Abstract
We have isolated and sequenced cDNAs for corticosteroid binding globulin (CBG) prepared from human liver and lung mRNAs. Our results indicate that CBG mRNA is relatively abundant in the liver but is also present in the lung, testis, and kidney. The liver CBG cDNA contains an open reading frame for a 405-amino acid (Mr 45,149) polypeptide. This includes a predominantly hydrophobic, leader sequence of 22 residues that precedes the known NH2-terminal sequence of human CBG. We, therefore, predict that the mature protein is composed of 383 amino acids and is a polypeptide of Mr 42,646. A second, in-frame, 72-base-pair cistron of unknown significance exists between the TAA termination codon for CBG and a possible polyadenylylation signal (AATAAA) located 16 nucleotides before the polyadenylylation site. The deduced amino acid sequence of mature CBG contains two cysteine residues and consensus sequences for the attachment of six possible N-linked oligosaccharide chains. The sequences of the human lung and liver CBG cDNAs differ by only one nucleotide within the proposed leader sequence, and we attribute this to a point mutation. No sequence homology was found between CBG and other steroid binding proteins, but there is a remarkable similarity between the amino acid sequences of CBG and of alpha 1-antitrypsin, and this extends to other members of the serpin (serine protease inhibitor) superfamily.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien T. G. Human corticosteroid binding globulin. Clin Endocrinol (Oxf) 1981 Feb;14(2):193–212. doi: 10.1111/j.1365-2265.1981.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., David E. V. Serum vitamin D-binding protein is a third member of the albumin and alpha fetoprotein gene family. J Clin Invest. 1985 Dec;76(6):2420–2424. doi: 10.1172/JCI112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defaye G., Basset M., Monnier N., Chambaz E. M. Electron spin resonance study of human transcortin: Thiol groups and binding site topography. Biochim Biophys Acta. 1980 Jun 26;623(2):280–294. doi: 10.1016/0005-2795(80)90256-1. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Law S. W., Dennison O. E. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):71–75. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faict D., De Moor P. Phylogenetic study of transcortin using monoclonal antibodies. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1037–1043. doi: 10.1016/s0006-291x(86)80386-2. [DOI] [PubMed] [Google Scholar]
- Fernlund P., Laurell C. B. A simple two-step procedure for the simultaneous isolation of corticosteroid binding globulin and sex hormone binding globulin from human serum by chromatography on cortisol-Sepharose and phenyl-Sepharose. J Steroid Biochem. 1981 Jun;14(6):545–552. doi: 10.1016/0022-4731(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Bailey T. J., Gustafson T. A., Markham B. E., Morkin E. Complete amino acid sequence of human thyroxine-binding globulin deduced from cloned DNA: close homology to the serine antiproteases. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7708–7712. doi: 10.1073/pnas.83.20.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H., Lehman N., Piette J., Hearst J. E. Purification of circular DNA using benzoylated naphthoylated DEAE-cellulose. DNA. 1985 Apr;4(2):157–164. doi: 10.1089/dna.1985.4.157. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Zeheb R., Yang A. Y., Rafferty U. M., Andreasen P. A., Nielsen L., Dano K., Lebo R. V., Gelehrter T. D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest. 1986 Dec;78(6):1673–1680. doi: 10.1172/JCI112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
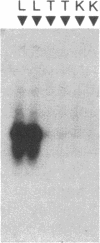
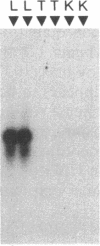
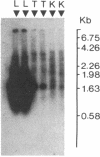
- Hammond G. L., Underhill D. A., Smith C. L., Goping I. S., Harley M. J., Musto N. A., Cheng C. Y., Bardin C. W. The cDNA-deduced primary structure of human sex hormone-binding globulin and location of its steroid-binding domain. FEBS Lett. 1987 May 4;215(1):100–104. doi: 10.1016/0014-5793(87)80121-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryb D. J., Khan M. S., Romas N. A., Rosner W. Specific binding of human corticosteroid-binding globulin to cell membranes. Proc Natl Acad Sci U S A. 1986 May;83(10):3253–3256. doi: 10.1073/pnas.83.10.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S., Aden D., Rosner W. Human corticosteroid binding globulin is secreted by a hepatoma-derived cell line. J Steroid Biochem. 1984 Feb;20(2):677–678. doi: 10.1016/0022-4731(84)90142-0. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Rosner W. Investigation of the binding site of human corticosteroid-binding globulin by affinity labeling. Demonstration of a cysteinyl residue in the binding site. J Biol Chem. 1977 Mar 25;252(6):1895–1900. [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Le Gaillard F., Han K. K., Dautrevaux M. Caractérisation et propriétés physico-chimiques de la transcortine humaine. Biochimie. 1975;57(5):559–568. doi: 10.1016/s0300-9084(75)80136-2. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Morinaga T., Sakai M., Wegmann T. G., Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4604–4608. doi: 10.1073/pnas.80.15.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M., Racadot O., Milgrom E. Specific localization of plasma corticosteroid-binding globulin immunoreactivity in pituitary corticotrophs. Endocrinology. 1984 Aug;115(2):559–569. doi: 10.1210/endo-115-2-559. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. A., Hammond G. L. Identification and characterization of a human corticosteroid binding globulin variant with a reduced affinity for cortisol. J Endocrinol. 1985 Feb;104(2):269–277. doi: 10.1677/joe.0.1040269. [DOI] [PubMed] [Google Scholar]
- Robinson P. A., Langley M. S., Hammond G. L. A solid-phase radioimmunoassay for human corticosteroid binding globulin. J Endocrinol. 1985 Feb;104(2):259–267. doi: 10.1677/joe.0.1040259. [DOI] [PubMed] [Google Scholar]
- Rosner W. Recent studies on the binding of cortisol in serum. J Steroid Biochem. 1972 Apr;3(3):531–542. doi: 10.1016/0022-4731(72)90100-8. [DOI] [PubMed] [Google Scholar]
- SEAL U. S., DOE R. P. VERTEBRATE DISTRIBUTION OF CORTICOSTEROID-BINDING GLOBULIN AND SOME ENDOCRINE EFFECTS ON CONCENTRATION. Steroids. 1965 Jun;41:827–841. doi: 10.1016/0039-128x(65)90174-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri P. K., Murai J. T., Hammond G. L., Nisker J. A., Raymoure W. J., Kuhn R. W. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strel'chyonok O. A., Avvakumov G. V., Akhrem A. A. Pregnancy-associated molecular variants of human serum transcortin and thyroxine-binding globulin. Carbohydr Res. 1984 Nov 15;134(1):133–140. doi: 10.1016/0008-6215(84)85028-4. [DOI] [PubMed] [Google Scholar]
- Strel'chyonok O. A., Avvakumov G. V. Evidence for the presence of specific binding sites for transcortin in human liver plasma membranes. Biochim Biophys Acta. 1983 Feb 22;755(3):514–517. doi: 10.1016/0304-4165(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Van Baelen H., Brepoels R., De Moor P. Transcortin Leuven: a variant of human corticosteroid-binding globulin with decreased cortisol-binding affinity. J Biol Chem. 1982 Apr 10;257(7):3397–3400. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]