Abstract
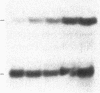
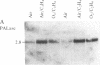
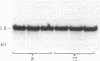
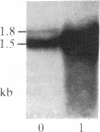
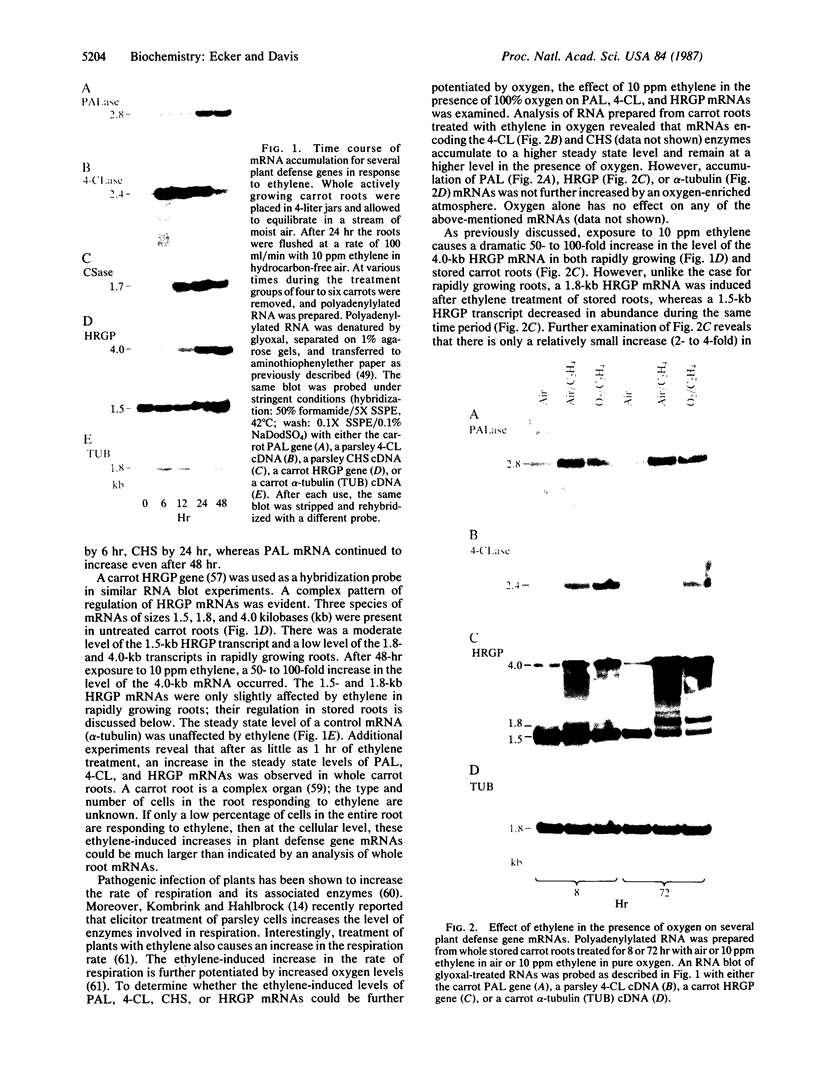
One of the earliest detectable events during plant-pathogen interaction is a rapid increase in ethylene biosynthesis. This gaseous plant stress hormone may be a signal for plants to activate defense mechanisms against invading pathogens such as bacteria, fungi, and viruses. The effect of ethylene on four plant genes involved in three separate plant defense response pathways was examined; these included (i and ii) genes that encode L-phenylalanine ammonia-lyase (EC 4.3.1.5) and 4-coumarate:CoA ligase [4-coumarate:CoA ligase (AMP-forming), EC 6.2.1.12], enzymes of the phenylpropanoid pathway, (iii) the gene encoding chalcone synthase, an enzyme of the flavonoid glycoside pathway, and (iv) the genes encoding hydroxyproline-rich glycoprotein, a major protein component(s) of plant cell walls. Blot hybridization analysis of mRNA from ethylene-treated carrot roots reveals marked increases in the levels of phenylalanine ammonia-lyase mRNA, 4-coumarate CoA ligase mRNA, chalcone synthase mRNA, and certain hydroxyproline-rich glycoprotein transcripts. The effect of ethylene on hydroxyproline-rich glycoprotein mRNA accumulation was different from that of wounding. Ethylene induces two hydroxyproline-rich glycoprotein mRNAs (1.8 and 4.0 kilobases), whereas wounding of carrot root leads to accumulation of an additional hydroxyproline-rich mRNA (1.5 kilobases). These results indicate that at least two distinct signals, ethylene and a wound signal, can affect the expression of plant defense-response genes.
Keywords: stress responses, plant hormone, wounding, RNA blot analysis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Robbins M. P., Dixon R. A. Metabolic changes in elicitor-treated bean cells. Enzymic responses associated with rapid changes in cell wall components. Eur J Biochem. 1985 May 2;148(3):571–578. doi: 10.1111/j.1432-1033.1985.tb08878.x. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen R. E., Laties G. G. Ethylene regulation of gene expression in carrots. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4060–4063. doi: 10.1073/pnas.79.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Mellon J. E., Helgeson J. P. Interaction of a hydroxyproline-rich glycoprotein from tobacco callus with potential pathogens. Plant Physiol. 1982 Aug;70(2):401–405. doi: 10.1104/pp.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols S. E., Laties G. G. Differential control of ethylene-induced gene expression and respiration in carrot roots. Plant Physiol. 1985 Mar;77(3):753–757. doi: 10.1104/pp.77.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions : VI. Elicitors of Ethylene from Colletotrichum lagenarium Trigger Chitinase Activity in Melon Plants. Plant Physiol. 1986 May;81(1):228–233. doi: 10.1104/pp.81.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom J. P., Staehelin L. A. Cross-linking patterns in salt-extractable extensin from carrot cell walls. Plant Physiol. 1986 May;81(1):234–241. doi: 10.1104/pp.81.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Mutagenesis of cultured plant cells. Genetics. 1976 Sep;84(1):51–57. doi: 10.1093/genetics/84.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Potentiating effect of pure oxygen on the enhancement of respiration by ethylene in plant storage organs: a comparative study. Plant Physiol. 1982 May;69(5):1031–1035. doi: 10.1104/pp.69.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C. B., Labavitch J. M., Yang S. F. The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol. 1986 Jul;81(3):929–930. doi: 10.1104/pp.81.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppan A., Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions : IV. Fungal Glycopeptides Which Elicit the Synthesis of Ethylene in Plants. Plant Physiol. 1984 Aug;75(4):1133–1138. doi: 10.1104/pp.75.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppan A., Roby D., Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions : III. In Vivo Effect of Ethylene on Hydroxyproline-Rich Glycoprotein Accumulation in the Cell Wall of Diseased Plants. Plant Physiol. 1982 Jul;70(1):82–86. doi: 10.1104/pp.70.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Laties G. G. Comparative effects of ethylene and cyanide on respiration, polysome prevalence, and gene expression in carrot roots. Plant Physiol. 1984 Jun;75(2):342–348. doi: 10.1104/pp.75.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]