Abstract
Study Objective
To describe changes in vaginal microbiota and pH over time among never sexually active adolescents at different menarcheal stages.
Design
A cohort of 49 sexually inexperienced Ugandan adolescents provided weekly self-collected vaginal swabs and behavioral/health information for up to two years. Menarcheal stage was classified as: not experiencing menarche during follow-up (premenarcheal, n=9), achieving menarche during follow-up (perimenarcheal, n=20), and being postmenarcheal (n=20) at enrollment. Vaginal microbiota were characterized as morphotypes of large Gram-positive rods, small Gram-negative or variable rods, and curved Gram-negative rods based on Nugent Gram-stain criteria. Baseline measures were compared using nonparametric tests. Mean changes (β) in morphotypes and pH over time were estimated using longitudinal mixed-effects models.
Results
The baseline median (IQR: interquartile range) Nugent score was 8 (7-8) in premenarcheal, 4.5 (1-8) in perimenarcheal, and 1 (0-3) in postmenarcheal girls (p=0.001). For each respective menarcheal stage, the median counts of (IQR) Gram-positive rods were 0 (0-0), 10 (0-30), and 30 (18-30) (p=0.002) and Gram-negative or variable rods were 30 (30-30), 16 (0.5-30), and 0.5 (0-2.5) (p=0.002) at enrollment. Counts of Gram-positive rods increased (β = 0.259, 95% CI: 0.156, 0.362) and Gram-negative or variable rods decreased (β = -0.201, 95% CI:-0.298,-0.103) significantly over time in premenarcheal girls, but not in other groups. Vaginal pH declined significantly in peri- and postmenarcheal girls only.
Conclusion
Vaginal microbiota composition varied by menarcheal stage at enrollment. Over time, significant changes in vaginal morphotypes occurred in premenarcheal girls, suggesting this may be an important period of transition.
Keywords: vaginal microbiota, pH, menarche, lactobacilli, bacterial vaginosis, Gram-stain, Nugent score
Introduction
The vaginal microbial environment is a complex and dynamic system and varies with different stages of biologic maturation. In postmenarcheal women, bacteria from the genus Lactobacillus are usually the predominant vaginal micro-organisms and have been associated with a healthy vaginal ecosystem (1). Lactobacilli are large Gram-positive rods that metabolize glycogen from the vaginal epithelium into lactic acid under estrogenic stimulation, which is required to maintain an acidic vaginal pH (4.0-4.5) and considered to be a primary mechanism for inhibiting the growth of pathogenic organisms (2-4). Bacterial vaginosis is characterized by the replacement of lactobacilli by an overgrowth of facultative and obligate anaerobic Gram-negative bacteria and genital mycoplasmas. Moreover, epidemiologic studies have suggested an increased risk of HIV and sexually transmitted infections (STIs) among women with BV and/or those who lack lactobacilli (5-8).
In prepubescent girls, the vaginal pH is alkaline and the vaginal microbiota is comprised of both anaerobic and aerobic rods and cocci with low frequencies of lactobacilli, Gardnerella vaginalis (G. vaginalis), and Mobiluncus (9-11). Throughout puberty, estrogen levels rise to reach concentrations found in mature females (12). Given that endocrine changes begin prior to onset of first menses and estrogen stimulates glycogen deposition in the vaginal epithelium, shifts in the composition of vaginal microbiota may precede first menses. One study among sexually active, postmenarcheal adolescents found a reduction in BV prevalence with increasing time since first menses (gynecologic age) and increasing breast and pubic hair development (13). However, since all participants were sexually active, the study could not disaggregate the effects of vaginal microbiota changes due to early sexual activity from the effects of pubertal maturation. In addition, we know of no studies that have investigated changes in vaginal microbiota over time in girls at or around the time of menarche, which may be an important transitional period for determining a woman's predominant vaginal microbiota in adulthood (14). In this paper, we present data on differences in vaginal microbiota assessed by Gram-stain using Nugent morphotype scoring criteria and vaginal pH by menarcheal status among girls aged 13 to 18 who had not initiated sexual activity.
Materials and Methods
Study Design and Data Collection
Between 2001 and 2003, the Rakai Health Science Program (RHSP) conducted a two-year cohort study of 312 consenting females aged 13-39 years in rural Rakai District, Uganda to assess weekly changes in vaginal microbiota and factors associated with BV progression and resolution. Women were included regardless of HIV status, current or prior pregnancy, or history of sexual experience. Girls between 13 and 19 years were oversampled compared to women in the older age groups in order to assess vaginal microbiota at younger ages. For this paper, a secondary analysis was conducted on 49 adolescents from this cohort who reported never having had sexual intercourse at enrollment (age range: 13-18 years). In these 49 females, the period of observation was truncated at the time of sexual debut.
For all women enrolled in the cohort, data collection was conducted in participants’ homes every week for 2 years. At each weekly visit, self-collected vaginal swabs and pH samples were collected and a short questionnaire was administered on sexual activity, menstrual history, and vaginal symptoms and treatment. At baseline and every six months, detailed data were collected on demographic characteristics, household environment, menstrual history, sexual behaviors, vaginal hygiene, and health status. Data on household dwelling characteristics and assets were collected at annual census visits.
Menarcheal stage was classified as: never experiencing menarche during follow-up (premenarcheal), achieved menarche during follow-up (perimenarcheal), and being postmenarcheal at enrollment. Gynecological age was estimated by subtracting the reported age at first menstruation from the age at enrollment (in years) in postmenarcheal girls and time to menarche was calculated as weeks from enrollment to reported first menses in perimenarcheal girls. To assess potential confounding of socioeconomic status, which has been associated with vaginal flora and age at menarche in the literature (15;16), we generated a relative household wealth index based on a sum of possession of modern objects (radio, bicycle, vehicle) and dwelling characteristics (materials used for roof, walls, and floor, and availability of electricity and latrine) weighted by the inverse of the prevalence within the population. This weight has been shown to have good agreement between other weighting methods for constructing wealth indices using binary variables (17). Vaginal hygiene behaviors included genital washing frequency, insertion of substances into the vagina, materials used for bathing, and water sources for bathing classified as protected (protected well, tap, or borehole), partially protected (unprotected well), and unprotected (rainwater or pond/lake) and based on the least protected source reported by the respondent out of 3 possible responses.
Vaginal swabs were rolled onto slides and air dried, Gram stained, and assessed for vaginal microbiota under oil immersion using the Nugent quantitative morphologic classification, which characterizes vaginal microbiota by the presence of morphotypes of large Gram-positive rods, small Gram-negative to variable rods, and curved Gram-variable rods (18). In postmenarcheal women, these morphotypes most often correspond to Lactobacillus, G. vaginalis or Bacterioides, and Mobiluncus spp., respectively; however, premenarcheal girls may differ in vaginal bacterial composition as described previously (9-11). The frequency of morphotypes were quantified as none (no organisms), less than 1, 1 to 4, 5 to 30, or more than 30 organisms per high power field and a morphotype score from 0 to 4 was assigned to large Gram-positive rods (weighted such that the absence yielded the highest score) and small Gram-negative to –variable rods and a score from 0 to 2 for curved Gram-negative rods. The morpotype scores were summed to obtain an overall Nugent score ranging from 0-10. A Nugent score of 7-10, which is typically used to diagnose BV in postmenarcheal women, was not used to diagnose BV in premenarcheal girls. In addition, we assessed large Gram-positive rods and small Gram-negative to variable rod morphotype scores separately. For each respective group, we assigned a count value of 0, 0.5, 2.5, 17.5, and 30 using the midpoints of the intervals corresponding to the original counts of the average number of organisms per field. Slides were read in batches within 2 weeks of collection by 2 independent laboratory technicians and discrepancies resolved by a 3rd senior technician. A 10% random sample was sent to Dr. Sharon Hillier's lab at the Magee-Women's Research Institute, University of Pittsburgh for external quality control.Vaginal pH was determined by BAKER-pHIX pH papers (pH 4.0-9.0, Phillipsburg, NJ) affixed to a spatula. Vaginal pH samples were not assessed during menstruation in the study and vaginal microbiota readings collected during menstruation were excluded from analyses.
This study was reviewed and approved by institutional review boards (IRBs) in Uganda (the Science and Ethics Committee of the Uganda Virus Research Institute) and the United States (the Johns Hopkins Bloomberg School of Public Health IRB and the Columbia University Medical Center IRB).
Statistical Methods
Baseline measures were compared by menarcheal status using Fishers exact tests for categorical variables and Kruskal Wallis tests for continuous variables. Longitudinal linear mixed-effects models using a normally distributed random intercept (mean=0) were used to estimate mean changes (β) in vaginal microbiota and pH over weekly follow-up. Confidence intervals were based on bootstrap estimations of the standard error with 500 replications (19). The interaction between menarcheal groups and time was assessed by pairwise comparisons of the beta coefficients using t-tests. Sub-analyses assessed whether quality of Gram-stained slides or controlling for relative wealth status had an impact on the estimates. Age and student status were considered to be collinear with menarcheal status and not adjusted for in the model.
To substantiate the model fit, vaginal microbiota and pH trajectories across weekly visits were examined by menarcheal groups using nonparametric locally weighted least squares scatterplot smoothing (lowess) techniques with varying bandwidths (20). A bandwidth of 0.8 was used for figures presented in this paper. Data were analyzed using Stata/SE 10.1 for Windows (Stata Corporation, College Station, TX).
Results
Baseline Characteristics
Nine participants remained premenarcheal throughout the study, 20 achieved menarche during follow-up (perimenarcheal), and 20 were postmenarcheal at enrollment (Table 1). The median age and interquartile range (IQR) in the premenarcheal (13 years; IQR: 13-14) and perimenarcheal (13 years; IQR: 13-14) were significantly younger than the postmenarcheal girls (16 years; IQR: 15-17, p<0.001). Postmenarcheal girls were also more likely to be out of school and have higher relative wealth compared to peri- and premenarcheal groups (Table 1). There were no statistically significant differences in religion or vaginal hygiene practices. The median age of menarche in the non-sexually active, postmenarcheal girls was 14 years (IQR: 13-15) (Table 1), which was equivalent to the median in sexually active women from the full cohort (14 years, IQR: 13-15). For all 49 girls, only one premenarcheal girl reported inserting substances into her vagina and one perimenarcheal girl reported symptoms of vaginal discharge (but no prior treatment) at enrollment.
Table 1.
Characteristics of never sexually active female adolescents aged 13-18 years at enrollment by menarcheal stage
| Characteristic | Premenarcheal | Perimenarcheal | Postmenarcheal | Total | p-valuea |
|---|---|---|---|---|---|
| Total | 9 | 20 | 20 | 49 | - |
| Sociodemographics | |||||
| Age, median (IQR)b | 13 (13-14) | 13 (13-14) | 16 (15-17) | 14 (13-15) | <0.001 |
| Student Status | |||||
| Current student | 9 (100) | 17 (85.0) | 9 (45.0) | 35 (71.4) | 0.002 |
| Out of school | 0 (0.0) | 3 (15.0) | 11 (55.0) | 14 (28.6) | |
| Religion | |||||
| Muslim | 1 (11.1) | 6 (30.0) | 5 (25.0) | 12 (24.5) | 0.62 |
| Christian | 8 (88.9) | 14 (70.0) | 15 (75.0) | 37 (75.5) | |
| Relative Wealth Tertiles | |||||
| Low | 6 (66.7) | 12 (60.0) | 3 (15.0) | 21 (42.8) | 0.01 |
| Medium | 0 (0.0) | 2 (10.0) | 7 (35.0) | 9 (18.4) | |
| High | 3 (33.3) | 6 (30.0) | 10 (50.0) | 19 (38.8) | |
| Vaginal Hygiene | |||||
| Sources of bathing water | |||||
| Protected | 2 (22.3) | 5 (25.0) | 4 (20.0) | 11 (22.5) | 0.98 |
| Partially protected | 3 (33.3) | 7 (35.0) | 6 (30.0) | 16 (32.7) | |
| Unprotected | 4 (44.4) | 8 (40.0) | 10 (50.0) | 22 (44.8) | |
| Genital washing materials | |||||
| Soap and water | 9 (100) | 14 (70.0) | 15 (75.0) | 38 (77.6) | 0.20 |
| Water only | 0 (0) | 6 (30.0) | 5 (25.0) | 11 (22.4) | |
| Daily genital washing frequency, median (IQR) | 2 (2-2) | 2 (2-3.5) | 3 (2-4) | 2 (2-3) | 0.18 |
| Menstrual Cycle (postmenarcheal only) | |||||
| Currently menstruating | - | - | 2 (10.0) | - | - |
| Age, at first menses, median (IQR)b | - | - | 14 (13-15) | - | - |
| Gynecologic age, median (IQR)b | - | - | 2 (1-2.5) | - | - |
NOTE. Data are presented as n (%) unless stated otherwise.
Fisher's exact test for categorical and Kruskal Wallis one-way analysis of variance with correction for ties for continuous variables
Chronologic age (Range=13-18); Genital washing frequency (Range=1-10); Menarche age (Range= 13-16 yrs.); Gynecologic Age (Range= 0-4)
The prevalence of a Nugent score of 7-10 was 78%, 35%, and 11% (p=0.003) and median (IQR) baseline Nugent score was 8 (7-18), 4.5 (1-8), and 1 (0-3) (p=0.001) in premenarcheal, perimenarcheal, and postmenarcheal girls, respectively (Table 2). Partitioning the Nugent score into morphotypes, the median (IQR) counts per field of large Gram-positive rods were 0 (0-0) in premenarcheal, 10 (0-30) in perimenarcheal, and 30 (17.5-30) (p=0.002) in postmenarcheal, and of small Gram-negative or variable rods were 30 (30-30), 16 (0.5-30), and 0.5 (0-2.5), respectively (p=0.002). There were no statistically significant differences in vaginal pH at enrollment (p = 0.12). The presence of curved Gram-variable morphotypes was rare with a morphotype score of 1 assigned to 2 (22.2%) premenarcheal, 1 (5.0%) peri-menarcheal, and 0 postmenarcheal girls.
Table 2.
Differences in vaginal microbiota and pH at enrollment and over time by menarcheal status
| Premenarcheal | Perimenarcheal | Postmenarcheal | Total | p-value | |
|---|---|---|---|---|---|
| Total | 9 | 20 | 20 | 49 | |
| Total # of weekly visits | 674 | 1,231 | 845 | 2,750 | |
| Median duration of visits a | 82 (78-86) | 71 (52-77) | 42 (17-70) | 66 (34-78) | 0.001 |
| Baseline Measures a | |||||
| Nugent score 7-10, n (%) | 7 (77.8) | 7 (35.0) | 2 (11.1) | 16 (34.0) | 0.003 |
| Nugent score | 8 (7-8) | 4.5 (1-8) | 1 (0-3) | 3 (1-8) | 0.001 |
| No. of Gram-positive rods | 0 (0-0) | 10 (0-30) | 30 (17.5-30) | 17.5 (0-30) | 0.002 |
| No. of Gram-negative or variable rods | 30 (30-30) | 16.3 (0.5-30) | 0.5 (0-2.5) | 2.5 (0.5-30) | 0.002 |
| Vaginal pH | 4.5 (4.5-5.0) | 4.5 (4.5-4.8) | 5 (4.5-5.0) | 4.5 (4.5-5.0) | 0.12 |
| Longitudinal changes b | β (95% CI) | β (95% CI) | β (95% CI) | ||
| Nugent score | -0.062 (-0.086,- 0.038)* | -0.014 (-0.039, 0.011) | 0.001 (-0.030,0.029) | - | - |
| No. of Gram-positive rods | 0.259 (0.156, 0.362)* | 0.061 (-0.032,0.155) | 0.012 (-0.110,0.135) | - | - |
| No. of Gram-negative or variable rods | -0.201 (-0.298,-0.103)* | -0.040 (-0.138, 0.059) | 0.025 (-0.099,0.148) | - | - |
| Vaginal pH | -0.006 (-0.015, 0.003) | -0.003 (-0.006,-0.001) | -0.003 (-0.005,-0.001) | - | - |
NOTE: Vaginal microbiota and pH samples collected during menstruation were excluded from baseline and longitudinal analyses
p<0.05 for the pairwise comparison of β coefficients in premenarcheal compared to peri- and postmenarcheal groups obtained using t-tests
Presented as median (IQR) unless stated otherwise; p-values based on Fisher's exact test for categorical and Kruskal Wallis rank test for continuous variables
Data are presented as the average change (β, 95% CI) in vaginal microbiota or pH per weekly visit
Longitudinal Changes in Vaginal Microbiota
The median (IQR) number of sexually inactive, non-menstruating visits was 82 (78-86), 71 (52-77), and 42 (17-70) in 9 pre-, 20 peri-, and 20 postmenarcheal girls (p=0.001), respectively (Table 2). The median time from enrollment to menarche was 52 weeks (IQR: 17.5-69) in perimenarcheal girls. One perimenarcheal girl and 7 postmenarcheal girls became sexually active over follow-up and their observations after sexual initiation were excluded. Only one postmenarcheal girl, who did not have BV by Nugent criteria, was treated for symptoms of discharge during follow-up.
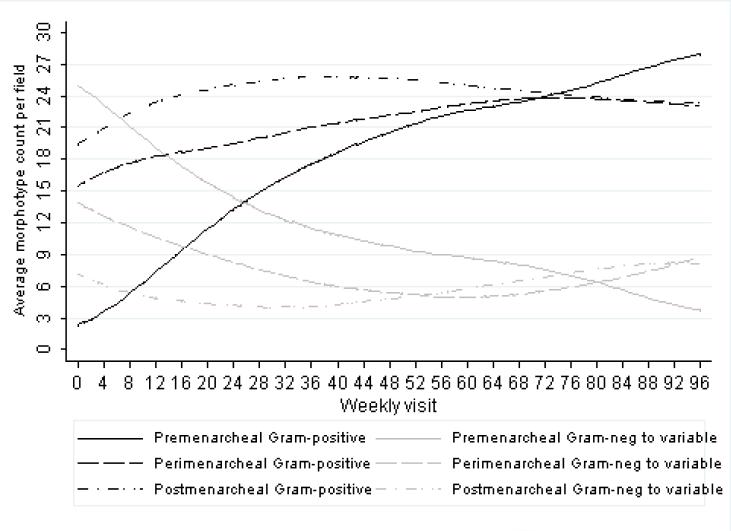
Figure 1 shows the nonparametric lowess-smoothed vaginal microbiota trajectories by menarcheal group for the average number of large Gram-positive organisms and small Gram-negative to variable rods per field across weekly visits. Consistent with Figure 1, regression analyses estimated a significant increase in counts of large Gram-positive rods per field (β = 0.259 counts/week, 95% CI: 0.156, 0.362) and a significant decline in small Gram-negative or variable rods per field (β = -0.201 counts/week, 95% CI:-0.298,-0.103) in premenarcheal girls over time (Table 2). However, there was no significant change in large Gram-positive rods per field (β=0.061 counts/week, 95% CI:-0.032, 0.155) or in small Gram-negative or variable rods per field (β= -0.040 counts/week, 95% CI:-0.138, 0.059) among the perimenarcheal girls or postmenarcheal girls (β=0.012 counts/week, 95% CI:-0.110, 0.135; β= 0.025 counts/week, 95% CI:-0.099, 0.148, respectively) over time. Consistent with morphotype trends, a statistically significant decline in overall Nugent score was seen in premenarcheal girls, but not in peri- and postmenarcheal girls (Table 2). Pairwise comparisons between menarcheal groups showed significant differences in ()in morphotype and Nugent score changes (β ) between the premenarcheal compared to perimenarcheal and postmenarcheal groups (p-value < 0.05 for all comparisons); however, there were no significant differences between the peri- and postmenarcheal groups. Less than 3% (77/2,750) of all weekly observations were assigned a morphotype score greater than 0 for curved Gram-variable rods.
Figure 1.
Lowess-smoothed average morphotype counts per field over weekly visits by menarcheal stage (bandwidth = 0.80). In the graph, large Gram-positive rod morphotype counts (black lines) and small Gram-negative or variable morphotype counts (grey lines) are stratified by premenarcheal (solid lines), perimenarcheal (dashed lines), and postmenarcheal (dashed-dotted lines) status.
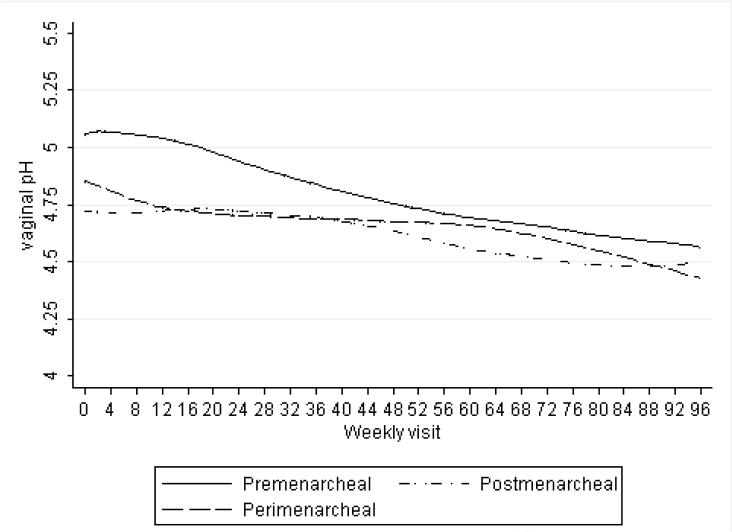
Vaginal pH declined in the premenarcheal girls (β = -0.006 units/week, 95% CI: -0.015, 0.003), but this was not statistically significant. There was a smaller, statistically significant decline in pH over time among those achieving menarche (β = -0.003 units/week, 95% CI: -0.006, -0.001) and postmenarcheal girls (β = -0.003 units/week, 95% CI: -0.005, -0.001). These results are consistent with the lowess-smoothed plots of vaginal pH decline shown in Figure 2. Pairwise comparisons of β coefficients showed no statistically significant differences between menarcheal groups. No substantial differences were observed when assessing the impact of slide quality and relative wealth status on estimates.
Figure 2.
Lowess-smoothed average vaginal pH over weekly visits by menarcheal stage (bandwidth = 0.80). In the graph, pre-, peri-, and postmenarcheal status is represented by solid, dashed, and dashed-dotted lines, respectively.
Discussion
In this study of never sexually active Ugandan adolescents, premenarcheal girls had low counts of large Gram-positive rods at enrollment that increased as they matured over time. A corresponding decrease was found in counts of Gram-negative or variable rods in this group. In contrast, changes in perimenarcheal and postmenarcheal girls were attenuated or relatively stable over time . All menarcheal groups experienced a decline in pH over-time; however, the decline was only significant in the perimenarcheal and postmenarcheal groups. Our findings are consistent with the hypothesis that rising estrogen levels preceding menarche may facilitate Lactobacillus growth, as evidenced by an increase in large Gram-positive rods, and the decline of other vaginal microbiota present in early puberty
This study investigated changes in vaginal microbiota and pH in never sexually active Ugandan adolescents accounting for different stages of menarche, whereas other studies were limited to sexually active postmenarcheal girls or prepubescent girls (11;13;21;22). Two cross-sectional studies by Brabin et al. among postmenarcheal, sexually active adolescents found that younger gynecologic age was associated with increased bacterial vaginosis (BV) but not with higher vaginal pH among sexually active adolescents attending a genitourinary clinic in Manchester, UK (13;21). In contrast, Stevens-Simon et al. found an inverse association between gynecologic age and vaginal pH among sexually active black women in the United States (22). However, it was unclear from these studies whether BV or vaginal alkalinity was exacerbated by sexual activity at a young age or whether the vaginal microbiota and pH had not been fully established among less mature adolescents.
Our findings suggest that vaginal microbiota transitioning appears to occur prior to menarche, which is a late event in puberty. Baseline results showed no difference in vaginal pH between groups, which was unexpected, and may be attributed to measurement variability in pH assessments. Smoothed averages of vaginal pH presented in Figure 2 may account for some of this variability and provide a better estimate for comparison between groups. While we did not find statistically significant differences between groups, we did observe higher smoothed averages and a greater magnitude of decline in vaginal pH in pre- compared to peri- and postmenarcheal groups. Other studies on the relationship between vaginal pH and gynecologic age are conflicting, as discussed above, and our findings may be attributed to other factors not measured in this study, such a glycogen deposition on vaginal epithelial cells, which may produce lactic acid even in the absence of lactobacilli (23). Finally, our results showed little change in vaginal microbiota assessed by Gram-stain over time among postmenarcheal adolescents, which is consistent with microbiologic evidence from a recent study that found similar composition of vaginal microbiota comparing postmenarcheal adolescents to adults using molecular methods (14). We are not aware of other studies that have evaluated changes in vaginal microbiota in pre- and postmenarcheal girls around the time of puberty. The longitudinal design and intensive weekly data collection on individuals provided a number of strengths. We were able to prospectively define menarcheal achievement, which is a more readily identifiable maturational event and is less variable to individual asynchronies in the sequence of pubertal changes. In addition, the high frequency of vaginal microbiota assessments across individuals has elements of both time-series and longitudinal data, which allowed for examination of time trends and achievement of statistical accuracy through model-based approaches despite fewer individuals per group. Finally, lowess-smoothed curves may better account for variability in vaginal microbiota and pH measurements compared to a single point in time (baseline) measure.
However, this was a secondary analysis of a larger observational cohort study and, we lacked direct measures of hormonal indicators. The possibility of misreporting sexual activity cannot be excluded; however, monthly testing of pregnancy was consistent with self-reported behaviors. In addition, we were limited to describing changes in morphotypes assessed by Nugent Gram-stain criteria rather than on specific species that may predominate in early vaginal colonization. The paucity of data on bacterial species among premenarcheal girls around the time of puberty, as compared to reproductive-aged women, did not allow us to infer the types of organisms present. Nevertheless, we documented changes in morphotypes of vaginal microbiota among adolescents at different menarcheal stages, suggesting this time period may be an important time for vaginal microbiota transitioning and potential colonization of the vagina with lactobacilli. Finally, estimation of means at later visits may be affected by fewer observations due to losses to follow-up or censoring due to sexual initiation in lowess-smoothed curves. However, the figures substantiate time trends assessed using model-based approaches for obtaining slopes and confidence intervals and observations from girls who remained sexually inactive over follow-up would likely be representative of those who initiated sex had they remained sexually inactive.
This study was conducted among a population of never sexually active rural Ugandan adolescents. The median age at menarche in this cohort was 14 years, which is approximately 1.5 years later than the median age in developed countries (15). Thus, our findings may only be generalizable to populations with later age at menarche.
Endocrine maturation and the establishment of a protective vaginal environment early in puberty may have implications for biological factors affecting adolescent susceptibility to vaginal infections. These trends should be confirmed in larger samples of pre, peri, and postmenarcheal girls and in different populations. Future studies should characterize the vaginal microbiota using 16S rRNA gene-based methods during pubertal maturation and by hormonal levels in order to elucidate changes in bacterial communities that may predispose girls to developing BV in later life.
Acknowledgements
We are grateful to the Rakai Health Sciences Program (RHSP) directors (David Serwadda, Fred Nalugoda, and Godfrey Kigozi) and RHSP data manager (Anthony Ndyanabo) for their work on this study.
Funding Sources
This work was supported in part by the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH grant R01AI47608 (to M.J.W); the NIH, National Institute for Allergy and Infectious Diseases (NIAID), NIH grant T32AI050056 (to J.M.Z), the Intramural Program of the Eunice Kennedy Shriver NICHD (to M.E.T), and the Cooperative Agreement number R36PS001104 (to M.E.T) from the Center for Disease Control and Prevention (CDC) [Its contents are solely the responsibility of the authors and do not represent the official views of the CDC].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marie E. Thoma, Eunice Kennedy Shriver National Institute of Child Health and Human Development (Rockville, MD, USA)
Ronald H. Gray, Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA)
Noah Kiwanuka, Makerere University School of Public Health and Rakai Health Sciences Program (Kampala, Uganda).
Simon Aluma, Rakai Health Sciences Program (Kalisizo, Uganda).
Mei-Cheng Wang, Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA).
Nelson Sewankambo, Makerere University College of Health Sciences (Kampala, Uganda).
Maria J. Wawer, Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA)
References
- 1.Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses. 1998 April;14(Suppl 1):S17–S21. [PubMed] [Google Scholar]
- 2.Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990 September;12(5):856–72. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 3.Hay P. Life in the littoral zone: lactobacilli losing the plot. Sex Transm Infect. 2005 April;81(2):100–2. doi: 10.1136/sti.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001 September;16(9):1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 5.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999 December;180(6):1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 6.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998 September 10;12(13):1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003 August 1;37(3):319–25. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 8.Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, Levine AM, Burk R, Palefsky JM, Moxley M, Ahdieh-Grant L, Strickler HD. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005 April 1;191(7):1129–39. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 9.Myhre AK, Bevanger LS, Berntzen K, Bratlid D. Anogenital bacteriology in non-abused preschool children: a descriptive study of the aerobic genital flora and the isolation of anogenital Gardnerella vaginalis. Acta Paediatr. 2002;91(8):885–91. doi: 10.1080/080352502760148586. [DOI] [PubMed] [Google Scholar]
- 10.Hammerschlag MR, Alpert S, Rosner I, Thurston P, Semine D, Mccomb D, Mccormack WM. Microbiology of Vagina in Children - Normal and Potentially Pathogenic Organisms. Pediatrics. 1978;62(1):56–62. [PubMed] [Google Scholar]
- 11.Hill GB, Claire KK, Gutman LT. Anaerobes predominate among the vaginal microflora of prepubertal girls. Clin Infect Dis. 1995;20(SUPPL. 2) doi: 10.1093/clinids/20.supplement_2.s269. [DOI] [PubMed] [Google Scholar]
- 12.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006 January;273(4):195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 13.Brabin L, Fairbrother E, Mandal D, Roberts SA, Higgins SP, Chandiok S, Wood P, Barnard G, Kitchener HC. Biological and hormonal markers of chlamydia, human papillomavirus, and bacterial vaginosis among adolescents attending genitourinary medicine clinics. Sex Transm Infect. 2005 April;81(2):128–32. doi: 10.1136/sti.2004.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Zhou X, Williams CJ, Hochwalt A, Forney LJ. Bacterial populations in the vaginas of healthy adolescent women. J Pediatr Adolesc Gynecol. 2009 February;22(1):11–8. doi: 10.1016/j.jpag.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 15.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003 October;24(5):668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 16.Bukusi EA, Cohen CR, Meier AS, Waiyaki PG, Nguti R, Njeri JN, Holmes KK. Bacterial vaginosis: risk factors among Kenyan women and their male partners. Sex Transm Dis. 2006 June;33(6):361–7. doi: 10.1097/01.olq.0000200551.07573.df. [DOI] [PubMed] [Google Scholar]
- 17.Howe LD, Hargreaves JR, Huttly SR. Issues in the construction of wealth indices for the measurement of socio-economic position in low-income countries. Emerg Themes Epidemiol. 2008;5:3. doi: 10.1186/1742-7622-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991 February;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diggle P, Heagarty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2 ed. Oxford University Press; 2002. [Google Scholar]
- 20.Cleveland WS, Devlin SJ. Locally Weighted Regression - An Approach to Regression-Analysis by Local Fitting. Journal of the American Statistical Association. 1988 September;83(403):596–610. [Google Scholar]
- 21.Brabin L, Roberts SA, Fairbrother E, Mandal D, Higgins SP, Chandiok S, Wood P, Barnard G, Kitchener HC. Factors affecting vaginal pH levels among female adolescents attending genitourinary medicine clinics. Sex Transm Infect. 2005 December;81(6):483–7. doi: 10.1136/sti.2005.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens-Simon C, Jamison J, McGregor JA, Douglas JM. Racial variation in vaginal pH among healthy sexually active adolescents. Sex Transm Dis. 1994 May;21(3):168–72. doi: 10.1097/00007435-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Stamey TA, Timothy MM. Studies of introital colonization in women with recurrent urinary infections. III. Vaginal glycogen concentrations. J UROL. 1975 August;114(2):268–70. doi: 10.1016/s0022-5347(17)67005-8. [DOI] [PubMed] [Google Scholar]