Abstract
BACKGROUND
A provocative finding from several double-blind clinical trials has been the association between greater adherence to placebo study medication and better health outcomes. We used data from the Studies of Left Ventricular Dysfunction (SOLVD) Treatment Trial (SOLVD-TT) and the SOLVD Prevention Trial (SOLVD-PT) to examine whether such associations could be validated and to examine several sources of bias and potential confounding.
METHODS
Survival analytic methods were used to estimate the association between placebo adherence and several health outcomes, employing a number of modeling techniques to test for the existence of alternative explanations for the association. Higher adherence was defined as having taken ≥75% of prescribed study medication.
RESULTS
Higher placebo adherence was associated with improved overall survival in both SOLVD-TT and SOLVD-PT [hazard ratio (HR) = 0.52, 95% confidence interval (CI): 0.35 to 0.79 and HR = 0.52, 95%CI: 0.38 to 0.71, respectively]. Associations were similar for fatal or non-fatal cardiovascular or coronary heart disease events. Adjustment for both modifiable and non-modifiable cardiac risk factors (including age, gender, diabetes, blood pressure, smoking, weight, alcohol use, and levels of education) had minimal effect on the strength of the association. Little evidence of bias was found as an explanation for this relationship.
CONCLUSIONS
In these two trials, better adherence to placebo was associated with markedly superior health outcomes, including total in-study mortality and incident cardiovascular events. No important confounders were identified. These data suggest there may exist strong but unrecognized determinants of health outcomes for which placebo adherence is a marker.
KEY WORDS: placebo, health outcome
INTRODUCTION
It is not surprising that better adherence to effective medication results in better health outcomes1,2. A provocative finding from the analysis of double-blind clinical trials, however, has been the discovery of a strong association between better adherence to placebo medication and improved survival. Post-hoc analyses of several clinical trials have found that placebo-allocated participants who were relatively more adherent had markedly improved survival compared to those who were relatively less adherent, with reductions in mortality ranging from approximately 40% to 75%3–11.
Why this should be so remains a mystery. The simplest explanation is that the published data represent only a select group of positive studies, rendering the published literature biased toward identifying this association. Other potential explanations include the possibility that medication adherence is only a proxy for healthier lifestyle and behaviors, that adherence to placebo is associated with adherence to life-prolonging medications, or that the association is confounded by participants who become ill and who are, therefore, both more likely to become non-adherent and more likely to die because of their illness (i.e., protopathic bias). Most published studies have examined potential confounding by a limited number of covariates, but have not thoroughly examined these other explanations, so the existence of this association is uncertain and, if true, its explanation remains largely unexplored.
The strength and apparent consistency of this relationship merit further investigation. Should the presence of the association be validated, the clinical and public-health implications are profound, given the extraordinarily strong protection associated with adherence to placebo. Understanding why medication adherence itself, independent of the medication's efficacy, would be associated with such markedly improved health outcomes could shed new light on powerful determinants of health and longevity. To examine this issue more carefully, we conducted a secondary data analysis of two large placebo-controlled clinical trials for which no prior examinations of this association had been performed; these analyses are part of a larger multi-study investigation of this remarkable relationship.
METHODS
Original Study and Data
We used data from the Studies Of Left Ventricular Dysfunction (SOLVD)12–14, a pair of large, double-blind, placebo-controlled randomized clinical trials of enalapril in patients with congestive heart failure (CHF). Participants had a cardiac ejection fraction <35% and were randomized into one of two separate trials that followed the same treatment protocol: those who were symptomatic were entered into the SOLVD Treatment Trial (SOLVD-TT)13, and those without symptoms were enrolled in the SOLVD Prevention Trial (SOLVD-PT)14. Participants were titrated to a dose of 10 mg of enalapril twice daily and followed every 4 months for a mean 37 to 41 months for the primary endpoint of all-cause mortality. Enalapril reduced mortality in both trials, though the effect was statistically significant only in the Treatment Trial13,14. Both studies found significantly fewer CHF-related hospitalizations in enalapril-allocated participants, and the Prevention Trial demonstrated a significant reduction in the incidence of symptomatic CHF13,14. Data for our analyses were obtained from the National Heart, Lung, and Blood Institute Data Repository of Epidemiology and Clinical Trials15.
Analytic Methods
The primary objective of these analyses was to obtain an unbiased and unconfounded estimate of the association between adherence to study medication and total in-study mortality among those participants randomized to the placebo group in both SOLVD studies. Secondary objectives included assessment of the association between placebo adherence and cause-specific mortality, including coronary heart disease (CHD) mortality, all cardiovascular disease (CVD) mortality (CHD, CHF, and stroke), and non-CVD mortality; the incidence of fatal or non-fatal CHD and CVD events was also investigated. We also examined several potential sources of bias and confounding.
We used survival analysis to assess the effects of placebo adherence16. We first generated Kaplan-Meier curves stratified by adherence and compared the adherence strata using log-rank tests. We used Cox proportional hazards models to obtain adjusted estimates of adherence effects and tested the proportionality assumption in the primary analysis using an interaction term containing the product of adherence and time16. Covariate adjustments included a pre-defined set of variables that included demographics, modifiable and non-modifiable risk factors and education (as the only psychosocial variable available). Baseline values of covariates were used for adjustment, as we did not have longitudinal measurements for most covariates. All analyses were performed with SAS v. 9.117.
The primary definition of placebo adherence was having taken at least 75% of prescribed placebo study medication. We also conducted sensitivity analyses in which we varied the cutoff point for higher adherence from 50% to 95%. For most analyses, adherence was treated as a fixed binary variable, defined as the total number of pills taken by a participant over each interval, as determined by pill counts, divided by the total number of pills that should have been taken during that interval; these percentages were then averaged over all intervals (i.e., the total mean adherence). For supplementary analyses, we calculated adherence as a cumulative variable using pill counts for the entire interval from baseline up to the most recent visit before each outcome event (i.e., at the determination of the survival probability at each failure time, adherence was re-calculated as the total placebo adherence up to the time of the event; this definition is termed the cumulative mean adherence). In another set of analyses, we also treated adherence as a simple time-dependent adherence variable using only the single adherence value for the most recent interval prior to each outcome event. Finally, one set of models used total mean adherence as a continuous variable.
Some participants had individual study visits in which raw adherence measurements exceeded 100% (participants received more study medication than needed for full adherence at the following visit). Percentages slightly greater than 100% were likely due to having lost or taken a few extra doses. Larger adherence values more likely arose from counting or data-entry errors. Accordingly, values between 101% and 125% were recoded as 100%, while values greater than 125% were set to missing for that visit.
In order to examine the possibility that both reduced adherence and mortality were due to some other serious, ultimately fatal illness, we repeated the analyses for total mortality after deleting each participant's last adherence measurement and last two measurements (these procedures reduce the effect of the adherence measurements in the 4 to 8 months before a participant's death), to test if the association attenuated. We also repeated the proportional hazards models, using a lagged adherence variable, which also diminishes the influence of the ultimate and penultimate adherence measurements; this procedure was conducted on both the cumulative mean-adherence variable and the time-dependent adherence variable. The time-dependent adherence definition would be the most sensitive to the lagged-variable procedure, since it is based on a single measurement (not averaged over several measurements) and provides the most sensitive test of the possibility of protopathic bias.
RESULTS
SOLVD Treatment Trial
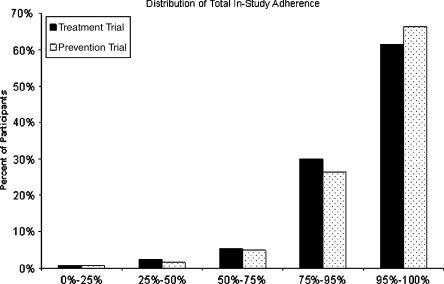
Among the placebo-allocated participants, 98 out of 1,255 (7.8%) took less than 75% of their prescribed study medication, and the distribution of adherence values was highly skewed (Fig. 1). Only 3% of visits had adherence values >125%; these were set to missing. No individuals were lost due to this data step. Other than small differences in the distribution of race, there were no significant differences in baseline characteristics between higher and lower adherent participants (Table 1). Overall, there were 491 in-study deaths (39.1% of randomized participants), with the great majority being due to cardiovascular causes (90.2% of all deaths; Table 2).
Figure 1.
Distribution of total mean adherence levels among placebo-allocated participants in the SOLVD studies.
Table 1.
Baseline Characteristics of Placebo-allocated Participants in Both SOLVD Studies, Overall and by Adherence Level
| Baseline characteristic | SOLVD-Treatment | SOLVD-Prevention | ||||
|---|---|---|---|---|---|---|
| Higher adherencea N = 1,157 | Lower adherence N = 98 | p-value | Higher adherence N = 1,951 | Lower adherence N = 141 | p-value | |
| Demographics | ||||||
| Age (years; mean, SD) | 60.6 (9.6) | 59.8 (10.8) | 0.30 | 58.9 (10.3) | 55.4 (12.2) | <0.001 |
| Gender, N (%) | ||||||
| M | 918 (79) | 85 (87) | 0.08 | 1,737 (89) | 121 (86) | 0.24 |
| F | 239 (21) | 13 (13) | 214 (11) | 20 (14) | ||
| Race, N (%) | ||||||
| White | 962 (82) | 59 (75) | 0.02 | 1,713 (88) | 101 (73) | <0.001 |
| African-American | 162 (14) | 18 (23) | 171 (9) | 34 (24) | ||
| Other | 51 (4) | 2 (3) | 65 (3) | 6 (4) | ||
| Married/partnered (%) b | 15 (79) | 177 (74) | 0.62 | 868 (76) | 53 (74) | 0.60 |
| Education | ||||||
| Less than high school | 500 (46) | 37 (44) | 0.90 | 416 (36) | 27 (35) | 0.88 |
| High school graduate | 360 (33) | 28 (33) | 379 (33) | 28 (36) | ||
| Greater than high school | 224 (21) | 19 (23) | 347 (30) | 23 (29) | ||
| Clinical characteristics | ||||||
| Systolic blood pressure, mmHg (mean, SD) | 124.8 (17.2) | 122.8 (18.3) | 0.37 | 125.7 (16.6) | 123.8 (18.1) | 0.12 |
| Concurrent medications (mean, SD) | 5.4 (1.9) | 5.8 (1.9) | 0.58 | 3.1 (2.0) | 3.1 (2.1) | 0.30 |
| Diabetes (%) | 310 (27) | 22 (23) | 0.38 | 288 (15) | 22 (21) | 0.06 |
| Weight, kg (mean, SD) | 79.5 (16.8) | 80.9 (16.0) | 0.53 | 81.7 (14.3) | 82.1 (14.7) | 0.62 |
| Current smoker (%) | ||||||
| Never | 280 (24) | 19 (20) | 0.43 | 411 (21) | 22 (16) | 0.05 |
| Current | 245 (21) | 25 (26) | 461 (24) | 45 (32) | ||
| Former | 632 (55) | 53 (55) | 1,079 (55) | 74 (52) | ||
| Avg alcohol in past 2 years (%) | ||||||
| None | 693 (60) | 48 (52) | 0.25 | 992 (52) | 65 (47) | 0.55 |
| 1–2 Drinks/day | 396 (34) | 39 (42) | 793 (41) | 63 (46) | ||
| >2 Drinks/day | 59 (5) | 6 (6) | 139 (7) | 9 (7) | ||
a“Higher adherence” defined as ≥75% total in-study placebo medication adherence
bAvailable on only one-fifth of SOLVD-TT participants and half of SOLVD-PT participants
Table 2.
Unadjusted Hazard Ratios for Association of Placebo Adherence with Mortality and Incident Events in the SOLVD Treatment Trial
| Lower adherent participants (N = 98) | Higher adherenta participants (N = 1,157) | |||
|---|---|---|---|---|
| Outcome | No. events | No. events | HR | 95% CI |
| Total mortality | 45 | 446 | 0.52 | 0.38–0.71 |
| CVD mortality | 41 | 402 | 0.52 | 0.38–0.72 |
| Non-CVD mortality | 4 | 44 | 0.53 | 0.19–1.48 |
| CHD mortality | 3 | 59 | 0.96 | 0.30–3.08 |
| Incident CVD events (fatal and non-fatal) | 85 | 855 | 0.46 | 0.36–0.57 |
| Incident CHD events (fatal and non-fatal) | 11 | 130 | 0.54 | 0.29–1.00 |
Legend:
CHD = Coronary heart disease
CVD = Cardiovascular disease
CI = Confidence interval
a“Higher adherent” defined as ≥75% total in-study placebo medication adherence
Significant differences (p < 0.05) indicated in bold type
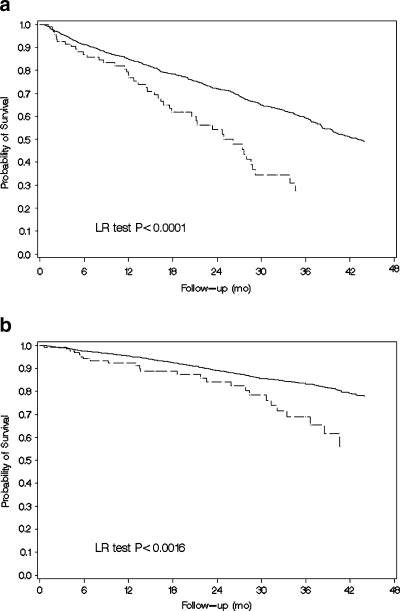
More adherent participants had significantly lower total mortality relative to less adherent participants (HR = 0.52, 95% CI: 0.38 to 0.71; Table 2, and Fig. 2a). The association was also found to be statistically significant for CVD mortality (which included CHF-related mortality), but not for CHD or non-CVD-related deaths, though the numbers of outcomes in these two subgroups were small. More adherent participants were also less likely to suffer either an incident CVD or CHD event, though the latter was of borderline significance (Table 2). The association between placebo adherence and total mortality persisted when total mean adherence was treated as a continuous measurement with HR = 0.89 (95% CI: 0.84 to 0.95) for every 10% increase in adherence. The association was similar when adherence was used as a time-dependent covariate (HR = 0.50, 95% CI: 0.38 to 0.66). When adherence was calculated as a cumulative variable (i.e., each participant's adherence was re-calculated at each visit using measurements only up to that point), the association remained significant (HR = 0.58, 95% CI: 0.43 to 0.79). Because the adherence cutoff value of 75% was somewhat arbitrary, we conducted a sensitivity analysis on the placebo adherence-mortality association at different cutoff points of adherence. The value of the total mean adherence for which the association with mortality was strongest was at a cutoff point of 55% adherence (HR = 0.42, 95% CI: 0.27 to 0.66).
Figure 2.
a Kaplan-Meier curves of cumulative survival for higher adherent (solid line) and lower adherent (dashed line) placebo-allocated study participants in the SOLVD Treatment Trial. b Kaplan-Meier curves of cumulative survival for higher adherent (solid line) and lower adherent (dashed line) placebo-allocated study participants in the SOLVD Prevention Trial.
Adjustment for potential confounders (demographics, modifiable and non-modifiable CVD risk factors, and education) did not result in a meaningful change in the association for any outcome (Table 3).
Table 3.
Adjusted Hazard Ratios (and 95% Confidence Intervals) for Association of Placebo Adherence with Mortality and Incident Events in the SOLVD Treatment Trial
| Outcome | Non-modifiable risk factorsa | Modifiable risk factorsb | All risk factorsc | Psychosocial measuresd | All covariatese |
|---|---|---|---|---|---|
| Mortality | |||||
| Total mortality | 0.54 (0.39, 0.74) | 0.53 (0.38, 0.74) | 0.56 (0.40, 0.77) | 0.55(0.39, 0.78) | 0.58 (0.41, 0.83) |
| CVD | 0.54 (0.39, 0.75) | 0.54 (0.38, 0.76) | 0.56 (0.40, 0.79) | 0.55 (0.38, 0.78) | 0.58 (0.40, 0.84) |
| Non-CVD | 0.56 (0.20, 1.58) | 0.45 (0.16, 1.27) | 0.51 (0.18, 1.44) | 0.63 (0.19, 2.03) | 0.61 (0.19, 2.01) |
| CHD | 0.92 (0.29, 2.97) | 0.85 (0.26, 2.72) | 0.82 (0.25, 2.64) | 0.82 (0.26, 2.63) | 0.74 (0.23, 2.40) |
| Morbidity | |||||
| Incident CVD events (fatal and non-fatal) | 0.57 (0.45, 0.72) | 0.56 (0.44, 0.70) | 0.56 (0.45, 0.71) | 0.55 (0.43, 0.70) | 0.54 (0.42, 0.69) |
| Incident CHD events (fatal and non-fatal) | 0.84 (0.45, 1.55) | 0.81 (0.43, 1.55) | 0.84 (0.44, 1.60) | 1.02 (0.50, 2.08) | 0.93 (0.46, 1.92) |
Legend:
CHD = Coronary heart disease
CVD = Cardiovascular disease
aNon-modifiable risk factors: age, sex, race
bModifiable risk factors: diabetes, alcohol, smoking, SBP, weight
cAll risk factors: age, sex, race, diabetes, alcohol, smoking, SBP, weight
dPsychosocial measures: education
eAll covariates: all of the above
Significant differences (p < 0.05) indicated in bold type
Several analyses were conducted to examine the possibility that the association between placebo adherence and mortality was the result of a serious and ultimately fatal illness, which caused both the participant's death and reduced adherence in the months prior to the participant's death. First, we estimated the association after eliminating the last and the last two adherence measurements; these procedures resulted in some attenuation in the association (HR = 0.75, 95% CI: 0.49 to 1.15 and HR = 0.77, 95% CI: 0.49 to 1.22, respectively). Next, we lagged the adherence variable in the survival models with cumulative mean adherence by one measurement (HR = 0.88, 95% CI: 0.57 to 1.35) and by two measurements (HR = 0.84, 95% CI: 0.53 to 1.34). However, the estimated effect of recent adherence as a time-dependent covariate was unaffected when it was calculated using data from the second (HR = 0.54, 95% CI: 0.34 to 0.85) or third (HR = 0.60, 95% CI: 0.36 to 0.98) most recent visit before each outcome event, rather than the most recent visit, as in the primary analysis.
SOLVD Prevention Trial
Among the 2,092 placebo-allocated participants in the SOLVD Prevention Trial, 141 (6.7%) were "lower adherent," and these participants tended to be slightly younger, current smokers, of African-American race, and diabetic (Table 1). Only 2% of visit adherence values were set to missing because they exceeded 125% adherence. Mortality was lower in the Prevention Trial (14.8%), with a distribution in causes of death similar to that in the Treatment Trial.
The hazard ratio for total mean placebo adherence and all-cause mortality was identical to that in SOLVD-TT (HR = 0.52, 95% CI: 0.35 to 0.79, Table 4 and Fig. 2b). Results were more consistent and significant across all causes of death compared to the Treatment Trial: with the exception of CHD mortality (in which the association was strong but not statistically significant), more adherent participants showed substantially greater survival (Table 4). Results were also similar regardless of whether adherence was treated as a cumulative variable (HR = 0.58, 95% CI: 0.39 to 0.88), a time-dependent variable (HR = 0.53, 95% CI: 0.36 to 0.77), or a continuous variable (HR = 0.90, 95% CI: 0.83 to 0.98 for each 10% increase in adherence). In the sensitivity analysis of the optimal adherence cutpoint, the association between placebo adherence and mortality was strongest using a cutoff point for adherence of 60% (HR = 0.42, 95% CI: 0.23 to 0.74).
Table 4.
Unadjusted Hazard Ratios for Association of Placebo Adherence with Mortality and Incident Events in the SOLVD Prevention Trial
| Lower adherent participants (N = 141) | Higher adherenta participants (N = 1,951) | |||
|---|---|---|---|---|
| Outcome | No. events | No events | HR | 95% CI |
| Total mortality | 25 | 292 | 0.52 | 0.35–0.79 |
| CVD mortality | 20 | 261 | 0.59 | 0.37–0.93 |
| Non-CVD mortality | 5 | 31 | 0.27 | 0.11–0.70 |
| CHD mortality | 4 | 47 | 0.49 | 0.18–1.37 |
| Incident CVD events (fatal and non-fatal) | 84 | 980 | 0.49 | 0.39–0.61 |
| Incident CHD events (fatal and non-fatal) | 17 | 177 | 0.42 | 0.26–0.70 |
Legend:
CHD = Coronary heart disease
CVD = Cardiovascular disease
CI = Confidence interval
a“Higher adherent” defined as ≥75% total in-study placebo medication adherence
Significant differences (p < 0.05) indicated in bold type
As in the Treatment Trial, multivariable adjustment had little effect on the results. The association between placebo adherence and mortality was similar in the bivariate and fully adjusted models for all outcomes except non-CVD mortality, for which adjustment caused substantial attenuation (Table 5).
Table 5.
Adjusted Hazard Ratios (and 95% Confidence Intervals) for Association of Placebo Adherence with Mortality and Incident Events in the SOLVD Prevention Trial
| Outcome | Non-modifiable risk factorsa | Modifiable risk factorsb | All risk factorsc | Psychosocial measuresd | All covariatese |
|---|---|---|---|---|---|
| Mortality | |||||
| Total mortality | 0.54 (0.35, 0.81) | 0.56 (0.36, 0.86) | 0.57 (0.37, 0.89) | 0.49 (0.31, 0.79) | 0.52 (0.31, 0.86) |
| CVD | 0.59 (0.37, 0.94) | 0.60 (0.37, 0.96) | 0.60 (0.37, 0.98) | 0.53 (0.31, 0.88) | 0.51 (0.30, 0.87) |
| Non-CVD | 0.31 (0.11, 0.70) | 0.37 (0.13, 1.05) | 0.42 (0.14, 1.28) | 0.31 (0.09, 1.04) | 0.60 (0.13, 2.83) |
| CHD | 0.49 (0.17, 1.39) | 0.51 (0.18, 1.44) | 0.50 (0.17, 1.44) | 0.40 (0.14, 1.12) | 0.38 (0.13, 1.14) |
| Morbidity | |||||
| Incident CVD events (fatal and non-fatal) | 0.70 (0.55, 0.88) | 0.73 (0.58, 0.92) | 0.71 (0.56, 0.90) | 0.67 (0.51, 0.89) | 0.67 (0.50, 0.89) |
| Incident CHD events (fatal and non-fatal) | 0.65 (0.39, 1.08) | 0.75 (0.45, 1.26) | 0.69 (0.41, 1.16) | 0.88 (0.45, 1.74) | 0.87 (0.44, 1.74) |
Legend:
CHD = Coronary heart disease
CVD = Cardiovascular disease
aNon-modifiable risk factors: age, sex, race
bModifiable risk factors: diabetes, alcohol, smoking, SBP, weight
cAll risk factors: age, sex, race, diabetes, alcohol, smoking, SBP, weight
dPsychosocial measures: education
eAll covariates: all of the above
Significant differences (p < 0.05) indicated in bold type
The Prevention Trial analyses did not support the concept that other serious illness was responsible for both the lower adherence and higher mortality. Unlike the Treatment Trial analyses, the association remained significant after dropping the final total mean adherence measurement (HR = 0.61, 95% CI: 0.38 to 0.96) or the final two adherence measurements (HR = 0.54, 95% CI: 0.34 to 0.88) in the models using total mean adherence. Results were similar in the models using adherence as a cumulative mean variable (HR = 0.55, 95% CI: 0.34 to 0.87 with a single lag and HR = 0.49, 95% CI: 0.30 to 0.79 with two lags in the adherence variable). Finally, the association was also essentially unchanged when estimated using adherence as a time-dependent variable and using the second (HR = 0.54, 95% CI: 0.35 to 0.86) or third (HR = 0.59 95% CI: 0.36 to 0.98) most recent visit.
DISCUSSION
It is surprising that better adherence to placebo should be associated with reduced mortality, since placebo, by definition, has no specific biologic or disease-modifying activity. Therefore, placebo adherence must be a marker for some other factors responsible for this extraordinary survival advantage, but what those factors might be remains a mystery. Adjustment for numerous available potential confounders did not appreciably attenuate the association, suggesting that the placebo adherence-mortality association is independent of these known risk factors. In addition, the association appeared to be generally present across all outcomes and for both mortality and incident events.
One possible explanation is that placebo adherence is merely a marker for adherence to other life-prolonging medications, and this possibility cannot be examined with the available data.
Another possibility is that some patients developed fatal illnesses with a prodrome (such as cancer), which could be responsible for both the participant's death and a reduction in their adherence to study medication. We investigated this possibility in several ways. We reasoned that, if the placebo adherence-mortality association was due to a decline in adherence just prior to a participant's death, then dropping the last one or two adherence measurements (or lagging the adherence variable by one or two visits) should result in substantial attenuation of the association. In fact, these procedures did result in some attenuation in the Treatment Trial, but not in the Prevention Trial results. Why these results differ is not entirely clear. While the mortality was higher in the Treatment Trial, there was sufficient power in the Prevention Trial such that all results retained statistical significance and the widths of the confidence intervals for SOLVD-PT were smaller than for SOLVD-TT. Finally, when we treated adherence as a simple time-dependent covariate, the association and its statistical significance remained essentially unchanged in both trials after lagging the adherence variable (this was the adherence measure that we hypothesized should be the most sensitive to protopathic bias as it is less influenced by prior adherence measurements). Taken together, these analyses suggest that, in the SOLVD trials, there was little support for the potential explanation that the placebo adherence-mortality association is a simple artifact of the presence of some other serious illness, though the inconsistency in the results between the SOLVD Treatment and Prevention studies merit further study in other datasets.
It is noteworthy that we had no prior knowledge of these results and that we resolved a priori to publish these findings regardless of the outcome. Most prior published studies are consistent with our findings, though potential publication bias must be considered. Study of more datasets, as part of this investigation, will be required to more definitively address this possibility.
Prior investigations have found comparable associations between adherence to placebo study medication and all-cause mortality. Following the publication of the original Coronary Drug Project analysis3, similar associations were seen in several other studies4–10. Two trials did not find this association18–20, though the latter was based on only nine arrhythmic deaths. When these data were combined in a meta-analysis, a strong association between placebo adherence and total mortality was observed, with a summary odds ratio = 0.56 (95% CI: 0.43 to 0.74)11. Three studies examined non-mortality endpoints: one did not find associations between placebo adherence and CHD or stroke incidence6, one did observe associations with sudden cardiac death and cardiac mortality9, and another found an association with hospitalization for CHF10.
Several limitations to these analyses should be noted. First, there were few psychosocial variables available for adjustment, and these characteristics may be important determinants of survival21–25, though whether such variables are also associated with adherence, fulfilling the definition of confounding, remains to be determined. Other, potentially important predictors of mortality in patients with CHF, such as exercise and depression, were not available for analysis and, if associated with adherence, may explain some of our findings. While the overall quality of the data was high, 2–3% of individual visit adherence values exceeded our data quality cutoff point and were set to missing. Finally, the lack of longitudinal data on potential confounders made it impossible to adjust for changes in these risk factors during the trials.
The implications of these findings are profound. The improved survival associated with greater adherence to placebo, if validated in other studies, is substantial and clinically meaningful. As noted, the factor(s) associated with this mortality reduction do not appear to be easily identifiable, but these analyses suggest that this survival advantage is not associated with risk factors that are commonly considered to predict survival, including smoking, hyperlipidemia, hypertension, and diabetes. Understanding for what placebo adherence is a marker may shed important light on strong determinants of health that go beyond traditional, well-accepted risk factors. Whether such determinants are intrinsic personal characteristics or represent potentially modifiable behaviors and psychological attributes that could be used to improve patients' health outcomes is a critical issue that deserves greater investigation and understanding.
Acknowledgments
Supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI), no. R01 HL081195.
The Studies of Left Ventricular Dysfunction (SOLVD) was conducted and supported by the NHLBI in collaboration with the SOLVD Study Investigators. This manuscript was prepared using a limited-access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the SOLVD or the NHLBI.
Conflict of Interest
None disclosed.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–30. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303(18):1038-41. [DOI] [PubMed]
- 4.Gallagher EJ, Viscoli CM, Horwitz RI. The relationship of treatment adherence to the risk of death after myocardial infarction in women. JAMA. 1993;270(6):742–4. doi: 10.1001/jama.270.6.742. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336(8714):542–5. doi: 10.1016/0140-6736(90)92095-Y. [DOI] [PubMed] [Google Scholar]
- 6.Glynn RJ, Buring JE, Manson JE, LaMotte F, Hennekens CH. Adherence to aspirin in the prevention of myocardial infarction. The physicians' health study. Arch Intern Med. 1994;154(23):2649–57. doi: 10.1001/archinte.1994.00420230032005. [DOI] [PubMed] [Google Scholar]
- 7.Cornfield J. The university group diabetes program. A further statistical analysis of the mortality findings. JAMA. 1971;217(12):1676–87. doi: 10.1001/jama.217.12.1676. [DOI] [PubMed] [Google Scholar]
- 8.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 9.Irvine J, Baker B, Smith J, Jandciu S, Paquette M, Cairns J, et al. Poor adherence to placebo or amiodarone therapy predicts mortality: Results from the CAMIAT study. arrhythmia trial. Psychosom Med. 1999;61(4):566–75. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–11. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 11.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The SOLVD Investigators Studies of left ventricular dysfunction (SOLVD)–rationale, design and methods: Two trials that evaluate the effect of enalapril in patients with reduced ejection fraction. Am J Cardiol. 1990;66(3):315–22. doi: 10.1016/0002-9149(90)90842-O. [DOI] [PubMed] [Google Scholar]
- 13.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 14.The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 15.National Heart Lung and Blood Institute. NHLBI limited access data sets. National Institutes of Health; 2004. Clin Trials Dec. 2004;1(6):517–24. [DOI] [PubMed]
- 16.Kleinbaum DG, Klein M. Survival analysis. 2. New York: Springer; 2005. [Google Scholar]
- 17.SAS Institute I. SAS statistical software. 2008;9.2.
- 18.The Lipid Research Clinics Program The lipid research clinics coronary primary prevention trial results: I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–64. doi: 10.1001/jama.251.3.351. [DOI] [PubMed] [Google Scholar]
- 19.The Lipid Research Clinics Program The lipid research clinics coronary primary prevention trial results: II. The relationship of reduction in incidence of coronary heart disease of cholesterol lowering. JAMA. 1984;251:365–74. doi: 10.1001/jama.251.3.365. [DOI] [PubMed] [Google Scholar]
- 20.Obias-Manno D, Friedmann E, Brooks MM, Thomas SA, Haakenson C, Morris M, et al. Adherence and arrhythmic mortality in the cardiac arrhythmia suppression trial (CAST) Ann Epidemiol. 1996;6(2):93–101. doi: 10.1016/1047-2797(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: Psychosocial predictors from a 20-year follow-up of women in the Framingham study. Am J Epidemiol. 1992;135(8):854–64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 22.Fry PS, Debats DL. Perfectionism and the five-factor personality traits as predictors of mortality in older adults. J Health Psychol. 2009;14(4):513–24. doi: 10.1177/1359105309103571. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz JE, Friedman HS, Tucker JS, Tomlinson-Keasey C, Wingard DL, Criqui MH. Sociodemographic and psychosocial factors in childhood as predictors of adult mortality. Am J Public Health. 1995;85(9):1237–45. doi: 10.2105/AJPH.85.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (second international study of infarct survival) collaborative group. Lancet. 1988 Aug 13;2(8607):349-60. [PubMed]
- 25.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13,648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364(9438):953–62. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]