Abstract
Background
Reflectance confocal microscopy (RCM) images skin at cellular resolution and has shown utility for the diagnosis of nonmelanoma skin cancer in-vivo. Topical application of Aluminum Chloride (AlCl3) enhances contrast in RCM images by brightening nuclei.
Objective
To investigate feasibility of RCM imaging of shave biopsy wounds using AlCl3 as a contrast agent.
Methods
AlCl3 staining was optimized, in terms of concentration versus immersion time, on excised tissue ex-vivo. RCM imaging protocol was tested in patients undergoing shave biopsies. The RCM images were retrospectively analyzed and compared to the corresponding histopathology.
Results
For 35% AlCl3, routinely used for hemostasis in clinic, minimum immersion time was determined to be 1 minute. We identified 3 consistent patterns of margins on RCM mosaic images by varying depths: epidermal margins, peripheral dermal margins, and deep dermal margins. Tumour islands of basal cell carcinoma were identified at peripheral or deep dermal margins, correlating on histopathology with aggregates of neoplastic basaloid cells. Atypical cobblestone or honeycomb pattern were identified at the epidermal margins, correlating with a proliferation of atypical keratinocytes extending to biopsy margins.
Conclusions
RCM imaging of shave biopsy wounds is feasible and demonstrates the future possibility of intra-operative mapping in surgical wounds.
Keywords: Confocal Microscopy, Surgical wound, Basal cell carcinoma, Squamous cell carcinoma, Aluminum Chloride
Introduction
Over two million individuals are treated annually for non-melanoma skin cancers (NMSCs) in the US with costs exceeding 1 billion dollars.1,2 Since NMSCs often develop on the face, Mohs micrographic surgery (MMS) is used as tissue-preserving technique. During MMS, tumour is excised with narrow margins and the surgeon assesses margins on frozen histopathological sections; any residual tumour is mapped and corresponding tissue is further excised. Processing frozen sections takes 20–45 minutes per stage of MMS. An intra-operative imaging modality which permits direct detection of residual tumours in surgical wounds may potentially expedite MMS.
To this end, reflectance confocal microscopy (RCM), a non-invasive imaging technique with cellular-level resolution, 3,4 has shown promise for diagnosis of NMSC. RCM was found to have sensitivity of 92% and specificity of 97% for pre-surgical in-vivo diagnosis of basal cell carcinoma (BCC). 5 Tannous et al demonstrated in a small case series the potential of intra-operative RCM to guide MMS; 6 the investigators also found that Aluminum Chloride (AlCl3), routinely used for hemostasis during skin surgery, enhances contrast between aggregates of BCC and surrounding dermis in RCM images. Recent work also demonstrated the feasibility of ex-vivo mosaicing confocal microscopy to rapidly detect BCCs in skin excision specimens from MMS, using exogenous fluorescent contrast agents to stain nuclear morphology. 7–9 However, use of fluorescent contrast agents for in-vivo imaging is still being tested for efficacy and toxicity10,11 and is not yet ready for clinical use. Thus, reflectance-based RCM imaging continues to advance more rapidly toward clinical applications. 12–16
Building upon Tannous’ initial observations,6 we performed a prospective study to more rigorously investigate the feasibility of RCM for intra-operative assessment of tumour margins in a larger series of surgical wounds. First, the utility of AlCl3 was further investigated on excised tissue. Next, we imaged shave biopsy wounds as a model for initial stages of MMS. Mosaicing, a recent advance in RCM imaging, 17–19 was used; mosaicing allows observation of larger fields-of-view of surgical wound margins, akin to low magnification histopathology. Establishing imaging protocols and identifying current imaging performance and challenges are necessary translational steps, toward the long-term goal of developing RCM as a guide to surgery.
Materials and methods
Pre-clinical study of AlCl3 as contrast stain
Freshly excised specimens were obtained from MMS performed at Memorial Sloan-Kettering Cancer Center (MSKCC). During MMS, excised specimens are frozen and Haematoxylin-and-Eosin (H&E)–stained sections are prepared and examined. Remaining tissue, which is routinely discarded, was collected for this study, under Institution Review Board approval.
Each specimen was thawed, rinsed in normal saline and imaged ex-vivo using a previously described bench-top RCM (VivaScope 2000, Lucid Inc., Rochester, NY). 17, 19 Subsequently, specimens were rinsed with saline, immersed in AlCl3 and re-imaged. Concentrations of AlCl3 and immersion times were varied to determine optimal imaging conditions. Concentrations tested were 20% in anhydrous ethyl alcohol, 35% in purified water, 50% in purified water and 50% in equal volumes of isopropanol and purified water. Immersion times were 5, 10, 30 and 45 seconds and 1, 2, 3 and 5 minutes. Minimum immersion time was defined as time required for all nuclei in RCM mosaic to appear consistently brightened. Each condition was retested on minimum of 5 specimens.
RCM imaging study on shave biopsy wounds
Patients
Participants were recruited from patients undergoing shave biopsy for diagnosis of suspicious skin lesions at MSKCC. All patients were 18 years or older. Written consent was obtained prior to enrolment. The research protocol was approved by MSKCC Institutional Review Board.
Instrumentation
For imaging patients, a commercially-available, previously described RCM (Vivascope 1500, Lucid Inc., Rochester, NY) was used. 4, 20 Briefly, RCM uses near-infrared laser at 830nm. A 30X objective lens allows imaging with optical sectioning of 3μm and lateral resolution of 1μm. Contact between the objective lens and skin is achieved with a tissue ring. The RCM acquires images of en face optical sections with 500 ×500μm2 field-of-view (equivalent to 30× magnification). An automated stepper was used to acquire up to 12×12 contiguous images into a “mosaic” which displays a 6×6mm2 field-of-view (equivalent to 3× magnification). RCM images can be acquired to depth of approximately 300μm.
RCM imaging protocol
A pilot was conducted during the first 8 cases to qualitatively assess the best imaging conditions and construct a protocol. For immersion medium in the wound cavity, sterile Surgilube gel (Fougera, Melville, NY) was used. The gel’s viscosity was found to be advantageous compared to sterile saline; the gel was better retained in the wound when imaging patients in recumbent position. Sterile conditions were ensured by draping the wound with transparent dressing (Tegaderm, 3M, St. Paul, Minnesota, USA). Imaging was tested through a 1 mm-thick disposable optical window made of polycarbonate disk (General Electric Company, Fairfield, CT); image quality with the polycarbonate disk was qualitatively better than that obtained with a glass window. Following the results of our pre-clinical study, we used AlCl3 solution as contrast agent in RCM images.
The final protocol was as follows:
The wound was swabbed with AlCl3 using sterile applicators.
The cavity was filled with sterile gel (Fig. 1).
The wound was sealed with sterile transparent adhesive dressing.
A drop of Crodamol STS oil (Croda Inc., Edison, NJ) was applied over the dressing.
The tissue ring with polycarbonate window was attached to the surface of the dressing. The ring covered part of the wound cavity and part of the wound edge.
Ultrasound gel was used as immersion medium between the tissue ring and the objective lens.
RCM images and mosaics were acquired at a minimum of three levels (Fig. 1): level of intact epidermis surrounding the wound (“epidermal margin”), level of superficial dermis in the wound (“peripheral dermal margin”) and base of the wound (“deep dermal margin”).
Figure 1.

Setup for attachment of reflectance confocal microscope (RCM) objective lens to shave biopsy wound. The skin at the site of imaging includes the surgical wound cavity with adjacent intact surrounding epidermis (“se”) and dermis (“sd”). The surgical wound cavity is filled with sterile gel and covered by transparent sterile dressing. Following the application of an oil drop onto the dressing, the tissue ring is attached to the dressing, to cover both a portion of the wound cavity and its margins. The top, concave side of the tissue ring is filled with ultrasound (“US”) gel as the immersion medium. The tissue ring serves as a docking template for the RCM objective lens for locating and stabilizing the site to be imaged. The three depth levels of en face RCM imaging are shown, including the “epidermal margin” at the level of the surrounding epidermis, “peripheral dermal margin” at the level of the surrounding superficial dermis, and “deep dermal margin” at the level of the base of the wound.
Using the final protocol, 39 additional lesions undergoing shave biopsy were included. Histopathological diagnoses for these lesions were BCC (n=10), squamous cell carcinoma (SCC, n=10), actinic keratosis (n=1), irritated seborrheic keratosis/lichen planus-like keratosis (n=11), melanoma (n=3), naevus (n=1) irritated verrucae (n=2) and neurofibroma (n=1).
Assessment of RCM images and histopathologic correlation
Images were jointly assessed by 2 dermatologists (AS and KN), one of whom (KN) is a MMS surgeon. Image quality was assessed as acceptable or poor. A mosaic image was considered acceptable if at least 75% of images that show wound margins displayed adequate resolution and contrast as previously defined. 21 For every RCM mosaic, the observers assessed the presence of the following structures – surrounding epidermis; surrounding dermis; bright keratinocytes, bright adnexal epithelium, collagen bundles and inflammatory cells within the wound; and tumour aggregates.
All biopsy specimens were routinely processed with formalin fixation and paraffin embedding, followed by vertical sectioning and H&E staining. Diagnoses were retrieved from the hospital information system. Slides were also examined (by A.S.) for findings which appeared to best correlate with RCM structures under analysis.
Results
Pre-clinical study of AlCl3 as contrast stain
RCM imaging of normal epidermis from excised tissue is shown (Fig. 2). Under unstained conditions, nuclei of keratinocytes appear dark and cytoplasm and intercellular borders between keratinocytes appear as bright polygonal outlines resulting in a honeycomb pattern at spinous and granular layers of the epidermis (Fig. 2A and Table 1). After immersion in AlCl3, nuclei of keratinocytes appear bright (Figs 2B-D) with enhanced nuclear-to-cytoplasm contrast, resulting in a cobblestone pattern (Table 1). The minimum immersion time for various AlCl3 concentrations is also shown (Table 2).
Figure 2.

Aluminum chloride (AlCl3)-stained epidermis showing nuclear brightening and enhanced nuclei-to-dermis contrast for different immersion times and AlCl3 concentrations. The epidermis is vertically-sectioned. (a) Unstained control; (b) 20% AlCl3, 3 minutes of immersion; (c) 35% AlCl3, 1 minute of immersion; and (d) 50% AlCl3 in water, 10 seconds of immersion. While the unstained image (a) shows a honeycomb pattern of dark nuclei and bright cellular outlines in the epidermis as normally seen with in vivo RCM, all AlCl3-stained tissue specimens (b–d) present a cobblestone pattern of bright nuclei in the epidermis.
Table 1.
Definitions and histopathological correlations of RCM terms
| RCM term | Morphologic description | Margin levels; wound tissue vs. surrounding skin | Histopathological correlation |
|---|---|---|---|
| Honeycomb pattern | A pattern whose lines are formed by well-demarcated, polygonal outlines of keratinocytes and whose holes are formed by the dark central nuclei of keratinocytes. | Epidermal margins; surrounding epidermis. | Normal keratinocytes of spinous and granular layers |
| Atypical honeycomb pattern | There is an irregular pattern formed by lines that vary in thickness and brightness and holes that vary in size and shape. There are cells that appear wholly bright, without central dark nucleus. | Epidermal margins; surrounding epidermis. | Atypical keratinocytes with nuclei that are crowded, pleomorphic and hyperchromatic; dyskeratotic keratinocytes; abnormal maturation of epidermis. |
| Cobblestone pattern | Small bright round to oval nuclei of keratinocytes that are uniformly sized and spaced and are separated by less refractile cytoplasm of keratinocytes. | Epidermal and peripheral dermal margins; epidermis within the wound. | Normal keratinocytes of basal, spinous and granular layers |
| Atypical cobblestone pattern | Bright nuclei of keratinocytes that are irregularly crowded and display variability in the size of nuclei and the presence of abnormally large nuclei | Can be seen at all margin levels in some cases of SCC; epidermis within wound | Atypical keratinocytes with nuclei that are crowded, pleomorphic and hyperchromatic; dyskeratotic keratinocytes; abnormal maturation of epidermis. |
| Collagen | Very bright discrete fibrillar structures (papillary dermis) or elongated bundles (reticular dermis) with no cellular component and no visible nucleus. | Peripheral and deep dermal margins; highly refractile in dermis within wound, blurred and less refractile in surrounding dermis | Collagen of the papillary or reticular dermis |
| Solar elastosis | Bright, thickened, blurred wavy fibrillar structures with less refractile amorphous background. | Peripheral and deep dermal margins; refractile in dermis within wound, blurred and less refractile in surrounding dermis | Solar elastosis in dermis |
| Bright stellate cells and small bright spots | Larger (>20μm), irregularly shaped bright cells and smaller bright round structures (<20μm). These cells are usually irregularly spaced (in contrast to cobblestone pattern). | Peripheral and deep dermal margins; refractile in dermis within wound | Lymphocytes and histiocytes |
Table 2.
The minimum immersion time versus concentration of aluminum chloride to attain consistent nuclear brightening.
| Concentration of aluminum chloride | Minimum time (sec) |
|---|---|
| 20% | 180 |
| 35% | 60 |
| 50% in isopropanol + water | 10 |
| 50% in purified water | 10 |
The results of the pre-clinical study were empirically translated to the protocol for imaging shave biopsy wounds in patients. The routinely used concentration of AlCl3 in our clinic is 35% for which the minimum immersion time was determined to be 1 minute (Fig. 2C). This was translated to swabbing the wound with sterile applicator 4 times with AlCl3 (each swab was used for about 15 seconds), resulting in immersion time of 1 minute.
RCM imaging study on shave biopsy wounds
Correlation of confocal and histopathologic features of normal skin
Using the final protocol, 39 lesions undergoing shave biopsy were imaged with RCM and analyzed. Bright nuclei of keratinocytes within the wound cavity were seen in 21 lesions (54%). These bright nuclei were uniformly spaced, forming a regular cobblestone pattern (Table 1, Fig. 3A). Since the epidermis overlying the surgical wound lacks a stratum corneum, the keratinocytes within the wound cavity were exposed to AlCl3 application. In contrast, the epidermis surrounding skin showed a honeycomb pattern (Table 1, Fig. 3B), similar to appearance of unstained epidermis in the preclinical study and similar to in-vivo imaging of epidermis of normal skin. 21 The regular cobblestone and honeycomb patterns correlate with normal pattern of keratinocytes at the spinous and granular layers (Fig. 3C). The transition between honeycomb and cobblestone patterns was often notable and demarcated the margins of the wound cavity at the level of the epidermis (Fig. 3D).
Figure 3.
Histopathological correlation of RCM features seen at shave biopsy margins. (a) Nuclei of keratinocytes that line the wound cavity appear bright, following application of aluminum chloride; this is referred to as cobblestone pattern. (b) Normal keratinocytes in the surrounding epidermis adjacent to the wound cavity display a different pattern, referred to as honeycomb pattern, whereby the nuclei are dark and the cellular outlines of keratinocytes are bright; (c) both patterns correlate with spinous and granular layers without atypia of keratinocytes. (d) Honeycomb pattern adjacent to cobblestone pattern; the dashed line of demarcation indicates the edge of the wound at the level of the epidermal margin. (e) Bright linear and curved structures in the dermis with a background of amorphous brightness seen on RCM correlated with collagen bundles in solar-altered dermis on histopathology (f). (g) Small round bright features and bright stellate cells in the dermis seen on RCM correlated with an infiltrate of lymphocytes and histiocytes on histopathology (h). Scale = 100μm on RCM images.
Bright adnexal structures were seen in only 2 lesions (5%). These were round structures in the dermis composed of uniformly spaced bright nuclei of adnexal epithelium. Bright linear or curved structures were seen in the dermis in 25 (64%) lesions. The bright structures were arranged in a retiform arrangement or in parallel (bundles). These structures correlated with dermal collagen. In some cases, these linear structures were apparent in the background of amorphous brightness (Fig. 3E), which correlated with solar elastosis (Fig. 3F). Bright stellate cells and small bright dots in the dermis were seen in 25 (64%) lesions (Fig. 3G), correlating with inflammatory cells (histiocytes and lymphocytes, Fig. 3H).
Evaluation of shave biopsy wound margins with RCM
Evaluating the RCM mosaics of the surgical wounds, we identified 3 margin levels:
“Epidermal margin” - mosaics acquired at the level of surrounding epidermis (Fig. 4A). The central portion of the wound cavity appeared dark. Peripheral to it, bright nuclei forming a cobblestone pattern were seen; these were compatible with nuclei of epidermal keratinocytes in the outer, most superficial perimeter of the wound cavity. Peripheral to wound, the epidermis in the surrounding skin showed a honeycomb pattern (Fig. 3D).
“Peripheral dermal margin” – mosaics acquired at the level of the superficial dermis (Fig. 4B). The centre of the wound still appeared dark. Around it, within the wound cavity area, a bright area of tissue was seen. Depending on the level of the mosaic, this bright tissue area displayed either a cobblestone pattern of bright nuclei of keratinocytes (in the more superficial mosaics); or refractile structures in retiform or parallel arrangement (in the deeper mosaics) compatible with dermal collagen. Peripheral to the bright area, the surrounding dermis appeared less refractile, showing either dermal papillae of the dermal-epidermal junction or dermal collagen of the papillary dermis. Collagen in the surrounding dermis appeared more blurred and less refractile than that within the exposed wound area. In addition, in some cases, with progressive imaging depth from the level of “peripheral dermal margins” to that of “deep dermal margins”, mosaics showed only a bright strip of wound margin at the focal plane of imaging. Central to this bright strip was the dark wound cavity, and peripheral to the bright strip, the surrounding skin appeared minimally to non-refractile due to the imaging depth.
“Deep dermal margins” – mosaics acquired at the level of the base of the wound (Fig. 4C). The wound cavity that appeared dark at the level of “peripheral dermal margin” now appeared bright, showing mostly highly refractile collagen bundles. The surrounding skin and superficial edges of the wound appeared dark.
Figure 4.
Wound margins at varying depths. The level of imaging is depicted by the dashed line in the drawings at the top of each column. (a–b) Epidermal margin; the RCM mosaic (a, 4×4 mm) is taken at the level of the spinous layer of the epidermis. The surrounding epidermis (“SE”) displays a honeycomb pattern. The dark area represents the gel-filled wound cavity. At higher magnification RCM (b, 0.5×0.5 mm) a regular honeycomb pattern in the surrounding epidermis (yellow arrow) can be seen, as well as few bright nuclei in cobblestone pattern within the wound cavity (white arrow). The demarcation between honeycomb and cobblestone patterns, denoting the wound edge, is shown (yellow dashed line). (c–d) Peripheral dermal margin; in this RCM mosaic (c, 4×4 mm) the surrounding skin (‘SS”) is blurred since imaging is performed through intact stratum corneum, resulting in loss of backscattered detected light with increasing depth. In contrast, the exposed dermis (“ED”) in the wound shows bright reticulated collagen of the papillary dermis. The base of the wound is still below the plane of imaging and therefore appears dark. At higher magnification RCM (d, 0.5×0.5 mm), there is demarcation (dashed yellow line) between the blurred surrounding skin (asterisk) and the exposed wound tissue showing bright dermal-epidermal junction (white dashed arrows) as well as bright, in-focus collagen in the superficial dermis (dashed yellow arrows). (e–f) Deep dermal margin; on RCM mosaic (e, 4×4 mm), the surrounding dermis appears blurred and dark because of deeper imaging level. However, the RCM focal plane reaches the exposed dermis (“ED”) at the base of the wound, which appears bright. At higher magnification RCM (f, 0.5×0.5 mm) the bright collagen bundles at the base of the wound can be seen (dashed yellow arrow).
For mosaics with acceptable imaging quality, epidermal margin was visible in 23 of 39 lesions (59%), peripheral dermal margin was visible in 23 lesions (59%), and deep dermal margin was visible in 23 lesions (59%). In 13 lesions (33%), all 3 margins were visible. Reasons for unacceptable quality of mosaic images included air bubbles obscuring images, over-saturation of image brightness compromising resolution, and inaccurate software stitching of images in the mosaic.
Correlation of confocal and histopathologic features of skin neoplasms
In 4 lesions (10%), bright tumour islands were seen at deep dermal margins (n=1) and peripheral dermal margins (n=3). When tumour islands were observed in exposed wound margins, they were composed of bright closely aggregated nuclei (Figs 5A&C, Fig. 6D). When tumour islands were located deeper in tissue, under the exposed surface or in the surrounding dermis, they appeared less refractile and individual nuclei were not discernible (Fig. 6E). Tumour islands correlated on histopathology with aggregates of basaloid cells showing peripheral palisading of nuclei (Figs 5B&D, Fig. 6F). In the surrounding dermis, a stroma composed of bright collagen bundles and bright stellate cells and small bright dots was observed (Fig. 6D); these bright cells correlated on histopathology with histiocytes and lymphocytes, respectively (Fig. 6F). In these 4 cases, histopathological diagnosis proved to be BCC, nodular in 2 lesions and superficial in 2 lesions.
Figure 5.
Basal cell carcinoma (BCC). Images a&b are from one lesion, images c&d from another lesion. (a) RCM imaging at the level of the dermal-epidermal junction (peripheral dermal margin) shows an aggregate of neoplastic cells appearing as a focus of bright nuclei (asterisk), well delineated from the adjacent epidermis (“E”) and dermis (“D”). (b) An aggregate of basaloid cells with peripheral palisading of nuclei (asterisk) is emanating from the undersurface of the epidermis (“E”) and protruding into the superficial dermis (“D”). The diagnosis is superficial BCC. (c) RCM imaging at the level of the dermis (deep dermal margin) shows a dermal aggregate of neoplastic cells (‘T”) displaying focal peripheral palisading of nuclei (solid arrow); the aggregates are well demarcated from the surrounding dermis by dark clefts (dashed arrow). (d) On histopathology, aggregate of basaloid cells (“T”) are seen in the reticular dermis with palisading of nuclei (solid arrow) and subtle clefting (dashed arrow), diagnostic of nodular BCC. Notably, the tumours were transected during biopsy and appear at the exposed wound surface. Since the neoplastic epithelial cells have been exposed to aluminum chloride application, the nuclei appear bright and help to delineate the tumor aggregates in RCM images.
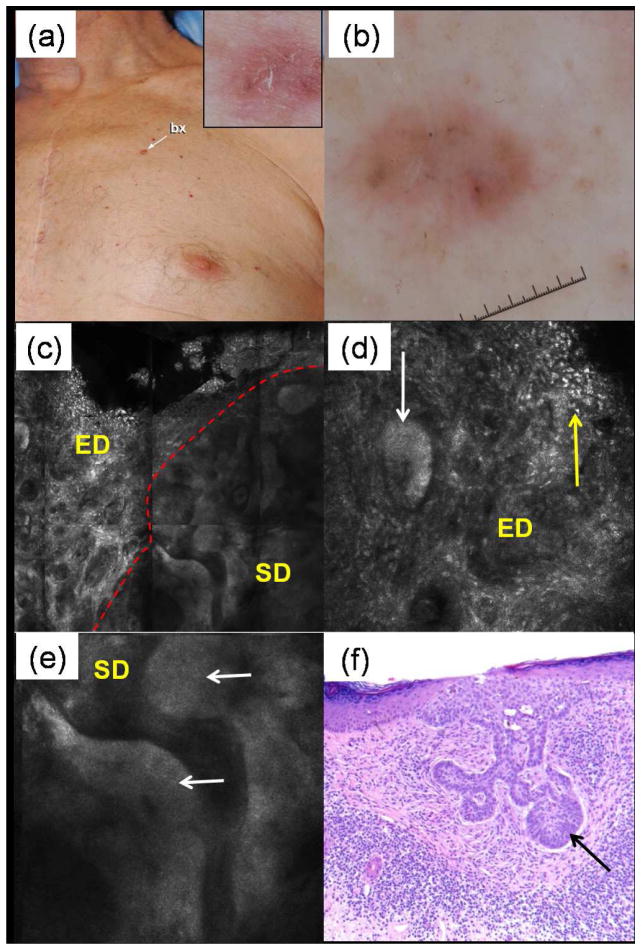
Figure 6.
Basal cell carcinoma (BCC). (a) This patient presented an 8 mm papule on the chest (inset shows close-up clinical image); (b) dermoscopy revealed gray dots and gray-brown ovoid nests, suspicious for BCC. (c) RCM mosaic (1.5×1.5 mm) of the shave biopsy wound is shown at the peripheral dermal margin level; the red dashed line demarcates the darker surrounding dermis (“SD”) from the brighter exposed dermis (“ED”). (d) Individual RCM image (500×500 μm) at the same level showing a bright tumor aggregate (white arrow) in the exposed dermis (“ED”) that also displays numerous bright spots and bright stellate cells (yellow arrow). (e) Individual RCM image (500×500 μm) in the surrounding dermis (“SD”) at the same level also showing tumor aggregates (white arrows). Note that these tumor aggregates and the surrounding dermis (e) appear less bright and with lower resolution than in the exposed dermis (d). (f) On histopathology, an aggregate of basaloid cells with peripheral palisading of nuclei (arrow) is emanating from the undersurface of the epidermis, diagnostic of superficial BCC. Note the dense inflammatory infiltrate which correlates with the numerous bright spots and bright stellate cells seen on RCM.
In 3 lesions (8%), atypical honeycomb was observed in epidermal margins (Fig. 7G); histopathological diagnosis proved to be SCC. In 1 SCC lesion, an atypical cobblestone pattern was seen in peripheral and deep dermal margins (Figs 7E&F); this atypical cobblestone pattern displayed crowding of nuclei, variability in size and brightness of nuclei and the presence of abnormally large nuclei (Fig. 7H). On histopathology, crowding and pleomorphism of nuclei, hyperchromatic nuclei and disordered maturation of the epidermis were seen; the proliferation of atypical keratinocytes extended to the base and peripheral margins of the biopsy (Fig. 7I). Of note, in another case, while an atypical honeycomb was observed in epidermal margins, the histopathological diagnosis proved to be lichen planus-like keratosis; in this case, mild atypia of keratinocytes, interpreted to be reactive to the lichenoid inflammation, was observed on histopathology.
Figure 7.
Squamous cell carcinoma (SCC). (a) This patient presented with a 1.5 cm red, keratotic plaque on the back. (b) A close-up clinical image post shave biopsy showing in the remaining scale in the surrounding skin (arrow). (c) Dermoscopy showing a pink blush and dotted vessels (arrow) in the skin surrounding the shave biopsy wound. (d) RCM mosaic (1.5×1.5 mm) at the level of epidermal margin (level indicated in the drawing above) showing a honeycomb pattern in the surrounding epidermis (“SE”), to the left of the dashed line. Note the enhanced brightness of nuclei in the exposed epidermis (“EE”, right of dashed line) producing a cobblestone pattern. (e) RCM mosaic (1.5×1.5 mm) at the level of peripheral dermal margin (level indicated in the drawing above). Both surrounding dermis (“SD”) and surrounding epidermis (“SE”) are seen to the left of the dashed line, indicating imaging is at the level of the dermal-epidermal junction. In the wound cavity, a cobblestone pattern is seen (asterisk) as well as dermal papillae (arrow). (f) RCM mosaic (1.5×1.5 mm) at the level of deep dermal margin, base of the wound (level indicated in the drawing above). The finding of cobblestone pattern (asterisk) in the dermis where blood vessels can be seen (dashed arrow), is clearly abnormal. (g) RCM individual image (500×500 μm), akin to higher magnification microscopy, of the surrounding skin at epidermal margin level shows an atypical honeycomb pattern, with outlines that vary in thickness and brightness and dark holes (dashed red arrow) that vary in size and shape. There are cells that appear wholly bright, without central dark nucleus (solid red arrow). (h) RCM individual image (500×500 μm), of the exposed epidermis at the epidermal margin level shows an atypical cobblestone pattern, with bright nuclei of keratinocytes (yellow arrows) that are irregularly crowded and display variability in the size of nuclei. (i) On histopathology, there is full thickness atypia of keratinocytes with jumbling, crowding and pleomorphism of nuclei, hyperchromatic nuclei and necrotic keratinocytes (black arrow), diagnostic of SCC. The proliferation of atypical keratinocytes extended to the peripheral margins (“PM”) and base of the biopsy (“base”).
Discussion
One potential application of RCM is intra-operative imaging to assess cancer margins. We showed recently that BCC can be detected ex-vivo in tissue excised during MMS. 8,9 Intra-operative margin mapping can further expedite MMS by obviating the need to wait for frozen pathology before proceeding with further surgery. However, RCM imaging of surgical wounds is challenging. In-vivo RCM imaging is normally performed on intact skin which presents a flat surface, while intra-operative mapping requires imaging of crater-shaped wounds. Development of an RCM protocol for imaging contoured surfaces and use of RCM mosaics to evaluate wound margins has not been previously described.
AlCl3 was used to improve visibility of tumours under RCM, as it was previously shown to enhance nuclear contrast. 6 We found that 20% AlCl3, previously used by Tannous et al, 6 requires longer immersion time of 3 minutes; in comparison, 35% AlCl3 that is routinely used in our clinic requires only 1 minute. While 50% AlCl3 achieved nuclear brightening even more rapidly, its use would require additional efficacy and safety study, as even lower concentrations of AlCl3 were shown to interfere with wound healing. 22 Previous studies showed that AlCl3 causes DNA to compact 23–25 accounting for increased light backscatter of nuclei under RCM. This mechanism is similar to that observed with acetic acid (“acetowhitening”). 26,27
Nuclear morphology of normal keratinocytes within the wound was enhanced by AlCl3, producing a cobblestone pattern. The difference in nuclear morphology, being bright (cobblestone pattern) in exposed keratinocytes within the wound, and dark (honeycomb pattern) in keratinocytes in surrounding skin, allows for identification of epidermal wound margins. In addition, collagen bundles and inflammatory cells within the wound appeared much brighter than in surrounding dermis, allowing recognition of peripheral and deep dermal margins. Taken together, reproducible patterns were created by these bright tissue structures and were used as landmarks to identify RCM wound margin levels.
We showed feasibility of identifying residual neoplastic aggregates in shave biopsy wound margins. Nuclear brightening was seen in aggregates of BCC within the wound cavity; the RCM pattern of tumour islands was closely correlated with histopathologic findings. Similarly, the nuclear enhancement of atypical keratinocytes in SCC allowed assessment of cellular criteria similar to those used in histopathology, such as nuclear crowding and pleomorphism of size. Of note, the effects of AlCl3 were limited to the wound cavity; in surrounding skin, brightening of nuclei was not seen and residual tumours were only recognized using previously described criteria for RCM diagnosis of BCC and SCC in intact skin (e.g., atypical honeycomb in SCC).
The present study also identified limitations of current RCM. The device is relatively bulky and imaging is slow. Since 6×6 mm2 mosaics were acquired in approximately 90 seconds, the minimum time to image each lesion was about 5 minutes. The use of adhesive tissue ring limits access to one edge of the wound and precludes surveying the entire perimeter. Re-attaching the ring would wrinkle the taut transparent dressing, introducing imaging artefacts. Only superficial wounds can be imaged to the base with current RCM. Wounds with steep walls yielded en face mosaics with thin ring of bright contrast and dark, blank spaces at the centre and periphery; these lesions required more mosaics to assess margins and therefore prolonged imaging time. To become practical, imaging surgical wounds requires new instrumentation in the form of flexible handheld probe and small objective lens, to enable full access and rapid imaging of the entire wound margin. Future software developments may include dynamic mosaicing algorithms that only image wound margins with bright contrast, where the mosaic provides diagnostic information, and avoids imaging blank spaces, reducing imaging time. Additionally, acceptable mosaic images in all 3 margins were obtained in only one third of cases. This reflects limitation of instrumentation, as well as the learning curve for using the wound imaging protocol. We anticipate that with improved instrumentation, the majority of lesions will be better assessed at all 3 wound margin levels.
In conclusion, RCM imaging of superficial surgical wounds is feasible. The application of AlCl3 enhances nuclear contrast of normal keratinocytes, inflammatory cells and neoplastic cells of BCC and SCC. The anatomic level of wound margins can be consistently identified in RCM mosaics. Conceivably, with advances in instrumentation and accumulation of experience among users, RCM imaging can serve as a routine tool to evaluate cancer margins during surgery in the skin and other tissues.
Bulleted Statements.
Previous research showed that skin cancer can be diagnosed pre-operatively with in vivo confocal of intact skin, and intra-operatively with ex-vivo confocal of excised tissue.
Herein, we showed feasibility of intra-operative, in vivo confocal imaging of shave biopsy wounds using aluminum chloride as contrast agent.
We developed imaging protocol for concave wounds; characterized wound margins by confocal landmarks; and detected residual skin cancer in wound margins.
Possible manuscript referees.
Dr Harold Rabinovitz, University of Miami, Florida, USA Harold@admcorp.com
Prof Scott W. Menzies, University of Sydney, Sydney, Australia, scott.menzies@sswahs.nsw.gov.au
Prof H. Peter Soyer, University of Queensland, Brisbane, Australia, p.soyer@uq.edu.au
Dr. John Strasswimmer, Dermatology Associates of the Palm Beaches, Delray Beach Florida, USA, delraymohs@me.com
Acknowledgments
Funding sources: This research was funded in parts by the National Institutes of Health (NIH) grant R44 CA093106 from the National Cancer Institute, NIH grant R01EB002715 from the Image-Guided Interventions program of the National Institute of Biomedical Imaging and Bioengineering and a grant from the Byrne Fund, Department of Medicine at Memorial Sloan-Kettering Cancer Center.
We thank Dr. Allan Halpern and Dr. Stephen Dusza for help in designing the study protocol, Dr Ashfaq Marghoob for help with accrual of patients, and the staff at Lucid Inc. (Dr. Jay Eastman, Christi Alessi-Fox, William Fox and Zachary Eastman) for technical support.
Footnotes
Conflicts of interest: Dr. Milind Rajadhyaksha is a former employee and owns equity in Lucid Inc., the company that makes and sells the VivaScope confocal microscope. The VivaScope is the commercial version of an original laboratory prototype of a confocal scanning laser microscope that was developed by Dr. Rajadhyaksha when he was in the Department of Dermatology at Massachusetts General Hospital, Harvard Medical School. All other authors have no conflicts of interest to declare.
References
- 1.Bialy TL, Whalen J, Veledar E, et al. Mohs micrographic surgery vs traditional surgical excision: a cost comparison analysis. Arch Dermatol. 2004;140:736–42. doi: 10.1001/archderm.140.6.736. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 4.Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 5.Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923–30. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Tannous Z, Torres A, González S. In vivo real-time confocal reflectance microscopy: a noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol Surg. 2003;29:839–46. doi: 10.1046/j.1524-4725.2003.29219.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Arashi MY, Salomatina E, Yaroslavsky AN. Multimodal confocal microscopy for diagnosing nonmelanoma skin cancers. Lasers Surg Med. 2007;39:696–705. doi: 10.1002/lsm.20578. [DOI] [PubMed] [Google Scholar]
- 8.Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242–50. doi: 10.1111/j.1365-2133.2009.09141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gareau DS, Karen JK, Dusza SW, et al. Sensitivity and specificity for detecting basal cell carcinomas in Mohs excisions with confocal fluorescence mosaicing microscopy. J Biomed Opt. 2009;14:034012. doi: 10.1117/1.3130331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makhlouf H, Gmitro AF, Tanbakuchi AA, et al. Multispectral confocal microendoscope for in vivo and in situ imaging. J Biomed Opt. 2008;13:044016. doi: 10.1117/1.2950313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udovich JA, Besselsen DG, Gmitro AF. Assessment of acridine orange and SYTO 16 for in vivo imaging of the peritoneal tissues in mice. J Microsc. 2009;234:124–9. doi: 10.1111/j.1365-2818.2009.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlgrimm-Siess V, Massone C, Scope A, et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br J Dermatol. 2009;161:1307–16. doi: 10.1111/j.1365-2133.2009.09289.x. [DOI] [PubMed] [Google Scholar]
- 13.Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766–72. doi: 10.1001/archdermatol.2009.134. [DOI] [PubMed] [Google Scholar]
- 14.Braga JC, Scope A, Klaz I, et al. The significance of reflectance confocal microscopy in the assessment of solitary pink skin lesions. J Am Acad Dermatol. 2009;61:230–41. doi: 10.1016/j.jaad.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann-Wellenhof R, Wurm EM, Ahlgrimm-Siess V, et al. Reflectance confocal microscopy--state-of-art and research overview. Semin Cutan Med Surg. 2009;28:172–9. doi: 10.1016/j.sder.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Rajadhyaksha M. Confocal microscopy of skin cancers: translational advances toward clinical utility. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3231–3. doi: 10.1109/IEMBS.2009.5333600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gareau DS, Patel YG, Li Y, et al. Confocal mosaicing microscopy in skin excisions: a demonstration of rapid surgical pathology. J Microsc. 2009;233:149–59. doi: 10.1111/j.1365-2818.2008.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001. doi: 10.1117/1.2981828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027. doi: 10.1117/1.2750294. [DOI] [PubMed] [Google Scholar]
- 20.Gareau DS, Patel YG, Rajadhyaksha M. Basic principles of reflectance confocal microscopy. In: Gonzalez S, Gill M, Halpern Ac, editors. Reflectance confocal microscopy of cutaneous tumours. London: Informa Healthcare; 2008. pp. 1–6. [Google Scholar]
- 21.Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644–58. doi: 10.1016/j.jaad.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Sawchuk WS, Friedman KJ, Manning T, Pinnell SR. Delayed healing in full-thickness wounds treated with aluminum chloride solution. A histologic study with evaporimetry correlation. J Am Acad Dermatol. 1986;15:982–9. doi: 10.1016/s0190-9622(86)70261-2. [DOI] [PubMed] [Google Scholar]
- 23.Karlik SJ, Eichhorn GL, Lewis PN, Crapper DR. Interaction of aluminum species with deoxyribonucleic acid. Biochemistry. 1980;19:5991–8. doi: 10.1021/bi00567a008. [DOI] [PubMed] [Google Scholar]
- 24.Karlik SJ, Eichhorn GL. Polynucleotide cross-linking by aluminum. J Inorg Biochem. 1989;37:259–69. doi: 10.1016/0162-0134(89)85001-9. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa Y, Kanbe T, Yoshikawa K. Compaction and multiple chain assembly of DNA with the cationic polymer poly(aluminum chloride) (PAC) Langmuir. 2004;20:6439–42. doi: 10.1021/la036392f. [DOI] [PubMed] [Google Scholar]
- 26.Drezek RA, Collier T, Brookner CK, et al. Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid. Am J Obstet Gynecol. 2000;182:1135–9. doi: 10.1067/mob.2000.104844. [DOI] [PubMed] [Google Scholar]
- 27.Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323–31. doi: 10.1117/1.1646175. [DOI] [PubMed] [Google Scholar]
- 28.Ren H, Waltzer WC, Bhalla R, et al. Diagnosis of bladder cancer with microelectromechanical systems-based cystoscopic optical coherence tomography. Urology. 2009;74:1351–7. doi: 10.1016/j.urology.2009.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh AC, Tresser NJ, Shen SS, Lerner SP. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology. 2008;72:133–7. doi: 10.1016/j.urology.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Shakhov AV, Terentjeva AB, Kamensky VA, et al. Optical coherence tomography monitoring for laser surgery of laryngeal carcinoma. J Surg Oncol. 2001;77:253–8. doi: 10.1002/jso.1105. [DOI] [PubMed] [Google Scholar]
- 31.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29:1457–68. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagaya N, Yamazaki R, Nakagawa A, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195:850–3. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Eljamel MS, Leese G, Moseley H. Intraoperative optical identification of pituitary adenomas. J Neurooncol. 2009;92:417–21. doi: 10.1007/s11060-009-9820-9. [DOI] [PubMed] [Google Scholar]
- 35.Lin WC, Toms SA, Johnson M, et al. In vivo brain tumour demarcation using optical spectroscopy. Photochem Photobiol. 2001;73:396–402. doi: 10.1562/0031-8655(2001)073<0396:ivbtdu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Toms SA, Lin WC, Weil RJ, et al. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery. 2005;57:382–91. doi: 10.1227/01.neu.000176855.39826.2d. [DOI] [PubMed] [Google Scholar]