Abstract
OBJECTIVE
Functional telomerase is essential to the replicative longevity of vascular cells. To gain insights into mechanisms by which intimal hyperplasia interferes with the repair process, expression and function of the telomerase catalytic subunit (TERT) were investigated following vascular injury.
METHODS AND RESULTS
We found that TERT was de novo activated in intima of the injured arteries, involving activation of the nuclear factor κB (NF-κB) pathway. Stimulation of the isolated intimal smooth muscle cell (SMC) by basic fibroblast growth factor or tumor necrosis factor α resulted in increased TERT activity. This depends on the activation of c-Myc signaling since mutation of the E-box in the promoter or over-expression of MAD1, a c-Myc competitor, abrogated the transcriptional activity. Inhibition of NF-κB in both intimal SMC and in the injured artery attenuated TERT transcriptional activity through reduction of c-Myc expression. Pharmacological blockade of TERT led to SMC senescence. Finally, depletion of telomerase function in mice resulted in severe intimal SMC senescence following vascular injury.
CONCLUSIONS
These results support a model whereby vascular injury induces de novo expression of TERT in intimal SMC via activation of NF-κB and up-regulation of c-Myc. The resumed TERT activity is critical for intimal hyperplasia.
Keywords: telomerase, nuclear factor-κB, smooth muscle cell, intimal hyperplasia
Introduction
Telomerase is a complex of ribonucleoproteins containing two core components, a catalytic telomerase reverse transcriptase (TERT) and a telomerase RNA component (Terc). Activation of TERT is implicated in the synthesis of new telomeric DNA repeats, thereby overcoming telomeric DNA attrition from the ends of the cell’s chromosomes during each round of division1, 2. Although TERT activity is low or absent in the majority of human adult somatic cells3, many lines of evidence suggest that TERT can be reactivated in some organs such as liver and spleen with self-renewal capacity 4-6. Moreover, TERT activity has also been found in inflamed lungs7, injured liver8 and hypertensive blood vessels9, indicative of a potential role for TERT in the process of tissue repair and remodeling. More recent studies indicate that TERT is involved in vascular smooth muscle cells (SMC) proliferation both in vitro10 and in vivo11, 12. However, the regulation of TERT and its function in the artery remain elusive.
Vascular SMC can be activated from quiescent into proliferative status in atherogenesis and in response to vessel injury. Proliferation of SMC is modulated by proinflammatory cytokines and growth factors expressed in atherosclerosis and restenotic lesions through activation of nuclear factor κB (NF-κB) signal transduction pathway13-17. We have recently shown that NF-κB plays a crucial role in intimal hyperplasia18. Given the fact that TERT expression in many tissues could be activated by mitogenic stimuli, we assessed the TERT expression and regulation by NF-κB activation in vascular cells using a rat model of carotid artery injury and the functional relevance of telomerase using arterial ligation model in Terc-/- mice.
Materials and Methods
An extended version of the Materials and Methods section can be found in the supplemental materials.
Rat model of angioplastic injury and inhibition of NF-κB
All procedures were approved by the regional ethical committee for animal research at Karolinska Institute. Male Sprague-Dawley rats (300-350g) were subjected to angioplastic injury to the left common carotid artery under general anesthesia by intraperitoneal injection of pentobarbital (2 mg/kg) plus Hypnorm® (50 mg/kg, Janssen Pharmaceutica, Belgium) as previously described19. Carotid arteries were transduced with 50 μL adenovirus encoding dominant-negative IKKβ (dnIKKβ) or E coli β-Galactosidase (β-Gal) at concentration 4×1010 pfu/mL and incubated for 40 minutes after the injury. The animals were sacrificed by overdosing pentobarbital two weeks after the injury and common carotid arteries were excised.
Carotid artery ligation model in Terc-/- mice
G4 mTerc-/- mice were used in this study20. Briefly, Mice were anesthetized with intraperitoneal injections of Hypnorm/Dormicum (Roche, Basel Schweiz) solution, the right common carotid artery and its bifurcation were exposed after mid cervical incision, and is subsequently subject to ligation. All surgery and post surgery treatment was carried out on heating pads. Three weeks after surgery the mice were sacrificed, the carotid arteries were dissected out for different purposes. Five cryosections between 200 to 1000 μm of ligated arteries were subjected to hematoxylin and eosin (H&E) staining and analysed for intimal area using Leica Qwin image analysis software (Leica Cambridge, UK).
Statistics
Statistical analyses were performed using the 2-tailed Student’s t test or Mann-Whitney test, for experiments comparing 2 groups, and ANOVA with Tukey’s Multiple Comparison post test, for 3 or more group experiments and values of p<0.05 were considered statistically significant.
Results
Activation of TERT expression is via NF-κB in the injured artery
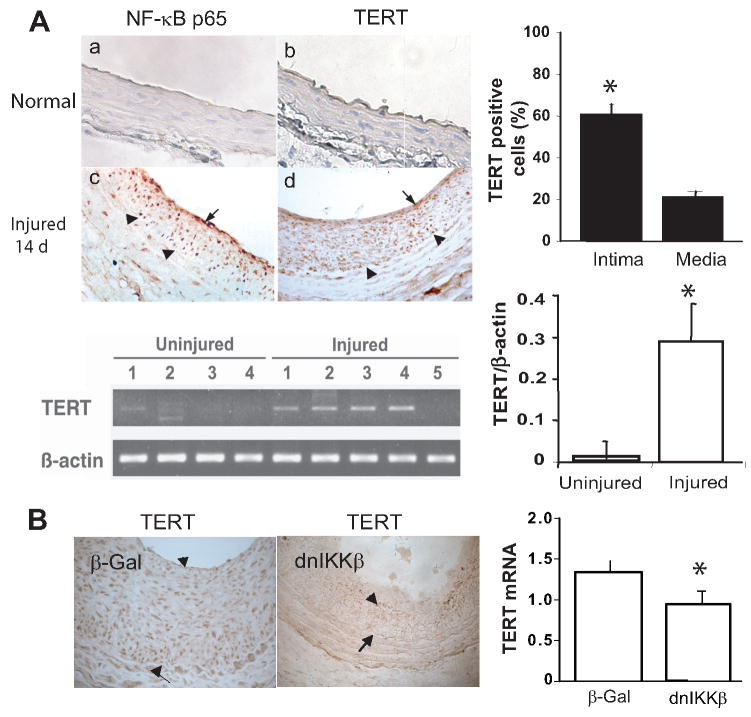
Using a rat carotid artery injury model, expression of TERT was examined in normal and injured vessels. Immunostaining analysis showed that TERT protein was highly induced in the injured vessels at day 14, and predominantly localized in intimal lesion (60% of intimal cells vs. 20% of medial cells, Fig 1A, top left panel, d and top right panel, p<0.05). Yet it was not detected in normal vessels, (Fig 1A, top left panel, b). To confirm the immunostaining findings, TERT transcript levels were analyzed by RT-PCR. Consistent with the results of TERT protein, levels of TERT mRNA were increased about 30-fold in the injured artery at day 14 when compared with normal artery (Fig 1A, lower panel, p<0.05). Intriguingly, immunostaining for NF-κB p65 displayed the similar expression as TERT in the intima (Fig 1A, c). Due to the pivotal role that NF-κB serves in vascular remodeling, we assessed the possibility that activation of NF-κB signal transduction pathway is involved in regulation of TERT expression. Utilizing adenovirus encoding dnIKKβ, the carotid artery was transduced at the time of balloon injury. This resulted in inhibition of the injury-induced NF-κB activation as described previously18, 21, as well as marked suppression of TERT expression at both transcriptional and protein levels relative to that of β-Gal transduced injured vessels at day 14 (Fig 1B).
Figure 1. Activation of TERT expression in the injured artery is NF-κB dependent.
A: Top panel left: Immunohistochemistry for TERT and NF-κB p65 in the rat carotid artery. The arrowheads indicate internal elastic lamina, and the arrows indicate cells showing representative positive signals for TERT and p65, respectively (brown color). (Original magnification: X400). Lower panel left: RT-PCR analysis of TERT and β-actin from uninjured and injured carotid arteries. B: Left panel: Immunohistochemistry for TERT in artery transduced with adenovirus carrying either E coli β -Galactosidase (β-Gal) or dominant-negative mutant of IκB kinase β (dnIKKβ) at day 14, the arrow heads indicate TERT positive cells and the arrows indicate internal elastic lamina. Right panel: qRT-PCR analysis of TERT from the injured carotid arteries transduced with β-Gal or dnIKKβ at day 14, respectively (n=5 to 7). TERT transcripts normalized to Hprt, data are presented as mean ± SEM *p<0.05.
Induction of TERT activity in isolated vascular SMC by bFGF or TNFα
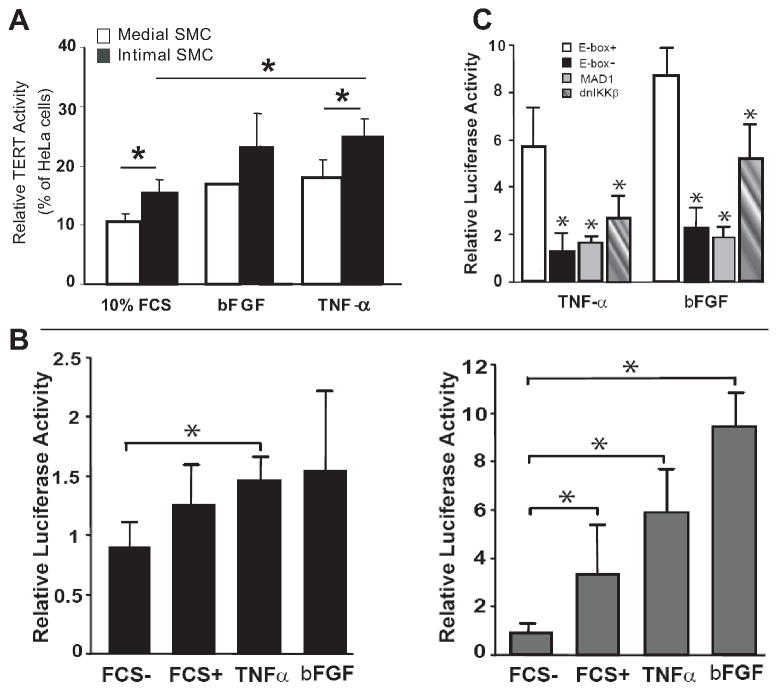
To directly assess TERT activity in SMC, isolated intimal and medial SMC were stimulated with bFGF or TNFα in vitro. As shown in Fig 2A, by TRAP analysis, a low level of basal TERT activity was detected in medial SMC, but higher level was observed in intimal SMC. Nevertheless, TERT activity was further enhanced by both TNFα and bFGF in medial SMC and to even greater levels in intimal SMC.
Figure 2. Enzymatic and transcriptional activity of TERT in medial and intimal SMC.
A: Induction of TERT activity in medial and intimal SMC by bFGF and TNFα. The TERT activity was analyzed by TRAP assay (see Materials and Methods). Results are expressed as relative activity to HeLa cells as a positive control. *p<0.05 B: Modulation of TERT promoter activity by bFGF and TNFα Intimal SMC were transfected with the long (1.4 kbp, left panel) or the proximal (181 bp, right panel) TERT promoter. Luciferase activity was determined in the cells 18 h after treatment with 0.5% FCS (FCS-), 10% FCS (FCS+), bFGF or TNFα, respectively. *p<0.05 vs FCS-. C: E-box signaling for the transcriptional activity of TERT. Intimal SMC were transfected with the proximal TERT promoter bearing mutated E-box (E-box-) or with intact E-box (E-box+) plus co-transfection with pEF-MAD1 (MAD1) or, Adv-dnIKKβ in intimal SMC in response to TNFα or bFGF. Luciferase activity was analyzed 18 h after being treated with bFGF or TNFα. P*< 0.05 vs E-box+. Data in (B) and (C) are corrected for transfection efficiency using Renilla luciferase, and the levels of luciferase activity are expressed in relative to the unstimulated cells transfected with empty pcDNA3. Data are mean ± SEM from three independent experiments.
E-box in TERT promoter is crucial for bFGF and TNFα in transcriptional regulation of TERT in intimal SMC
To clarify molecular mechanisms for the transactivation of the TERT gene upon vascular injury, intimal SMC were transiently transfected with a 1.4 kb (encompassing -1353 to +9 of the transcriptional start site) TERT promoter-reporter or a 181 bp short proximal promoter-reporter 22, 23. Our data showed that the long TERT promoter conferred minor transcriptional activity in intimal SMC upon either bFGF or TNFα stimulation (Fig 2B, left panel). In contrast, the short proximal TERT promoter was strongly activated by serum, bFGF or TNFα, consistent with a previous report (Fig 2B, right panel)22. These data indicate that the short proximal TERT promoter, so called core promoter, confers the essential activity for the upregulation of TERT expression by bFGF or TNFα, therefore, was further investigated.
Previous studies show that an E-box element (located at -165 within the proximal promoter region), which functions as the binding motif of transcription factor c-Myc, is conserved in both murine and human TERT gene promoter, and vital to transcriptional activation of the gene 23. To determine the functional role of E-box in the bFGF and TNFα- induced activation of TERT, we examined activity of TERT core promoter in the condition with intact or mutated E-box. As shown in Fig 2C, TNFα and bFGF caused 6- and 9-fold increase of the promoter activity with the intact E-box, respectively in intimal SMC. Abrogation of the E-box by mutation, however, resulted in marked decrease in the promoter activity to both TNFα and bFGF. Similarly, enforced expression of MAD1, a competitor of MAX that forms a heterodimer with c-Myc for the subsequent binding to E-box 24, could also attenuate the promoter activity. These data suggest that bFGF and TNFα induced c-Myc activation and subsequent binding to the E-box in the TERT promoter contribute to the transactivation of TERT in the intimal SMC. We also examined the involvement of NF-κB in transcriptional regulation of TERT in intimal SMC by overexpressing NF-κB inhibitor, dnIKKβ. Interestingly, despite no consensus NF-κB response element in TERT proximal promoter region, dnIKKβ markedly diminished both bFGF and TNFα-induced promoter activity in intimal SMC (Fig 2C).
NF-κB regulates c-Myc expression and subsequent binding to the E-box in the TERT promoter in intimal SMC
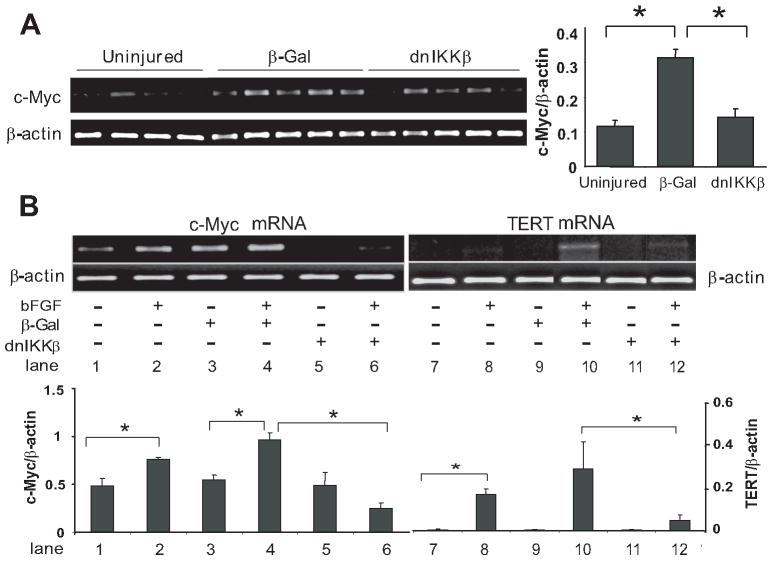
Given the functional importance of c-Myc in TERT expression, the hypothesis that NF-κB regulates c-Myc and consequently the TERT expression in the vascular repair process was tested. Our in vivo data showed that c-Myc mRNA levels increased 3-fold in the carotid artery at day 14 after injury, but was reduced to nearly pre-injury levels in the vessels transfected with dnIKKβ (Fig 3A). Consistently, dnIKKβ infection also resulted in suppression of c-Myc and TERT expression in vitro in intimal SMC subjected to bFGF stimulation. (Fig 3B, lane 6 versus lane 4; lane 12 versus lane 10)
Figure 3. Regulation of c-Myc and TERT expression by NF-κB.
A: RT-PCR analysis of c-Myc and β-actin mRNA from uninjured or injured arteries transduced with β-Gal or dnIKKβ at day 14, respectively. Right panel: Quantification of c-Myc transcripts normalized to β-actin. Data are presented as mean ± SEM, n= 4-5 rats *p<0.05. B: upper panel: RT-PCR products corresponding to c-Myc, TERT and β-actin (as an internal control) from intimal SMC stimulated with or without bFGF for 2 h or 24 h, respectively, prior to infection with or without β-Gal or dnIKKβ for 1 h. Lower panel: Quantification of c-Myc and TERT transcripts normalized to β-actin. Data are presented as mean ± SEM from three independent experiments, *p<0.05.
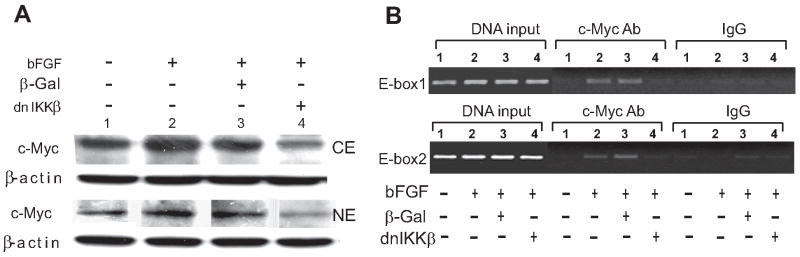
Analysis of c-Myc by western blotting validated that blockade of NF-κB by dnIKKβ led to reduction of both cytoplasmic and nuclear c-Myc protein (Fig 4A, lane 4 versus lane 2 or 3). Subsequently, the binding of c-Myc onto two different E-box sequences in TERT gene promoter was assessed by ChiP assay in the chromatin context of intimal cells exposed to bFGF stimulation. E-box 1 designates the sequence -114 to +108 in the proximal region of rat TERT gene promoter, while E-box 2 sequence is retrieved from -690 to -468 in the distal region corresponding to the E-box included in the long promoter (Fig 4B). We showed that increased in vivo c-Myc binding to E-box 1 was detected in the intimal cells exposed to bFGF for 2h (Fig 4B, c-Myc antibody precipitated panel, namely C-Myc ab, lanes 2 and 3), while it remained undetectable in untouched (lane 1 under c-Myc ab panel) and dnIKKβ infected cells (lane 4 under c-Myc ab panel), indicating that dnIKKβ treatment abrogates c-Myc binding to TERT promoter. Similar results were also observed for the binding of c-Myc to E-box 2 (Fig 4B bottom middle c-Myc ab panel).
Figure 4. Regulation of c-Myc expression and its binding to E-box by NF-κB.
A: Western blotting of c-Myc in intimal SMC stimulated with bFGF for 12 h in the presence of β-Gal or dnIKKβ (see Material and Methods). Cytoplasmic and nuclear extracts (CE and NE) were immunoblotted with c-Myc antibody, reprobed with β-actin antibody for loading control. A representative from three independent experiments is shown. B: Chromatin immunoprecipitation assay: Intimal SMC were exposed to bFGF stimulation or not for 2 h in the presence of β-Gal or dnIKKβ (lane 1: control; 2: bFGF stimulation; 3: β-Gal infection and bFGF stimulation; 4: dnIKKβ infection and bFGF stimulation). Chromatin extracts were immunoprecipitated with specific antibody to c-Myc or an IgG control. Panels of DNA INPUT, c-Myc ab and IgG correspond to the control genomic DNA input, c-Myc antibody or IgG pull-down respectively. The detection of captured TERT promoter was performed by PCR as described in Materials and methods.
Both inhibition of TERT and Terc knockout results in replicative senescence of intimal SMC
To assess the relevance of TERT reactivation to SMC proliferation, intimal SMC were treated with bFGF in conjunction with different doses of TERT inhibitor BIBR1532. Supplemental figure IA shows that BIBR1532 at 1 μmol/L significantly impeded bFGF-induced intimal cell proliferation at 3, 7 and 14 days and at 5 μmol/L fully inhibited the cell proliferation at all time points. However, 10 μmol/L of this inhibitor revealed cell toxicity characterized by massive cell death at day 3 (data not shown). Morphologic changes of the cells exposed to BIBR1532 were also evaluated microscopically. As shown in supplemental figure IB, at day 7, BIBR1532 (1 μmol/L)-treated intimal cells became flat and enlarged in morphology, a characteristic of cellular senescence, and apparently growth arrested when compared to bFGF stimulated intimal SMC. At day 14, BIBR1532-treated intimal cells displayed signs of apoptosis as indicated by membrane blebbing.
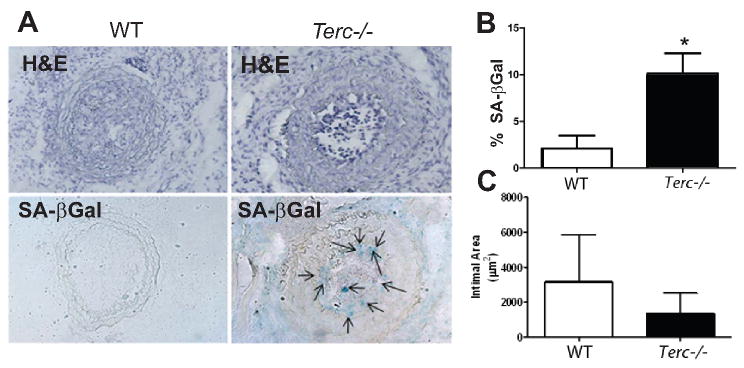
To further assess in vivo function of TERT during vascular repair process, we utilized a telomerase deficient mouse model by knocking down the RNA template of telomerase (Terc-/- mice). As early generations of these mice, up to G3, display relatively normal phenotype, compared to later generations25 we chose G4 Terc-/- mice, and ligated common carotid artery, after 3 weeks, mice were sacrificed and arteries removed for analysis. Fig 5A and B show that Terc-/- mice displayed a severe cellular senescence restricted to the intimal area as detected by SA-βGal staining, compared to wild type mice. Intimal lesion was determined from 200 μm to 1000 μm from ligation site, and no clear distinction in the lesion size was observed up to 800 μm between the two groups of mice (data not shown). However a tendency of intimal reduction, albeit not statistically significant incomparison with the wild type mice, was noticed at 1000 μm level in Terc-/- mice (Fig 5C).
Figure 5. Terc deficiency in mice induces intimal cellular senescence in the ligation induced artery injury model.
A: H&E (top panel) and SA-βgal stainings (bottom panel) for carotid arteries after ligation for 3 weeks in Terc-/- mice and wild type mice. The arrows indicated SA-βgal positive staining cells. B: quantificaiton of senescence visible (positive area of SA-βgal stainings) in the carotid arteries of Terc-/- mice (n=8) and wild type mice (n=6). Data are expressed as mean±SEM, *p<0.05. C: quantificaiton of intimal area for carotid arteries at 1000 μm from the ligation from Terc-/- (n=8) and wild type mice (n=6).
Discussion
The present study reports for the first time two interesting findings. First, vascular injury, through activation of NF-κB, induces transcriptional upregulation of TERT selectively in intimal SMC, and c-Myc has been identified as NF-κB modulated transcription factor directing TERT transactivation upon vascular injury. Secondly, depletion of Terc in mice results in severe intimal SMC senescence following vascular injury. Our study supports the published work11 that TERT was reactivated following vascular injury, and this also further extend our knowledge on NF-κB signaling modulating TERT in other cells types26-28. Our findings establish activation of TERT in intimal SMC by the injury induced NF-κB signaling as a basic mechanism underlying regulation of intimal hyperplasia.
Molecular mechanism that regulates intimal hyperplasia remains elusive. We demonstrated here that a cell type specific activation of TERT expression in intimal SMC undertakes in the process of intimal hyperplasia, and is tightly associated with activation of NF–κB signaling. To understand the regulation of TERT expression in SMC following vascular injury, we established an in vitro model in which TERT activity can be easily assessed in medial and intimal SMC exposed to TNFα or bFGF. Previous studies demonstrate that angioplastic injury to the artery provokes a pronounced inflammatory response, including induction and activation of the mitogen bFGF as well as proinflammatory cytokines such as TNFα, implicated in modulating SMC migration, growth and apoptosis 29-32. In addition, both TNFα and bFGF are able to directly activate NF-κB 16, 32. Initial analysis of TERT activity reveals that although both intimal and medial SMC possess a very low level of basal TERT activity, this can be substantially enhanced, in particular in the intimal SMC, when exposed to TNFα or bFGF, suggesting that TERT in SMC could be regulated by bFGF and TNFα produced by activated SMC and other inflammatory cells in the injured vessel. This view also supports a recent study that bFGF induces TERT expression in rat lung fibroblasts33. However, bFGF and TNFα may regulate TERT through different mechanisms. bFGF strongly activates the TERT promoter and induces the gene expression in intimal SMC, indicating that it regulates TERT primarily via activation of gene transcription. Conversely, TNFα is unable to induce TERT transcription in SMC (data not shown), although this cytokine could induce TERT activity and to some extent activate the TERT promoter. In view of recent findings that TNFα can rapidly activate TERT translocation from the cytoplasm to the nucleus26, we postulate that TNFα regulates TERT activity via an alternative mechanism probably acting on posttranslational events.
Regulation of TERT activity can be achieved at various levels, including transcriptional27 and post-transcriptional26, 33-35. A recent study10 has shown that an increased TERT activity was due to phosphorylation of TERT in the proliferative vascular SMC, the present data, however, show that TERT in the injured vessel is modulated primarily via transcription regulation and involves NF-κB activation. Immunostaining analysis shows that both TERT and activated NF-κB signal were localized in the intima, and TERT expression was diminished when inhibiting NF-κB by dnIKKβ. These findings indicate that NF-κB activation serves as a critical regulatory mechanism contributing to de novo activation of TERT transcription. Previous studies have clarified that TERT transcription could be directed by several transcription factors, including c-Myc, NF-κB and STAT3 22, 23, 36-39, suggesting that TERT expression may be regulated by different factors in different cellular context40. To gain further insight into the mechanism by which NF-κB modulate TERT expression in SMC, we assessed the activity of the TERT gene promoter in intimal SMC upon bFGF and TNFα stimulation. Study on TERT gene promoter has identified that activation of an E-box element in the proximal core promoter by Myc/Max dimer is essential for transcriptional activation in immortalized and cancer cells22 24, 41, and also required for the TERT transcription in normal epithelial and fibroblast cells22, 42, 43. Results of the present promoter assay indicate that the transcription activity of TERT to a great extent depends on c-Myc function and E-box in the TERT core promoter region since mutation of the E-box or overexpression of MAD1 abolishes the promoter activity. Intriguingly, blockade of NF-κB also substantially attenuates the activity of bFGF and TNFα and since no NF-κB element exists in the tested TERT core promoter, these data imply an indirect rather than a direct role for NF-κB in the regulation of TERT gene transcription.
In this situation, activation of c-Myc has also been established as a central mechanism. We showed that c-Myc is induced in the injured vessels in an NF-κB dependent manner. Furthermore, overexpression of dnIKKβ in cultured intimal SMC blocks the cytokine-induced rapid c-Myc expression, and TERT expression. ChiP assay demonstrates NF-κB-mediated direct binding of c-Myc onto the TERT promoter in the chromatin context of living cells exposed to bFGF treatment. Taken together, the established correlation between activation of NF-κB and up-regulation of c-Myc and transcriptional activation of TERT gene provides an indirect role of NF-κB through c-Myc in the regulation of TERT gene expression following vascular injury.
Activation of telomerase is believed to be critical for cell proliferation and immortalization; finally, we explored the functional role of TERT reactivation in intimal hyperplasia in two ways. First, pharmacological inhibition of TERT in intimal SMC restrains the cell growth suggesting a critical role for TERT in supporting intimal SMC proliferation. Second, mimicing vascular injury by performing carotid artery ligation of Terc deficient mice induced dramatic cellular senescence in the intima of Terc-/- mice in comparison to wild type mice, affirming that reactivating SMC TERT upon injury is required for intimal development. These data may provide new understanding and solution for restenosis.
In conclusion, the present data demonstrate that expression of TERT can be de novo activated in vascular SMC in response to injury and that this is mediated by the activation of the NF-κB signal transduction pathway. In view of the critical role of NF-κB in the regulation of intimal hyperplasia and TERT activity, advances in the understanding of the regulation of TERT activity may open new avenues for therapeutic intervention of intimal hyperplasia.
Supplementary Material
Acknowledgments
a) We thank Dr. Andrew H Lichtman (Department of pathology, Brigham and Women’s Hospital) for supporting on testing conjugated telomerase and NF-κB antibodies.
b) Sources of Funding - This work was supported in part by grants from the Swedish Research Council (2004-3469 and 2007-3337 to ZQY), the Swedish Research Link (2005-6333 to ZQY), the Swedish Heart-Lung Foundation (20080844 to ZQY), EU FP6 IMMUNATH (LSHM-CT-2006-037400), and EU FP7 AtheroRemo (201668).
Footnotes
c) Disclosure - The authors have declared that no conflict of interest exists.
References
- 1.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 3.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Rivera L, Herrera E, Albar JP, Blasco MA. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci U S A. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozawa K, Kurumiya Y, Yamamoto A, Isobe Y, Suzuki M, Yoshida S. Up-regulation of telomerase in primary cultured rat hepatocytes. J Biochem (Tokyo) 1999;126:361–367. doi: 10.1093/oxfordjournals.jbchem.a022458. [DOI] [PubMed] [Google Scholar]
- 6.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- 8.Tsujiuchi T, Tsutsumi M, Kido A, Takahama M, Sakitani H, Iki K, Sasaki Y, Denda A, Konishi Y. Induction of telomerase activity during regeneration after partial hepatectomy in the rat. Cancer Lett. 1998;122:115–120. doi: 10.1016/s0304-3835(97)00378-9. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Li H, Mu FT, Ebisui O, Funder JW, Liu JP. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. Faseb J. 2002;16:96–98. doi: 10.1096/cj.01-0447fje. [DOI] [PubMed] [Google Scholar]
- 10.Minamino T, Kourembanas S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res. 2001;89:237–243. doi: 10.1161/hh1501.094267. [DOI] [PubMed] [Google Scholar]
- 11.Gizard F, Nomiyama T, Zhao Y, Findeisen HM, Heywood EB, Jones KL, Staels B, Bruemmer D. The PPARalpha/p16INK4a pathway inhibits vascular smooth muscle cell proliferation by repressing cell cycle-dependent telomerase activation. Circ Res. 2008;103:1155–1163. doi: 10.1161/CIRCRESAHA.108.186205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellas RE, Lee JS, Sonenshein GE. Expression of a constitutive NF-kappa B-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J Clin Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourcier T, Sukhova G, Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 15.Landry DB, Couper LL, Bryant SR, Lindner V. Activation of the NF-kappa B and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol. 1997;151:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H. Regulation of vascular smooth muscle cell proliferation by nuclear factor-kappaB and its inhibitor, I-kappaB. J Biol Chem. 2000;275:883–889. doi: 10.1074/jbc.275.2.883. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Gonzalez J, Llorente-Cortes V, Badimon L. Cellular and molecular biology of atherosclerotic lesions. Rev Esp Cardiol. 2001;54:218–231. doi: 10.1016/s0300-8932(01)76294-x. [DOI] [PubMed] [Google Scholar]
- 18.Bu DX, Erl W, de Martin R, Hansson GK, Yan ZQ. IKKbeta-dependent NF-kappaB pathway controls vascular inflammation and intimal hyperplasia. Faseb J. 2005;19:1293–1295. doi: 10.1096/fj.04-2645fje. [DOI] [PubMed] [Google Scholar]
- 19.Yan ZQ, Yokota T, Zhang W, Hansson GK. Expression of inducible nitric oxide synthase inhibits platelet adhesion and restores blood flow in the injured artery. Circ Res. 1996;79:38–44. doi: 10.1161/01.res.79.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 21.Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am J Pathol. 2006;169:2245–2253. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horikawa I, Cable PL, Afshari C, Barrett JC. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999;59:826–830. [PubMed] [Google Scholar]
- 23.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 24.Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 25.Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Telomere biology in cardiovascular disease: the TERC-/- mouse as a model for heart failure and ageing. Cardiovasc Res. 2009;81:244–252. doi: 10.1093/cvr/cvn337. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63:18–21. [PubMed] [Google Scholar]
- 27.Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K, Eto Y, Yamada H, Anderson KC. TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochem Biophys Res Commun. 2004;316:528–532. doi: 10.1016/j.bbrc.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 28.Sinha-Datta U, Horikawa I, Michishita E, Datta A, Sigler-Nicot JC, Brown M, Kazanji M, Barrett JC, Nicot C. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood. 2004;104:2523–2531. doi: 10.1182/blood-2003-12-4251. [DOI] [PubMed] [Google Scholar]
- 29.Jackson CL, Reidy MA. Basic fibroblast growth factor: its role in the control of smooth muscle cell migration. Am J Pathol. 1993;143:1024–1031. [PMC free article] [PubMed] [Google Scholar]
- 30.Koyama H, Olson NE, Dastvan FF, Reidy MA. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ Res. 1998;82:713–721. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- 31.Agrotis A, Kanellakis P, Kostolias G, Di Vitto G, Wei C, Hannan R, Jennings G, Bobik A. Proliferation of neointimal smooth muscle cells after arterial injury. Dependence on interactions between fibroblast growth factor receptor-2 and fibroblast growth factor-9. J Biol Chem. 2004;279:42221–42229. doi: 10.1074/jbc.M408121200. [DOI] [PubMed] [Google Scholar]
- 32.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Nozaki Y, Phan SH. Regulation of telomerase activity in rat lung fibroblasts. Am J Respir Cell Mol Biol. 2002;26:534–540. doi: 10.1165/ajrcmb.26.5.4668. [DOI] [PubMed] [Google Scholar]
- 34.Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res. 2002;62:3876–3882. [PubMed] [Google Scholar]
- 36.Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 37.Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, Ariga H, Inoue M. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT) Nucleic Acids Res. 2000;28:669–677. doi: 10.1093/nar/28.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin L, Hubbard AK, Giardina C. NF-kappa B regulates transcription of the mouse telomerase catalytic subunit. J Biol Chem. 2000;275:36671–36675. doi: 10.1074/jbc.M007378200. [DOI] [PubMed] [Google Scholar]
- 39.Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 2005;65:6516–6520. doi: 10.1158/0008-5472.CAN-05-0924. [DOI] [PubMed] [Google Scholar]
- 40.Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 41.Cerni C. Telomeres, telomerase, and myc. An update. Mutat Res. 2000;462:31–47. doi: 10.1016/s1383-5742(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 42.Braunstein I, Cohen-Barak O, Shachaf C, Ravel Y, Yalon-Hacohen M, Mills GB, Tzukerman M, Skorecki KL. Human telomerase reverse transcriptase promoter regulation in normal and malignant human ovarian epithelial cells. Cancer Res. 2001;61:5529–5536. [PubMed] [Google Scholar]
- 43.Semov A, Marcotte R, Semova N, Ye X, Wang E. Microarray analysis of E-box binding-related gene expression in young and replicatively senescent human fibroblasts. Anal Biochem. 2002;302:38–51. doi: 10.1006/abio.2001.5515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.