min the protective effect of catalase-SKL was largely overwhelmed (20.40 ± 0.67 vs. 17.79 ± 0.77%; p=not significant).
Effect of extracellular catalase on HR injury in NRVM (Figure 4C)
The data presented to this point suggests catalase-SKL offers intracellular antioxidant prophylaxis. Catalase lacking a means of cellular uptake, that is, largely extracellular catalase, is incapable of affording such benefits. To demonstrate this latter point, we treated myocytes with “extracellular” catalase at a concentration of 100 uM throughout the duration of the HR treatment (3 or 6 hr) and compared the results to values obtained with control cells (without any catalase added). The data presented in Figure 4C demonstrate that the amount of cell death after 3 and 6 hr of HR was almost identical to the data from Figure 4A documenting the reproducibility of the HR assay system. Furthermore, extracellular catalase had no effect in reducing cell injury compared to untreated myocytes (44.6 ± 1.4 vs. 47.0 ± 1.6%; 50.3 ± 0.7 vs. 51.1 ± 1.7%; 3 hr and 6 hr, respectively; p=not significant at both time points).
Discussion
The present study describes several experimental findings that indicate catalase-SKL may represent a new option in the treatment of oxidative stress-induced cell death such as occurs in myocardial IR injury. First, the data show that catalase-SKL is taken up by myocytes in significant quantities (after as little as one hour) and that the enzyme is concentrated in the membrane fraction of the myocyte. Secondly, transduced catalase-SKL exhibits antioxidant activity and inhibits the additional significant oxidative stress induced by administration of exogenous H2O2. Third, catalase-SKL provides substantially better protection against HR injury than unimported, i.e. extracellular, catalase.
Summary of efforts to ameliorate IR injury
Throughout the past 20 years, hundreds of interventions, both pharmacologic and non-pharmacologic, have been shown to reduce the cell death associated with IR. Many of these interventions have been shown to be protective in animal models of IR although even in animal studies not all agents produced uniformly positive results. Some of the most promising drugs were sent to clinical trials where nearly all failed to show significant benefit including hyaluronidase, corticosteroids, recombinant SOD, prostacyclin, fluosol, cariporide, nitrates, anti-P-selectin, and antileukocyte interventions (anti-CD-18 monoclonal antibodies) (4). Two interventions showed some promise, adenosine and glucose-insulin-potassium, but still did not provide statistically significant protection against acute MI (8, 19, 25). Moreover, adenosine given at reperfusion has produced mixed results in animal studies (29).
In reviewing the published failed clinical trials, one common theme is that the agents were delivered late in the ischemic phase or at the time of reperfusion without knowledge of the effectiveness of drug delivery. It is possible that higher concentrations of certain drugs would have proved protective if the method of delivery was capable of achieving an effective drug concentration to the heart without causing local or systemic toxicity. To truly know whether anti-ischemic drugs will have a significant benefit in reducing infarct size requires delivery of effective cardioprotective drugs at the optimal concentration and during the correct time frame. Previous efforts at increasing the effective dose of antioxidant drugs to the heart have been limited to studies attempting to extend the plasma half-life of SOD by conjugation to polyethylene glycol but these efforts have not been tried in clinical trials. New approaches to target delivery and/or intracellular trafficking of anti-ischemic drugs to specific subcellular areas where the action is most effective is a rational approach to improve the efficacy of antioxidant therapy in myocardial IR injury.
Catalase-SKL
Catalase-SKL utilizes a relatively new approach called protein transduction to deliver the protein across the cell membrane and target it specifically to the peroxisome where catalase is normally stored in myocardium.
The data presented in figure 1 showed that after one hour of incubation, myocytes contained significantly more catalase and that the catalase was concentrated in the membrane/organelle fraction and not in the soluble fraction. Furthermore, after one hour of treatment, the amount of catalase activity increased over two-fold compared to control myocytes. These data indicate that catalase-SKL is capable of entering myocytes and being appropriately trafficked to organelles.
Effect of catalase-SKL on oxidative stress and cell injury
In order to be effective, the delivered catalase must be biologically active at the site of action and capable of reducing oxidative stress. To determine if catalase-SKL could reduce oxidative stress, we administered exogenous H2O2, a well known oxidative stress, to cultured NVRM and determined the effect on both oxidation and cell injury. The data in Figure 3 showed that myocytes treated with catalase-SKL reduced H2O2- induced oxidative stress. These data, along with the data presented in Figure 4C, demonstrate that catalase-SKL’s action in reducing cell death is most likely due to reduction in oxidative stress/damage inside the myocyte. However, other oxidative species, including other oxygen derived free radicals (i.e. superoxide and/or hydroxyl radical) and/or nitrous species (i.e. nitric oxide), were not directly inhibited by catalase-SKL and therefore the role they play in overall cell injury in these experiments is not clear. For example, the elimination of cellular protection after 60 minutes of simulated ischemia/reperfusion (Figure 4B) may be the result of persistent or even enhanced activity of such species despite the presence of higher catalase levels.
Mechanism of catalase-SKL protection
Catalase is the major endogenous enzyme involved in the breakdown of H2O2 and therefore has an obvious and potent antioxidant effect. H2O2 is a powerful oxidative agent whose role in cell injury is well documented. It can cause rapid cell damage through lipid peroxidation of membranes, cross-linking of proteins, and nuclear fragmentation. In this study catalase-SKL was specifically targeted to the intracellular compartment of myocytes where it likely was concentrated in the peroxisome; the normal subcellular site for catalase. Although any reduction in intracellular oxidative stress is likely to be beneficial to long term cell survival, it is possible that catalase may have acted through a more specific mechanism. For example, recent studies have stressed that breakdown of H2O2 inhibits activation of apoptotic pathways that in turn reduce cell death. Other studies have shown that catalase improved intracellular calcium handling and therefore perhaps the enzyme can reduce cell death by reducing intracellular calcium overload (24). One recent study hypothesized that alterations in the oxidative environment of cells via breakdown/scavenging of free radicals may protect myocytes through increasing the ability of mitochondria to phosphorylate adenosine diphosphate (ADP) and therefore maintain adequate ATP levels for prolonged cell survival (16). Although all of these are interesting hypotheses, the precise mechanism by which increased catalase reduced cell death in this study was not specifically studied.
Summary and conclusion
In summary, the present study shows that catalase-SKL is selectively taken up by myocytes into the membrane subfraction, increases the endogenous antioxidant capacity of treated myocytes in response to a known oxidative stress, and reduces cell death in response to both HR and simulated IR injury. Extracellular catalase alone was not protective. Further studies are indicated to determine if catalase-SKL can be exploited in a more clinically relevant model system to reduce myocyte cell death from IR injury.
Acknowledgments
Research supported by RO1-HL-84405-A1 from the NHLBI to RVH.
Footnotes
Conflict of Interest: SRT is a cofounder of EXT Life Sciences Inc., a Michigan-based biotechnology company owned, in part, by Wayne State University. SRT retains an equity interest in the company. Transducible catalase-SKL is covered by a United States patent (#7601366), which is owned by Wayne State University. Other authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction of experimental infarct size by recombinant human superoxide dismutase: Insights in the pathophysiology of reperfusion injury. Circulation. 1986;74:1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 3.Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med. 2006;41:835–848. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Zhu W-X, Hartley CJ, Michael LH, Repine J, Hess ML, Kukreja RC, Roberts R. Attenuation of dysfunction in the postischemic “stunned” myocardium by dimethylthiourea. Circulation. 1987;76:458–468. doi: 10.1161/01.cir.76.2.458. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Saari JT, Kang YJ. Repression of hypoxia-reoxygenation injury in the catalse-overexpressing heart of transgenic mice. Proc Soc Exp Biol Med. 1997;216:112–116. doi: 10.3181/00379727-216-44162b. [DOI] [PubMed] [Google Scholar]
- 7.Chi L, Tamura Y, Hoff PT, Macha M, Gallagher KP, Schork MA, Lucchesi BR. Effect of superoxide dismutase on myocardial infarct size in the canine heart after 6 hours of regional ischemia and reperfusion: A demonstration of myocardial salvage. Circulation Research. 1989;64:665–675. doi: 10.1161/01.res.64.4.665. [DOI] [PubMed] [Google Scholar]
- 8.Diaz R, Goyal A, Mehta SR, Afzal R, Xavier D, Pais P, Chrolavicius S, Zhu J, Kazmi K, Liu L, Budaj A, Zubaid M, Avezum A, Ruda M, Yusuf S. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA. 2007;298:2399–2405. doi: 10.1001/jama.298.20.2399. [DOI] [PubMed] [Google Scholar]
- 9.Doroshow JH, Locker GY, Myers CE. The enzymatic defenses of the mouse heart against reactive metabolites. J Clin Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher KP, Buda AJ, Pace DP, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;74:1424–1433. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- 11.Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circulation Research. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 12.Herzog V, Fahimi HD. Microbodies (peroxisomes) containing catalase in the myocardium: morphological and biochemical evidence. Science. 1974;185:271–273. doi: 10.1126/science.185.4147.271. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Akerboom TPM, Sies H. Role of key defense systems in target organ toxicity. In: Cohen GM, editor. Target Organ Toxicity. Boca Raton, FLA: CRC Press; 1986. pp. 129–143. [Google Scholar]
- 14.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 15.Koepke J, Nakrieko KA, Wood CS, Boucher KK, Terlecky LJ, Walton PA, Terlecky SR. Restoration of peroxisomal catalase import in a model of human cellular aging. Traffic. 2007;8:1590–1600. doi: 10.1111/j.1600-0854.2007.00633.x. [DOI] [PubMed] [Google Scholar]
- 16.Korge P, Weiss JN. Redox regulation of endogenous substrate oxidation by cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2006;291:H436–H445. doi: 10.1152/ajpheart.01292.2005. [DOI] [PubMed] [Google Scholar]
- 17.Legakis J, Koepke J, Jedeszco C, Barlaskar F, Terlecky LJ, Walton PA, Terlecky SR. Peroxisome senescence in human fibroblasts. Molecular Biology of the Cell. 2002;13:4243–4255. doi: 10.1091/mbc.E02-06-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Chen Y, Saari JK, Kang YJ. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 20.Naslund U, Haggmark S, Johansson G, Marklund SL, Reiz S, Oberg A. Superoxide dismutase and catalase reduce infarct size in a porcine myocardial occlusion-reperfusion model. Journal of Molecular and Cellular Cardiology. 1986;18:1077–1084. doi: 10.1016/s0022-2828(86)80294-2. [DOI] [PubMed] [Google Scholar]
- 21.Nejima J, Knight DR, Fallon JT, Uemura N, Manders WT, Canfield DR, Cohen MV, Vatner SF. Superoxide dismutase reduces reperfusion arrhythmias but fails to salvage regional function of myocardium at risk in conscious dogs. Circulation. 1989;79:143–153. doi: 10.1161/01.cir.79.1.143. [DOI] [PubMed] [Google Scholar]
- 22.Ooiwa H, Jordan MC, Bylund-Fellenius A, Downey JM. PEG SOD fails to limit infarct size in reperfused rabbit heart. Circulation. 1989;80:II–294. [Google Scholar]
- 23.Price M, Terlecky SR, Kessel D. A role for hydrogen peroxide in the proapoptotic effects of photodynamic therapy. Photochemistry and Photobiology. 2009;85:1491–1496. doi: 10.1111/j.1751-1097.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren J, Li Q, Wu S, Li S-Y, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mechanisms of Ageing and Development. 2007;128:276–285. doi: 10.1016/j.mad.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 26.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Storrie B, Madden EA. Isolation of subcellular organelles. Methods in Enzymology. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- 28.Terecky SR, Koepke J, Walton PA. Peroxisomes and aging. Biochimica et Biophysica Acta. 2006;1763:1749–1754. doi: 10.1016/j.bbamcr.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vander Heide RS, Reimer KA. Effect of adenosine therapy at reperfusion on myocardial infarct size in dogs. Cardiovascular Research. 1996;31:711–718. doi: 10.1016/0008-6363(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 30.Wood CS, Koepke J, Teng H, Boucher KK, Katz S, Chang P, Terlecky LJ, Papanayotou I, Walton JA, Terlecky SR. Hypocatalasemic fibroblasts accumulate hydrogen peroxide and display age-associated pathologies. Traffic. 2006;7:97–107. doi: 10.1111/j.1600-0854.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 31.Yokoda S, Fahimi HD. Immunohistochemical localization of catalase in rat liver. J Histochem Cytochem. 1981;29:805–812. doi: 10.1177/29.7.6790603. [DOI] [PubMed] [Google Scholar]
- 32.Young CN, Koepke J, Terlecky LJ, Borkin MS, Savoy LB, Terlecky SR. Reactive oxgyen species in TNF-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. Journal of Investigative Dermatology. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Q, Terlecky SR, Lash LH. Diabetes increases susceptibility of primary cultures of rat proximal tubular cells to chemically induced injury. Toxicology and Appied Pharmacology. 2009;241:1–13. doi: 10.1016/j.taap.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. Journal of Histochemistry and Cytochemistry. 2000;48:585–594. doi: 10.1177/002215540004800502. [DOI] [PubMed] [Google Scholar]
- 35.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. ProcNatlAcadSciUSA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


