Abstract
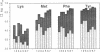
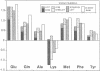
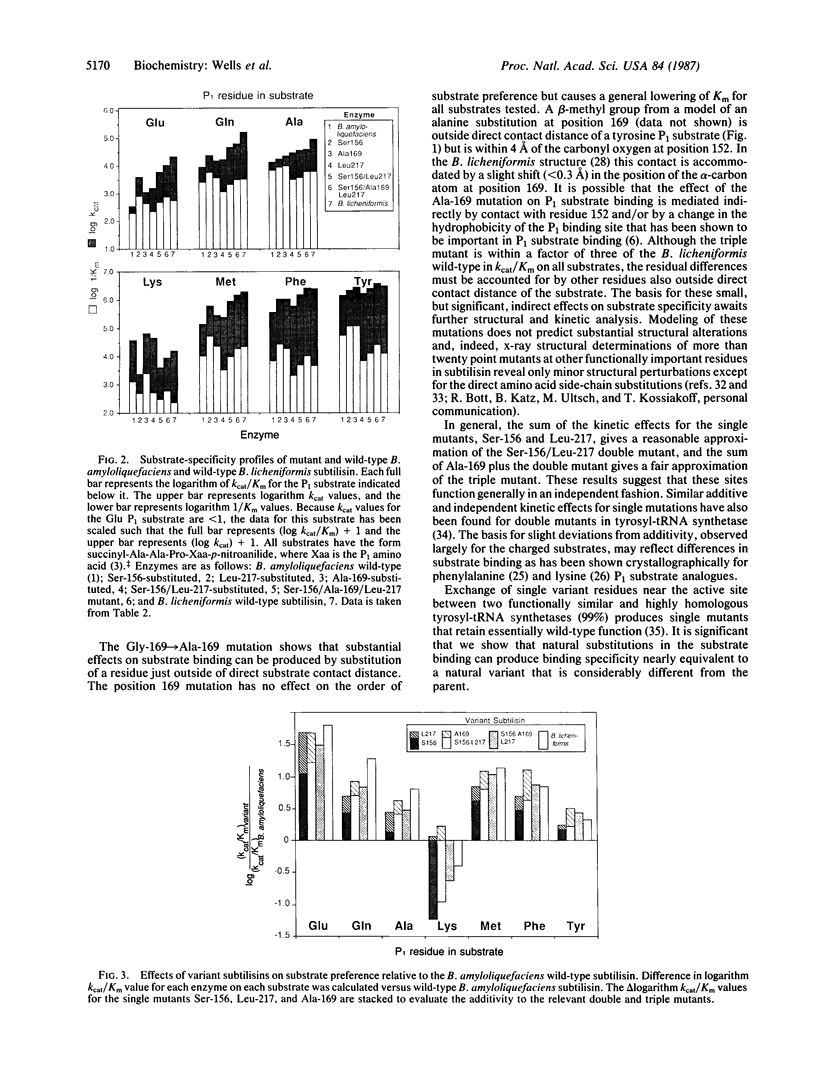
The Bacillus licheniformis and Bacillus amyloliquefaciens subtilisins differ by 31% in protein sequence and by factors of greater than 60 in catalytic efficiency, kcat/Km, toward various substrates. Despite large differences in sequence and substrate specificity for these serine proteases, only two amino acid substitutions (residues 156 and 217) occur within 4 A (contact distance) of modeled substrates, and a third substitution (residue 169) is within 7 A. The three B. licheniformis substitutions (Ser-156/Ala-169/Leu-217) were introduced into the wild-type B. amyloliquefaciens subtilisin (Glu-156/Gly-169/Tyr-217) by site-directed mutagenesis. The substrate specificity of the triple mutant approaches that of B. licheniformis enzyme when assayed with seven different substrates that vary in charge, size, and hydrophobicity. Thus, specificity properties of distantly related and functionally divergent enzymes can be exchanged by limited amino acid replacements, in this case representing less than 4% of the sequence differences.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band L., Henner D. J. Bacillus subtilis requires a "stringent" Shine-Dalgarno region for gene expression. DNA. 1984;3(1):17–21. doi: 10.1089/dna.1.1984.3.17. [DOI] [PubMed] [Google Scholar]
- Bode W., Papamokos E., Musil D., Seemueller U., Fritz H. Refined 1.2 A crystal structure of the complex formed between subtilisin Carlsberg and the inhibitor eglin c. Molecular structure of eglin and its detailed interaction with subtilisin. EMBO J. 1986 Apr;5(4):813–818. doi: 10.1002/j.1460-2075.1986.tb04286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. J., Winter G., Wilkinson A. J., Fersht A. R. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus). Cell. 1984 Oct;38(3):835–840. doi: 10.1016/0092-8674(84)90278-2. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Largman C., Fletcher T., Roczniak S., Barr P. J., Fletterick R., Rutter W. J. Redesigning trypsin: alteration of substrate specificity. Science. 1985 Apr 19;228(4697):291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- Estell D. A., Graycar T. P., Miller J. V., Powers D. B., Wells J. A., Burnier J. P., Ng P. G. Probing steric and hydrophobic effects on enzyme-substrate interactions by protein engineering. Science. 1986 Aug 8;233(4764):659–663. doi: 10.1126/science.233.4764.659. [DOI] [PubMed] [Google Scholar]
- Estell D. A., Graycar T. P., Wells J. A. Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J Biol Chem. 1985 Jun 10;260(11):6518–6521. [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- GUTFREUND H., STURTEVANT J. M. The mechanism of the reaction of chymotrypsin with p-nitrophenyl acetate. Biochem J. 1956 Aug;63(4):656–661. doi: 10.1042/bj0630656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. J., L'Italien J. J., Pilacinski W. P., Glassman D. L., Krzyzek R. A. High-level expression in Escherichia coli of biologically active bovine growth hormone. DNA. 1985 Aug;4(4):273–281. doi: 10.1089/dna.1985.4.273. [DOI] [PubMed] [Google Scholar]
- Hirono S., Akagawa H., Mitsui Y., Iitaka Y. Crystal structure at 2.6 A resolution of the complex of subtilisin BPN' with streptomyces subtilisin inhibitor. J Mol Biol. 1984 Sep 15;178(2):389–414. doi: 10.1016/0022-2836(84)90150-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Eliasson M., Uhlén M., Flock J. I. Cloning, sequencing and expression of subtilisin Carlsberg from Bacillus licheniformis. Nucleic Acids Res. 1985 Dec 20;13(24):8913–8926. doi: 10.1093/nar/13.24.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Lowe D. M., Borgford T., Fersht A. R. Natural variation of tyrosyl-tRNA synthetase and comparison with engineered mutants. Biochemistry. 1986 Apr 22;25(8):1887–1891. doi: 10.1021/bi00356a008. [DOI] [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Katz B. A., Kossiakoff A. The crystallographically determined structures of atypical strained disulfides engineered into subtilisin. J Biol Chem. 1986 Nov 25;261(33):15480–15485. [PubMed] [Google Scholar]
- Mas M. T., Chen C. Y., Hitzeman R. A., Riggs A. D. Active human-yeast chimeric phosphoglycerate kinases engineered by domain interchange. Science. 1986 Aug 15;233(4765):788–790. doi: 10.1126/science.3526552. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer S. T., Kraut J. X-ray crystallographic study of boronic acid adducts with subtilisin BPN' (Novo). A model for the catalytic transition state. J Biol Chem. 1975 Sep 25;250(18):7120–7126. [PubMed] [Google Scholar]
- McPhalen C. A., Schnebli H. P., James M. N. Crystal and molecular structure of the inhibitor eglin from leeches in complex with subtilisin Carlsberg. FEBS Lett. 1985 Aug 19;188(1):55–58. doi: 10.1016/0014-5793(85)80873-5. [DOI] [PubMed] [Google Scholar]
- McPhalen C. A., Svendsen I., Jonassen I., James M. N. Crystal and molecular structure of chymotrypsin inhibitor 2 from barley seeds in complex with subtilisin Novo. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7242–7246. doi: 10.1073/pnas.82.21.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali C., Creaser E. H. Protein engineering of alcohol dehydrogenase--1. Effects of two amino acid changes in the active site of yeast ADH-1. Protein Eng. 1986 Oct-Nov;1(1):55–57. doi: 10.1093/protein/1.1.55. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Alden R. A., Freer S. T., Birktoft J. J., Kraut J. Polypeptide halomethyl ketones bind to serine proteases as analogs of the tetrahedral intermediate. X-ray crystallographic comparison of lysine- and phenylalanine-polypeptide chloromethyl ketone-inhibited subtilisin. J Biol Chem. 1976 Feb 25;251(4):1097–1103. [PubMed] [Google Scholar]
- Pähler A., Banerjee A., Dattagupta J. K., Fujiwara T., Lindner K., Pal G. P., Suck D., Weber G., Saenger W. Three-dimensional structure of fungal proteinase K reveals similarity to bacterial subtilisin. EMBO J. 1984 Jun;3(6):1311–1314. doi: 10.1002/j.1460-2075.1984.tb01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus J. D., Alden R. A., Birktoft J. J., Kraut J., Powers J. C., Wilcox P. E. An x-ray crystallographic study of the binding of peptide chloromethyl ketone inhibitors to subtilisin BPN'. Biochemistry. 1972 Jun 20;11(13):2439–2449. doi: 10.1021/bi00763a009. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Kraut J., Alden R. A., Birktoft J. J. Subtilisin; a stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972 Nov 7;11(23):4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Ferrari E., Henner D. J., Estell D. A., Chen E. Y. Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983 Nov 25;11(22):7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B., Bott R. R., Graycar T. P., Estell D. A. Designing substrate specificity by protein engineering of electrostatic interactions. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1219–1223. doi: 10.1073/pnas.84.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B. In vivo formation and stability of engineered disulfide bonds in subtilisin. J Biol Chem. 1986 May 15;261(14):6564–6570. [PubMed] [Google Scholar]
- Wells J. A., Vasser M., Powers D. B. Cassette mutagenesis: an efficient method for generation of multiple mutations at defined sites. Gene. 1985;34(2-3):315–323. doi: 10.1016/0378-1119(85)90140-4. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Brown E. L., Ptashne M. Substituting an alpha-helix switches the sequence-specific DNA interactions of a repressor. Cell. 1984 Sep;38(2):361–369. doi: 10.1016/0092-8674(84)90491-4. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Ptashne M. Changing the binding specificity of a repressor by redesigning an alpha-helix. Nature. 1985 Aug 15;316(6029):601–605. doi: 10.1038/316601a0. [DOI] [PubMed] [Google Scholar]
- Yang M. Y., Ferrari E., Henner D. J. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984 Oct;160(1):15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]