Summary
Background
Lowering of LDL cholesterol reduces major vascular events, but whether more intensive therapy safely produces extra benefits is uncertain. We aimed to establish efficacy and safety of more intensive statin treatment in patients at high cardiovascular risk.
Methods
We undertook a double-blind randomised trial in 12 064 men and women aged 18–80 years with a history of myocardial infarction. Participants were either currently on or had clear indication for statin therapy, and had a total cholesterol concentration of at least 3·5 mmol/L if already on a statin or 4·5 mmol/L if not. Randomisation to either 80 mg or 20 mg simvastatin daily was done centrally using a minimisation algorithm. Participants were assessed at 2, 4, 8, and 12 months after randomisation and then every 6 months until final follow-up. The primary endpoint was major vascular events, defined as coronary death, myocardial infarction, stroke, or arterial revascularisation. Analysis was by intention to treat. This study is registered, number ISRCTN74348595.
Findings
6031 participants were allocated 80 mg simvastatin daily, and 6033 allocated 20 mg simvastatin daily. During a mean follow-up of 6·7 (SD 1·5) years, allocation to 80 mg simvastatin produced an average 0·35 (SE 0·01) mmol/L greater reduction in LDL cholesterol compared with allocation to 20 mg. Major vascular events occurred in 1477 (24·5%) participants allocated 80 mg simvastatin versus 1553 (25·7%) of those allocated 20 mg, corresponding to a 6% proportional reduction (risk ratio 0·94, 95% CI 0·88–1·01; p=0·10). There were no apparent differences in numbers of haemorrhagic strokes (24 [0·4%] vs 25 [0·4%]) or deaths attributed to vascular (565 [9·4%] vs 572 [9·5%]) or non-vascular (399 [6·6%] vs 398 [6·6%]) causes. Compared with two (0·03%) cases of myopathy in patients taking 20 mg simvastatin daily, there were 53 (0·9%) cases in the 80 mg group.
Interpretation
The 6% (SE 3·5%) reduction in major vascular events with a further 0·35 mmol/L reduction in LDL cholesterol in our trial is consistent with previous trials. Myopathy was increased with 80 mg simvastatin daily, but intensive lowering of LDL cholesterol can be achieved safely with other regimens.
Funding
Merck; The Clinical Trial Service Unit also receives funding from the UK Medical Research Council and the British Heart Foundation.
Introduction
LDL cholesterol is an important cause of coronary heart disease. Observational studies indicate a continuous positive association between risk of coronary heart disease and LDL cholesterol concentration that extends throughout, and well below, the range seen in high-income populations.1,2 Taken together, several large randomised trials of statin therapy versus control have shown that lowering of LDL cholesterol reduces risk of occlusive vascular events.3 Benefits were seen even in participants who, before randomisation, had lower-than-average cholesterol concentrations, and the proportional risk reduction was related to the magnitude of the achieved cholesterol reduction.3,4 These findings suggest indirectly that larger reductions in LDL cholesterol would produce larger reductions in the risk of vascular events.
Previously, four randomised trials have directly compared the effects on clinical endpoints of more versus less potent statin regimens.5–8 Collectively, the results of those trials suggest that more intensive lowering of LDL cholesterol produces further reductions in vascular events,9 but concerns remain about the possibility of significant adverse effects.10 Moreover, high doses of particular statins have been associated with increases in liver enzyme concentrations and with increases in the rare but potentially serious side-effect of myopathy.5–8 In the SEARCH trial, we aimed to help establish reliably the balance of efficacy and safety of more intensive LDL-cholesterol-lowering therapy by comparing long-term treatment with 80 mg versus 20 mg simvastatin daily in a large population of patients at high risk of cardiovascular events.
Methods
Patients
The study objectives, design, and methods have been reported previously,11,12 and are summarised here. Men and women aged 18–80 years with a history of previous myocardial infarction were eligible provided they fulfilled the following criteria: either current statin use or clear indication for this treatment (and no clear indication for folic acid); total cholesterol of at least 3·5 mmol/L if already on a statin or 4·5 mmol/L if not; and no clear contraindications to the study treatments.11 Individuals with other predominant medical problems that could reduce compliance with long-term study treatment were also excluded. (As well as comparing different doses of simvastatin, a two-by-two factorial design allowed the separate assessment of folate-based homocysteine-lowering therapy.13)
Medical collaborators from 88 UK hospitals appointed senior nurses to run special clinics for the study (see Acknowledgments). Ethics committee approval was obtained from the South East Thames multicentre research ethics committee, along with local site-specific approval. Computerised hospital records were used to identify patients discharged previously with a diagnosis of myocardial infarction who, with the agreement of their general practitioners, were invited to attend the local study clinic. At the initial screening visit, eligible individuals were given detailed information about the study and asked for their written agreement to participate. Potentially eligible participants entered a prerandomisation run-in phase14 of treatment with 20 mg simvastatin daily (and placebo vitamins); they were instructed to stop taking any non-study statin.
Randomisation and masking
Compliant individuals who did not have a serious problem during the run-in, and agreed to participate, were randomly allocated to receive either 80 mg or 20 mg simvastatin daily with a double-dummy approach to mask the treatment allocation (and separately, in a two-by-two factorial design, 2 mg folic acid plus 1 mg vitamin B12 daily or matching placebo). The central telephone randomisation system used a minimisation algorithm15 to balance the treatment groups with respect to eligibility criteria and other major prognostic factors.11
Procedures
After randomisation between September 1998, and October 2001, participants were to be seen in study clinics at 2, 4, 8, and 12 months and then 6-monthly until final follow-up visits between October 2007, and June 2008. Those who became unable or unwilling to attend were to be contacted by telephone at the time of their scheduled follow-up (or, alternatively, follow-up was to be maintained via their general practitioner), but their allocated study simvastatin had to be stopped since blood safety monitoring could not be continued. Compliance with study treatment was assessed by review of the calendar-packed tablets remaining. For those who had stopped, the reasons for doing so were sought. Participants prescribed a non-study statin by their own doctor had the study simvastatin tablets stopped. To assess the effects of the simvastatin allocation on lipid profile, assays were done in blood from a sample of about 1000 participants each year, from all participants scheduled for follow-up between February and November 2003 (median of 2·5 years), and at all final visits. Differences in lipid concentrations were based on comparisons between all participants allocated 80 mg simvastatin and all allocated 20 mg simvastatin, irrespective of compliance (with any missing data imputed from the current use of study or non-study statin and the lipid concentrations following run-in on 20 mg simvastatin daily).
Information was recorded at each follow-up about any suspected myocardial infarction, stroke, vascular procedure, pulmonary embolus, cancer, or other serious adverse event (including all hospital admissions). Additionally, reports were systematically sought of muscle pain or weakness and of any serious or non-serious adverse events thought likely to be due to study treatment. Further details were sought from participants' general practitioners (plus hospital records when necessary) about reports that might relate to major vascular events or deaths, and from the Medical Research Information Service for England and Wales and the General Register Office for Scotland about the sites of any registered cancers and the certified causes of any deaths. All such information was reviewed by coordinating centre clinicians masked to study treatment allocation, and events coded according to prespecified criteria.11 Analyses were based on confirmed plus unrefuted reports; definite confirmation was available for 93% of non-fatal myocardial infarctions, 89% of non-fatal strokes, and 96% of revascularisations included.
Blood samples were taken at each follow-up visit for central laboratory assays of alanine aminotransferase and creatine kinase concentrations. Persistent increases in alanine aminotransferase concentration to more than three times the upper limit of normal (defined as two consecutive values) resulted in temporary discontinuation of study simvastatin and early follow-up visits according to a prespecified algorithm. Increases of creatine kinase to more than five times the upper limit of normal were followed by a repeat measurement within a week and a diagnosis of myopathy made if the concentration was more than ten times the upper limit of normal plus unexplained muscle symptoms. Rhabdomyolysis (a subset of myopathy) was defined as creatine kinase more than 40 times the upper limit of normal plus evidence of end-organ damage (eg, doubling of plasma creatinine). Patients with confirmed myopathy had their study treatment permanently stopped. During the study, we observed that smaller increases in creatine kinase combined with raised alanine aminotransferase values often occurred before development of myopathy. Hence, incipient myopathy was retrospectively defined as creatine kinase more than both three times the upper limit of normal and five times the participant's baseline value plus alanine aminotransferase more than 1·7 times their baseline value.12
Statistical analysis
The data analysis plan was prespecified either in the original protocol or in amendments made before any analyses of the effects on clinical outcomes were available to the steering committee (see study website and reference 11). In planning the trial, it had been anticipated that allocation to 80 mg versus 20 mg simvastatin daily would produce an average 0·5 mmol/L difference in LDL cholesterol,16,17 which might produce a 10–15% further reduction in the rate of major coronary events (defined as coronary death, myocardial infarction, or coronary revascularisation).18 On the basis of previous studies,18,19 we estimated that about 1900 such events would occur during median follow-up of about 4 years, which would provide 90% power at p<0·01 to detect a 15% risk reduction. The protocol prespecified that the steering committee could modify the study schedule on the basis of interim review of the unmasked cholesterol differences between the treatment groups and of the masked event rates in both treatment groups combined. During median follow-up of 3–4 years, both the LDL cholesterol difference between the treatment groups and the overall major coronary event rate were smaller than had been anticipated. Consequently, in 2004, the steering committee decided to change the primary outcome from major coronary events to major vascular events (defined as coronary death, myocardial infarction, any stroke, or any arterial revascularisation), and to continue until at least 2800 patients had a major vascular event to have 90% power at p<0·05 to detect a 10% risk reduction.11
Secondary outcomes were: major vascular events considered separately in the first year (when little difference was anticipated)3 and in the later years of the scheduled treatment period; major vascular events in participants subdivided into three similar-sized groups with respect to LDL cholesterol concentrations following run-in on 20 mg simvastatin daily (with the hypothesis that a greater LDL cholesterol may produce a greater risk reduction); major vascular events in the presence and the absence of the allocated study vitamins (expecting that the effects would be similar); major coronary events; and any type of stroke (excluding transient ischaemic attacks). Tertiary outcomes included: total and cause-specific mortality (considering deaths from vascular and non-vascular causes separately); vascular mortality excluding the first year after randomisation; coronary and non-coronary revascularisation; confirmed haemorrhagic and other strokes separately; pulmonary embolus; total and site-specific cancers; admissions to hospital for various other causes; and possible adverse effects of treatment (in particular, evidence of liver or muscle abnormalities).
Comparisons involved log-rank analyses of the first occurrence of particular events during the scheduled treatment period among all participants allocated 80 mg simvastatin daily versus all allocated 20 mg simvastatin daily (ie, intention to treat),20,21 except risk ratios for myopathy, which were estimated by Cox regression. Tests for heterogeneity or, if more appropriate, trend were to be used to help to establish whether the proportional effects noted in subcategories differed clearly from overall effects after due allowance for multiple comparisons.20,22 In-house software was used for all analyses.
This study is registered, number ISRCTN74348595.
Role of the funding source
The study was designed, undertaken, analysed, interpreted, and reported by the investigators independently of all funding sources. The writing committee had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
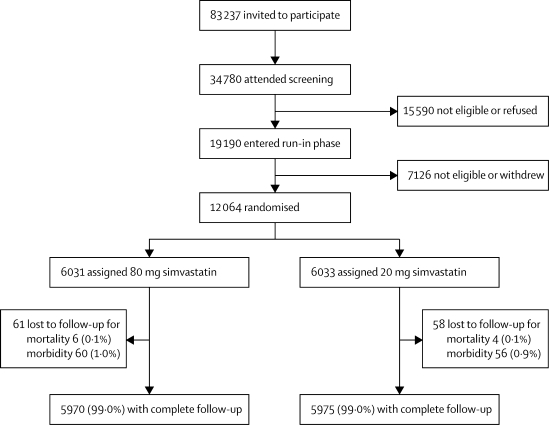
Invitations were sent to 83 237 potentially eligible survivors of myocardial infarction, of whom 34 780 attended the initial screening clinic visit, and 19 190 entered the prerandomisation run-in phase (figure 1). Reasons for withdrawal of the 7126 patients who entered the run-in but were not subsequently randomised have been reported previously;11 none had a serious adverse reaction or was excluded because of increases in liver or muscle enzyme concentrations. 12 064 individuals (10 012 men and 2052 women) with a history of myocardial infarction and an average age of 64·2 (SD 8·9) years, were randomly assigned to treatment groups. Previous coronary revascularisation was reported by 3962 participants (33%), non-coronary revascularisation by 279 (2%), cerebrovascular disease (stroke or transient ischaemic attack) by 837 (7%), diabetes by 1267 (11%), and treated hypertension by 5074 (42%). Nearly three-quarters were taking a statin before study entry: simvastatin, 5240 participants (43%); atorvastatin, 1563 (13%); pravastatin, 969 (8%); cerivastatin, 569 (5%); or fluvastatin, 347 (3%). At the end of the run-in on 20 mg simvastatin daily, mean non-fasting total cholesterol was 4·23 (SD 0·73) mmol/L, directly measured LDL cholesterol was 2·50 (0·61) mmol/L, HDL cholesterol was 1·04 (0·36) mmol/L, apolipoprotein A1 was 1·35 (0·22) g/L, and apolipoprotein B was 0·90 (0·17) g/L. The large study size and use of minimised randomisation produced good balance between the treatment groups.
Figure 1.
Trial profile
Numbers lost to follow-up relate to those without information to the end of the scheduled treatment period for mortality (as well as morbidity) and for morbidity alone.
Mean follow-up duration was 6·7 (SD 1·5) person-years: 40 129 person-years in those allocated 80 mg and 40 158 person-years in those allocated 20 mg simvastatin daily. Compliance was defined as at least 80% of the scheduled simvastatin tablets having been taken since the previous follow-up. Among participants allocated 80 mg simvastatin, 5275 (90%) were compliant after 12 months, and 2555 (77%) after 84 months (table 1). Compliance in patients allocated 20 mg simvastatin was similar after 12 months, but had dropped to 69% by 84 months, with an increasing proportion of patients having started a non-study statin. Table 2 shows that the main reason for discontinuation of study treatment in participants allocated 20 mg simvastatin was medical advice, generally because of a perceived need for more intensive cholesterol-lowering therapy. By contrast, slightly more of the patients allocated 80 mg simvastatin were likely to stop because of raised liver or muscle enzyme concentrations or to have reported muscle pain or weakness.
Table 1.
Compliance with study simvastatin (>80% taken) at scheduled follow-up visits
|
Patients allocated 80 mg simvastatin daily |
Patients allocated 20 mg simvastatin daily |
|||||
|---|---|---|---|---|---|---|
| All | Compliant | Other* | All | Compliant | Other* | |
| 12 months | 5832 | 5275 (90%) | 273 (5%) | 5815 | 5273 (91%) | 296 (5%) |
| 24 months | 5642 | 4939 (88%) | 431 (8%) | 5634 | 4863 (86%) | 528 (9%) |
| 36 months | 5455 | 4666 (86%) | 535 (10%) | 5440 | 4506 (83%) | 718 (13%) |
| 48 months | 5321 | 4425 (83%) | 669 (13%) | 5305 | 4199 (79%) | 917 (17%) |
| 60 months | 5126 | 4160 (81%) | 753 (15%) | 5143 | 3902 (76%) | 1053 (20%) |
| 72 months | 4895 | 3909 (80%) | 790 (16%) | 4901 | 3541 (72%) | 1153 (24%) |
| 84 months | 3325 | 2555 (77%) | 640 (19%) | 3252 | 2243 (69%) | 882 (27%) |
Data are number of patients (%); percentages are proportion of patients at each follow-up.
Non-compliant with study simvastatin, but taking non-study statin.
Table 2.
Reasons for stopping study simvastatin tablets before scheduled end of study
| 80 mg simvastatin daily (n=6031) | 20 mg simvastatin daily (n=6033) | |
|---|---|---|
| Medical advice* | 663 (11·0%) | 1105 (18·3%) |
| Other personal wish† | 736 (12·2%) | 801 (13·3%) |
| Raised liver or muscle enzyme concentrations | 104 (1·7%) | 30 (0·5%) |
| Muscle pain or weakness | 63 (1·0%) | 34 (0·6%) |
| Contraindicated drug started | 19 (0·3%) | 20 (0·3%) |
| Other symptoms | 92 (1·5%) | 100 (1·7%) |
| Other reasons | 106 (1·8%) | 96 (1·6%) |
| Total | 1654 (27·4%) | 2060 (34·1%) |
Data are number of patients (%).
Non-study statin was started in 92% of the participants who stopped because of medical advice.
Other personal wish excludes discontinuations that were also attributed to medical advice.
Table 3 shows the blood lipid differences achieved between participants allocated 80 mg versus 20 mg simvastatin. At 2 months, LDL cholesterol was reduced by 0·51 (SE 0·06) mmol/L more in those allocated 80 mg simvastatin (as originally anticipated), but that difference had decreased to 0·34 (0·02) mmol/L by 84 months (mainly because of increasing non-compliance with the allocated treatment), yielding a weighted average difference during the study of 0·35 (0·01) mmol/L. In parallel, apolipoprotein B concentrations were significantly reduced by a weighted average of 0·087 (0·004) g/L. No significant differences were observed in HDL cholesterol or apolipoprotein A1, but non-fasting triglycerides were reduced by a weighted average of 0·15 (0·02) mmol/L. The intention-to-treat analyses of the effects of treatment allocation on clinical outcomes should be interpreted in light of the achieved 0·35 mmol/L average difference in LDL cholesterol.
Table 3.
Mean differences in plasma concentrations of lipids during follow-up, by allocated treatment
| Total cholesterol (mmol/L) | LDL cholesterol (mmol/L) | HDL cholesterol (mmol/L) | Triglycerides (mmol/L) | Apolipoprotein A1 (g/L) | Apolipoprotein B (g/L) | |
|---|---|---|---|---|---|---|
| 2 months | −0·63 (0·07) | −0·51 (0·06) | −0·02 (0·04) | −0·22 (0·11) | −0·029 (0·022) | −0·127 (0·016) |
| 12 months | −0·45 (0·08) | −0·39 (0·06) | 0·02 (0·04) | −0·18 (0·11) | 0·013 (0·023) | −0·100 (0·018) |
| 24 months | −0·39 (0·03) | −0·34 (0·02) | 0·02 (0·01) | −0·15 (0·04) | 0·031 (0·023) | −0·102 (0·017) |
| 36 months | −0·43 (0·03) | −0·38 (0·03) | 0·04 (0·01) | −0·18 (0·04) | 0·007 (0·021) | −0·090 (0·017) |
| 48 months | −0·37 (0·07) | −0·33 (0·06) | 0·05 (0·03) | −0·17 (0·10) | 0·010 (0·021) | −0·100 (0·018) |
| 60 months | −0·30 (0·07) | −0·29 (0·06) | 0·04 (0·03) | −0·17 (0·08) | 0·011 (0·019) | −0·068 (0·018) |
| 72 months | −0·35 (0·05) | −0·30 (0·05) | −0·00 (0·03) | −0·04 (0·08) | 0·004 (0·017) | −0·073 (0·013) |
| 84 months | −0·39 (0·03) | −0·34 (0·02) | 0·02 (0·02) | −0·17 (0·04) | −0·005 (0·009) | −0·084 (0·007) |
| Average | −0·40 (0·01) | −0·35 (0·01) | 0·02 (0·01) | −0·15 (0·02) | 0·002 (0·005) | −0·087 (0·004) |
Data are mean (SE); differences for the 80 mg simvastatin group minus those for the 20 mg group. Comparison is by intention to treat, with any missing data imputed. At 2, 12, 24, 36, 48, 60, 72, and 84 months, the numbers of patients (including those with imputed values) contributing to total, LDL, and HDL cholesterol and triglycerides were 439, 412, 3337, 2812, 709, 576, 779, and 2924, and to apolipoproteins were 437, 412, 494, 557, 550, 576, 779, and 2924.
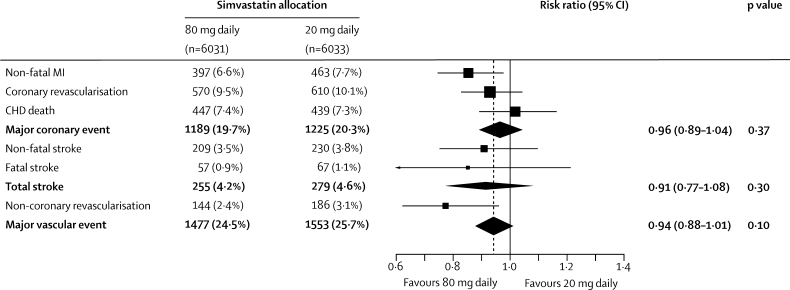
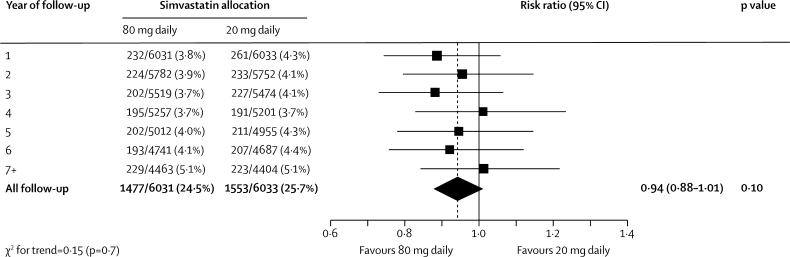
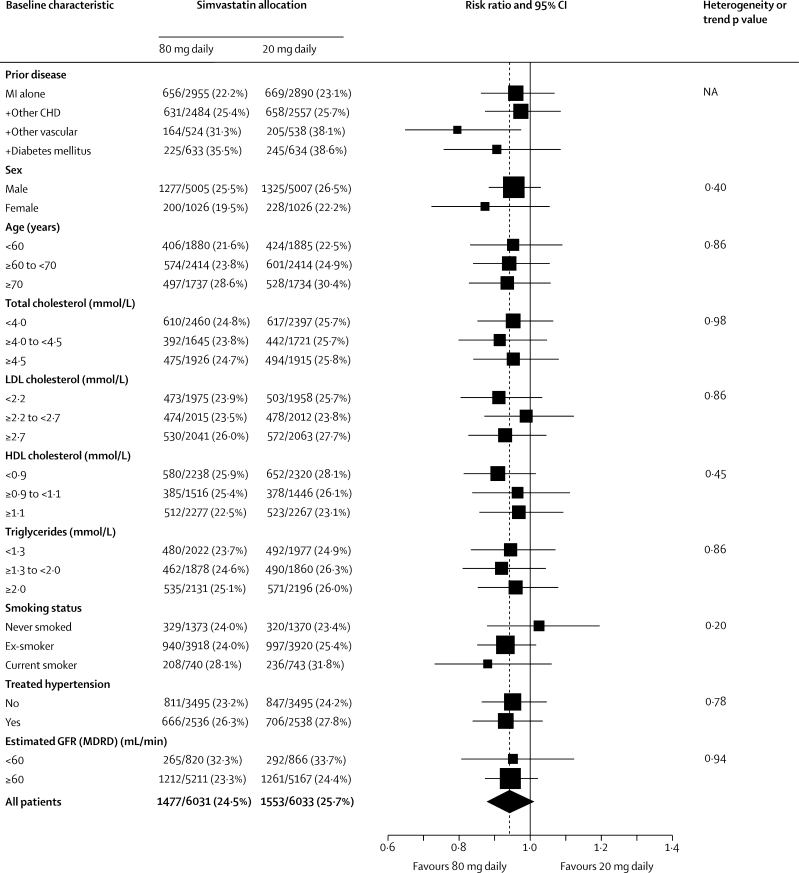
Major vascular events occurred during the scheduled treatment period in 1477 (24·5%) of the 6031 participants allocated 80 mg simvastatin versus 1553 (25·7%) of the 6033 allocated 20 mg simvastatin (risk ratio [RR] 0·94, 95% CI 0·88–1·01; p=0·10; figure 2). This non-significant reduction in risk did not increase significantly with duration of treatment (p value for trend=0·7; figure 3). Among participants in the low, middle, and high thirds of baseline LDL cholesterol (figure 4), allocation to 80 mg simvastatin produced further average reductions in LDL cholesterol of 0·30 (SE 0·02), 0·37 (0·02), and 0·36 (0·02) mmol/L, respectively, but no significant difference between the major vascular event reductions. Nor were there any significant differences in the effects of more intensive lowering of LDL cholesterol on major vascular events in any other subcategories examined (figure 4), including in the presence of the allocated vitamins (11% [SE 4·8] reduction) and in their absence (1% [5·2] reduction; heterogeneity p value=0·15).
Figure 2.
Effects of simvastatin dose allocation on first major vascular event
Analyses are of the numbers of participants having a first event of each type during follow-up (with non-fatal and fatal events considered separately), so there is some non-additivity between different types of event. Risk ratios are plotted comparing outcomes in participants allocated 80 mg simvastatin with those in participants allocated 20 mg simvastatin, along with 95% CIs. The dashed vertical line shows the overall risk ratio. MI=myocardial infarction. CHD=coronary heart disease.
Figure 3.
Effects of simvastatin dose allocation on first major vascular event by year of follow-up
Risk ratios are plotted comparing outcomes in participants allocated 80 mg simvastatin with those in participants allocated 20 mg simvastatin, along with 95% CIs. The dashed vertical line shows the overall risk ratio. Analyses are of numbers of participants having a first event during each year of follow-up and of those still at risk of a first event at the start of each year.
Figure 4.
Effects of simvastatin dose allocation on first major vascular event in different categories of participant
Risk ratios are plotted comparing outcomes in participants allocated 80 mg simvastatin with those in participants allocated 20 mg simvastatin, along with 95% CIs. The dashed vertical line shows the overall risk ratio. p values of χ2 tests are given for heterogeneity between RRs within dichotomous categories and for trend within other categories (except for previous disease categories since there is some overlap between them). Lipid categories relate to measured values at the randomisation visit after all participants had been taking 20 mg simvastatin daily for 2 months during the run-in. MI=myocardial infarction. CHD=coronary heart disease. GFR (MDRD)=glomerular filtration rate estimated with modification of diet in renal disease equation.
Compared with 20 mg simvastatin, allocation to 80 mg simvastatin was associated with proportional reductions in major coronary events of 4% (SE 4), in any stroke of 9% (8), and in non-coronary revascularisations of 23% (10; figure 2). The overall effect on major coronary events reflected the combination of RRs of 0·85 (95% CI 0·75–0·99) for non-fatal myocardial infarction, 0·94 (0·66–1·34) for coronary revascularisation, and 1·02 (0·89–1·16) for coronary death. The effect on any stroke reflected RRs of 0·91 (0·75–1·10) for non-fatal stroke and of 0·85 (0·60–1·21) for fatal stroke, with no apparent effect on definite haemorrhagic stroke (24 [0·4%] vs 25 [0·4%]) and RR of 0·91 (0·77–1·09) for the residual of presumed ischaemic stroke (233 [3·9%] vs 255 [4·2%]). For other vascular outcomes, there were no clear effects on the numbers of patients admitted to hospital for stable or unstable angina (743 [12·3%] allocated 80 mg simvastatin vs 718 [11·9%] allocated 20 mg simvastatin), admission to hospital or died because of heart failure (254 [4·2%] vs 254 [4·2%]), or reported to have had transient cerebral ischaemic attacks (154 [2·6%] vs 151 [2·6%]) or non-fatal or fatal pulmonary emboli (59 [1·0%] vs 53 [0·9%]).
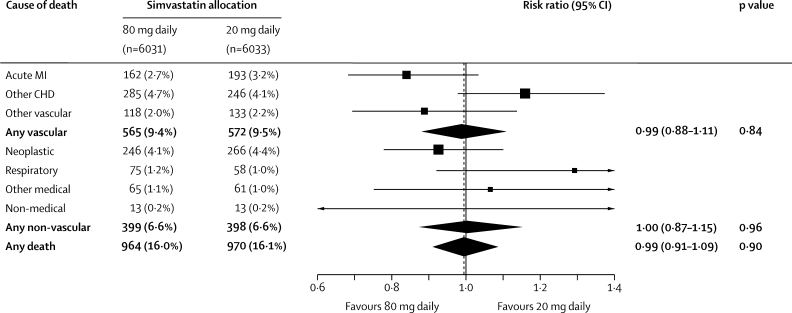
The numbers of deaths attributed to vascular causes (565 [9·4%] vs 572 [9·5%]) or non-vascular causes (399 [6·6%] vs 398 [6·6%]; figure 5) did not differ significantly between the treatment groups. Among the vascular deaths, allocation to more intensive therapy was associated with fewer deaths due to definite acute myocardial infarction (RR 0·84; 95% CI 0·68–1·03), but that difference was not significant and there was no apparent reduction in deaths from all other vascular causes. Among the non-vascular deaths, there were no significant differences in the numbers attributed to neoplastic, respiratory, other medical (including 11 vs four hepatic and three vs seven renal) or non-medical (including one vs two from suicide) causes.
Figure 5.
Effects of simvastatin dose allocation on cause-specific mortality
Risk ratios are plotted comparing outcomes in participants allocated 80 mg simvastatin with those in participants allocated 20 mg simvastatin, along with 95% CIs. The dashed vertical line shows the overall risk ratio. MI=myocardial infarction. CHD=coronary heart disease.
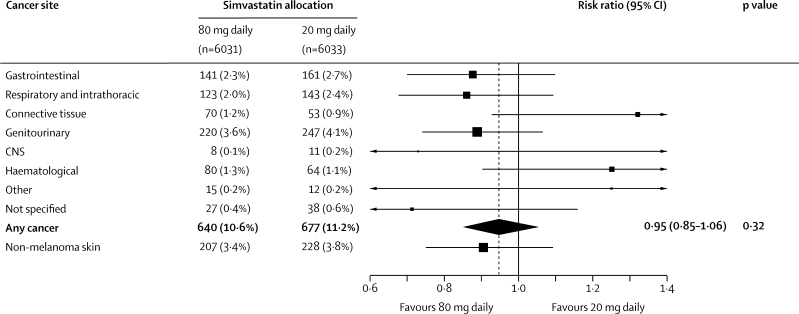
New primary cancers (excluding non-melanoma skin cancer) were diagnosed in 640 (10·6%) participants allocated 80 mg simvastatin versus 677 (11·2%) allocated 20 mg simvastatin (RR 0·95, 95% CI 0·85–1·06; figure 6), and caused death in 246 (4·1%) versus 266 (4·4%) participants (0·93, 0·78–1·10; figure 5). These differences were not significant, and nor were there significant differences between the treatment groups in the incidence of cancers in any particular body system. When cancer sites were more finely divided, a nominally significant excess of female breast cancer was observed (31 [3·0%] vs 17 [1·7%]; along with four vs one male breast cancers), but that difference is based on small numbers of events and was not significant after appropriate allowance was made for multiple comparisons (as prespecified). Moreover, the Cholesterol Treatment Trialists' updated meta-analysis of individual patient data does not indicate any excess risk of female breast cancer in the other trials of statin versus control (205 [1·2%] vs 195 [1·2%]) or of more intensive versus standard statin regimens (42 [1·5%] vs 37 [1·3%]).23
Figure 6.
Effect of simvastatin dose allocation on site-specific cancer incidence
Risk ratios are plotted comparing outcomes in participants allocated 80 mg simvastatin with those in participants allocated 20 mg simvastatin, along with 95% CIs. The dashed vertical line shows the overall risk ratio. Analyses are of the numbers of participants developing cancer at each site (excluding recurrences or new cancers at the same site), so there is some non-additivity between cancers at different sites. Connective tissue includes breast, melanoma, skin, and other connective tissue cancers, but not non-melanoma skin cancer (which was prospectively to be considered separately).
Persistent increases of alanine aminotransferase to four times the upper limit of normal were rare, and no significant difference was found between the treatment groups (14 [0·2%] allocated 80 mg simvastatin vs ten [0·2%] allocated 20 mg simvastatin; p=0·5; table 4). Only eight cases of hepatitis were reported (three vs five); all of these patients recovered, none had serious liver disease, and no deaths from hepatitis were reported. At each of the scheduled follow-up visits, about 7% of the participants reported unexplained muscle pain or weakness, but at no time was there any significant difference between the treatment groups. Similarly, at each scheduled follow-up in the Heart Protection Study of 40 mg simvastatin daily versus placebo, 6–7% of participants in each treatment group reported such symptoms.4 There was, however, a small excess in reports of such symptoms on at least one occasion during follow-up (43·5% allocated 80 mg vs 41·6% allocated 20 mg simvastatin; p=0·05).
Table 4.
Myopathy and raised alanine aminotransferase and creatine kinase concentrations
| 80 mg simvastatin daily (n=6031) | 20 mg simvastatin daily (n=6033) | ||
|---|---|---|---|
| Alanine aminotransferase | |||
| >2 to ≤4 times ULN | 197 (3·3%) | 119 (2·0%) | |
| >4 times ULN | 51 (0·8%) | 40 (0·7%) | |
| >4 times ULN on repeat visits | 14 (0·2%) | 10 (0·2%) | |
| Creatine kinase | |||
| >5 to ≤10 times ULN | 77 (1·3%) | 31 (0·5%) | |
| >10 to ≤40 times ULN * | 45 (0·7%) | 12 (0·2%) | |
| >40 times ULN * | 23 (0·4%) | 0 | |
| Myopathy | |||
| Incipient | 82 (1·4%) | 12 (0·2%) | |
| Definite† | 53 (0·9%) | 2 (0·0%) | |
| No rhabdomyolysis | 46 (0·8%) | 2 (0·0%) | |
| Rhabdomyolysis | 7 (0·1%) | 0 | |
Data are number of patients (%). ULN=upper limit of normal for laboratory.
20 (vs 11) patients with creatine kinase more than ten times ULN were asymptomatic (and so not classified as myopathy).
Five (vs one) of the patients with definite myopathy did not have a recorded measurement of creatine kinase more than ten times ULN, but presented with clinical myopathy.
Few of the participants were ever found to have raised creatine kinase, but there was an excess in those allocated 80 mg simvastatin (table 4). Myopathy was confirmed in 53 (1%) participants allocated 80 mg simvastatin compared with two (0·03%) allocated 20 mg simvastatin (RR 26·6, 95% CI 6·5–109·3; p<0·0001). This excess was highest in the first year after allocation to 80 mg simvastatin daily (four per 1000 person-years) and decreased in subsequent years (one per 1000 person-years). During the study, an increased risk of myopathy (based on eight cases) was found when 80 mg simvastatin daily was used in combination with amiodarone12,24 so, in January, 2003, all patients taking concurrent amiodarone were provided with 20 mg simvastatin daily (irrespective of their original allocation). Incipient myopathy was also detected in 82 (1·4%) participants allocated 80 mg simvastatin compared with 12 (0·2%) allocated 20 mg simvastatin (RR 6·9, 95% CI 3·8–12·6; p<0·0001). Participants with incipient myopathy were generally asymptomatic, and many remained on their study simvastatin; 12 (21%) of the 58 who kept taking the drug (52 allocated 80 mg vs six allocated 20 mg), but only one (3%) of the 36 who stopped (all on 80 mg simvastatin daily), subsequently developed definite myopathy. Consequently, if some of these patients had not stopped their study simvastatin, the rate of definite myopathy would have been somewhat greater than is shown in table 4.
Rhabdomyolysis was diagnosed in seven participants allocated 80 mg simvastatin versus none allocated 20 mg simvastatin. But, for another seven participants with creatine kinase higher than 40 times the upper limit of normal (all on 80 mg simvastatin), no information was available about end-organ damage, so rhabdomyolysis could not be confirmed or refuted. Consequently, the number of confirmed cases might underestimate the incidence of rhabdomyolysis with 80 mg simvastatin daily. All of the patients with myopathy recovered, although ten were admitted to hospital with (or at the time of) myopathy and two died within a few weeks of developing the disorder (one from heart failure, with rhabdomyolysis given as a contributory cause on the death certificate; and one from septicaemia with no mention of rhabdomyolysis).
Rates of admission to hospital for other outcomes did not differ significantly between the treatment groups (even before making allowance for the exploratory nature of such analyses). New diabetes was reported by 633 (10·5%) participants allocated 80 mg simvastatin versus 591 (9·8%) allocated 20 mg simvastatin (RR 1·07, 95% CI 0·96–1·20). Full blood counts were done at randomisation and at 1-year and 4-year follow-up visits, with a significant effect (p<0·0001) seen only for platelet count (219×103 [SD 58] vs 223×103 [59] cells per μL at 4 years). This small difference of 4·5×103 (SE 1·2) per μL did not seem to be clinically significant, with only four versus two reports of thrombocytopenia. Blood pressure at the final follow-up visit did not differ between the treatment groups. Since age-related hearing loss has been associated with vascular risk factors,25–27 hearing thresholds at 1 and 4 kHz were assessed28 in both ears at the final follow-up visit. Hearing thresholds increased with age at both the low and high frequencies, but there was no measurable effect at either frequency of the small additional LDL reduction produced by 80 mg simvastatin daily. Apart from the differences in study treatment discontinuations attributed to the perceived need for more intensive cholesterol-lowering therapy or to muscle problems, there were no significant differences attributed to any other adverse event (191 [3·2%] vs 187 [3·1%]; table 2).
Discussion
In an updated meta-analysis,23 the proportional reduction in the risk of major vascular events per 1 mmol/L LDL cholesterol reduction in the trials of more versus less intensive therapy was similar to that seen in the trials of statin versus control and, in the combined data from all of these trials, the proportional risk reduction was 22% (95% CI 20–24) per 1 mmol/L LDL cholesterol reduction. Although not significant on its own, the 6% (SE 3·5) proportional risk reduction in the SEARCH trial that was associated with a 0·35 mmol/L decrease in LDL cholesterol is compatible with the 7% reduction (ie, one third of 22%) expected on the basis of previous trials. Collectively, these results are consistent with a direct association between the achieved reductions in LDL cholesterol and the proportional reductions in major vascular events. In particular, the proportional reduction in risk per 1 mmol/L reduction in LDL cholesterol seems to be largely independent of the presenting cholesterol concentration. That is, the results show that lowering of LDL cholesterol from 4 mmol/L to 3 mmol/L reduces risk of vascular events by about a fifth, and that lowering it from 3 mmol/L to 2 mmol/L also reduces the (residual) risk by about a fifth.
Before SEARCH, the largest of the randomised trials of more versus less intensive statin therapy assessed the effects of lowering LDL cholesterol from an average of 2·6 mmol/L to an average of 2·0 mmol/L for up to 6 years in 10 001 patients.7 More intensive therapy was associated with a significant 22% (95% CI 11–31) reduction in major vascular events, but concerns were raised by a non-significant excess of non-vascular deaths (158 [3·2%] on 80 mg atorvastatin vs 127 [2·5%] on 10 mg atorvastatin; RR 1·25; 95% CI 0·99–1·57; p=0·07). This adverse trend was not supported by a subsequent randomised comparison of 80 mg atorvastatin versus 20–40 mg simvastatin for 5 years in 8888 patients (143 [3·2%] vs 156 [3·5%] non-vascular deaths; RR 0·92, 95% CI 0·73–1·15; p=0·47).8 In the larger and longer SEARCH trial, LDL cholesterol was reduced from an average of 2·52 mmol/L to an average of 2·17 mmol/L for about 7 years and, again, there were similar numbers of non-vascular deaths in both groups (399 [6·6%] on 80 mg simvastatin vs 398 [6·6%] on 20 mg simvastatin), as well as similar numbers with incident cancer (640 [10·6%] vs 677 [11·2%]). The higher absolute rate of non-vascular mortality in SEARCH reflects both the older age of the participants and the longer study duration.
Randomised trials are not needed to identify large effects on rare outcomes,22 and a higher than expected rate of myopathy was detected early in SEARCH.12 The absolute excess of myopathy was about four per 1000 during the first year of treatment with 80 mg simvastatin daily, and decreased thereafter to about one per 1000 per year,12 with about a fifth developing rhabdomyolysis. About half of these cases were detected as a result of the frequent and systematic monitoring procedures in SEARCH. Such monitoring, and detection at an early stage of myopathy, might have prevented the development of some cases of rhabdomyolysis with severe organ damage. SEARCH allowed the identification of a common variant (estimated population prevalence of 0·15) in the SLCO1B1 gene that was associated with a particularly high risk of myopathy.12 More than 60% of the myopathy cases could be attributed to the C variant at this location in the gene, which affects the coding of the organic anion-transporter polypeptide OAT1B1 responsible for statin uptake into the liver. These findings suggest that, if 80 mg simvastatin daily is to be prescribed, regular blood monitoring should be considered routinely to be able to detect early signs for the development of myopathy unless genetic testing can be used to identify individuals who are at low risk of myopathy.
In the Heart Protection Study,4 average compliance of about two-thirds to allocated treatment with 40 mg simvastatin daily yielded an average reduction in LDL cholesterol of 1·0 mmol/L and a 24% proportional reduction in the risk of major vascular events. We anticipated that allocation to 20 mg simvastatin daily in the SEARCH study population would reduce LDL cholesterol by at least 1·0 mmol/L, and that allocation to 80 mg simvastatin daily would reduce it by a further 0·5 mmol/L. But, during follow-up, LDL cholesterol treatment targets for the management of coronary heart disease were reduced substantially. For example, when recruitment started in 1998, international guidelines generally advocated LDL cholesterol targets of about 3 mmol/L, and the initiation of statin treatment only when the presenting LDL cholesterol was higher than about 2·5 mmol/L.29,30 After publication in 2002 of the Heart Protection Study results,4 which showed similar proportional reductions in risk in patients who presented with LDL cholesterol concentrations lower or higher than 2·5 mmol/L, many guidelines were modified.31,32
By 2005, LDL cholesterol targets had typically fallen to less than 2 mmol/L for the types of high-risk individual included in SEARCH.33 Moreover, the UK Government started to provide financial incentives to encourage the use of high doses of statin therapy for secondary prevention and, in 2008, recommended the use of 80 mg simvastatin daily for intensive LDL-cholesterol-lowering therapy.34 As a consequence, an increasing proportion of SEARCH participants had their allocated study simvastatin stopped and more intensive non-study regimens prescribed by their own doctors. This outcome was somewhat more likely to occur in patients allocated 20 mg simvastatin (presumably because their measured lipid concentrations were higher) than in those allocated 80 mg simvastatin. Consequently, whereas the LDL cholesterol difference was 0·5 mmol/L at 2–4 months after randomisation (when there was good compliance and little non-study statin use), it had fallen to about 0·3 mmol/L by the end of the scheduled treatment period, yielding a study-long average difference of 0·35 mmol/L. Even so, as shown here and in the accompanying paper,23 the results of SEARCH are consistent with previous statin trials and provide supporting evidence for the benefits of intensive LDL-cholesterol-lowering therapy in patients at sufficiently high risk.
After randomisation and long-term follow-up of large numbers of patients in trials of statin versus control and of more versus less intensive statin therapy (including SEARCH), there is clear evidence that lowering of LDL cholesterol concentrations lowers the risk of major vascular events and that intensive lowering of LDL cholesterol reduces risk more. The risk of statin-related myopathy with 20–40 mg simvastatin daily is low (about one per 10 000 patients per year), but is increased about ten times (to about one per 1000 patients per year4,35) with 80 mg simvastatin daily. This excess risk tends to occur during the first year after study treatment (when monitoring could be focused) and is largely confined to people who carry a particular genetic variant (which could be detected before starting treatment). Even so, for patients deemed to be at sufficient risk of major vascular events, a more appropriate strategy (by contrast with current guidance34) could be to consider regimens involving newer, more potent, statins (eg, 80 mg atorvastatin or 20–40 mg rosuvastatin daily) or the combination of standard doses of generic statins (eg, 40 mg simvastatin daily) with other agents that can lower LDL cholesterol substantially without producing such increases in the risk of myopathy.
Acknowledgments
Acknowledgments
We thank the participants in the study and the doctors, nurses, and administrative staff in hospitals and general practices throughout the UK who assisted with its conduct. The study was supported by a grant from Merck (manufacturers of the simvastatin and suppliers of the vitamins), and the Clinical Trial Service Unit also receives core support from the UK Medical Research Council and the British Heart Foundation.
Contributors
Rory Collins and Jane Armitage coordinated and, with Sarah Parish and Richard Peto, designed the study. Karl Wallendszus analysed the data and Louise Bowman, Richard Bulbulia, Richard Haynes, and Kazem Rahimi contributed to running the study, data collection, and interpretation. Jane Armitage and Rory Collins wrote the report and all members of the writing committee critically reviewed it.
SEARCH Study Collaborative Group
Writing committee: Jane Armitage, Louise Bowman, Karl Wallendszus, Richard Bulbulia, Kazem Rahimi, Richard Haynes, Sarah Parish, Richard Peto, Rory Collins.
Steering committee: T Meade and P Sleight (co-chairs), R Collins and J Armitage (principal investigators), L Bowman (clinical coordinator), S Parish and R Peto (statisticians), J Barton, C Bray, E Wincott, R Dayanandan (administrative coordinators), R Clarke, I Graham, D Simpson, C Warlow, D Wilken (expert advisors); and J Tobert and T Musliner (Merck observers).
Data monitoring committee: R Doll (chair; died 2005), L Wilhelmsen (vice-chair and then chair from 2005), K Fox, C Hill, P Sandercock.
Collaborators:
Aberdeen Royal J Webster, J Henderson, A Nixon, S Lackie, J Thompson; Addenbrooke's M Brown, S Blackwood, M Morgan; Barnsley District General W Rhoden (deceased), B Saeed, M Houghton, A Nicholson, C Simpson, B Hoburn; Bedford I Cooper, A Gallivan, E Pickerell, J Hancock, J Watkinson; Birmingham City B Ryder, S Jones, W Burbridge, M Kitchen, H O'Leary, C Verow, L Meynell, L Rollinson; Birmingham Heartlands S Bain, A Jones, C Jewkes, C Russon; Bishop Auckland General M Bateson, P Gill, J Nicol; Bristol Royal G Bayly, D Stansbie, G Andrews, M Halestrap, J Meredith; Burnley General R Best, D Appleyard, R Briggs, H Wareing, K Holmes, J Holt, M Kenyon; Castleford & Normanton C White, M Khalifa, D Newton, A Wass, R Watkinson; City General, Stoke-on-Trent J Creamer, S Anderson, A Bethell, C Butler, K Castro, M Washington, M Weston, K Cleaver, J Machin; Conquest, Hastings R Wray, J Sinclair, A Van Aalst; Coventry and Warwickshire M Been, R Mattu, D Bates, A Burke, L Gill, E Walton; Cumberland M Cowley, H Robson, A Graham, G Rose, M Kerr, J Mallinson, B Peascod; Derbyshire Royal R Donnelly, J Kalk, A Scott, T Gibson, J Hannah, L Henshaw, M Margetts, N Pearson, S Frost, S Murray; Derriford A Marshall, J Went, A Inman, J Simmonds, A Teasdale; Dewsbury T Kemp, G Roberts; Ealing J Kooner, S Cahill, M Lloyd, O Molloy, J Wigley, M Galvin, C Wilder; Edinburgh Royal R Lindley, S Shaw, C Swainson, L Hillis, J Johnston, A Johnstone, D Miller, M Kennedy; Fairfield, Bury S Mushahwar, M Savage, D Appleyard, G Ayer, J Schofield, S Greenhalgh, J Parks, S Speak; Frenchay, Bristol C Coulson, M Papouchado, R Carpenter, J Wisby; Glasgow Royal S Cobbe, C Campbell, J Hunter, H Young, M Gallacher; Gloucestershire Royal D Lindsay, A Halliday, S Godfrey, L O'Donahoo; Guy's & St Thomas' J Chambers, A Wierzbicki, A Jones, D Parkin, K Nwafor; Hairmyres, Glasgow K Oldroyd, B Vallance, N Cunningham, G Moreland, C Oldroyd, H Young, M Crawford; Hillingdon R Hillson, K Knott, N Mahabir, A Crouch, Y MacDonald; HM Stanley, St Asaph C Bellamy, J Green, L Brown, J Heron, N Jones, M Roberts, D Hainsworth, J Williams; Hope Hospital, Salford P Barnes, A Fitchet, C Longworth, J Davidson; Ipswich N Irvine, R Oliver, C Pond, M Nuttall; King's Mill, Sutton-in-Ashfield R Lloyd-Mostyn, M Brown, J Barrowcliffe, S Blackburn, W Furnell, S Webster, L Wheatley; Leicester General I Hudson, J Pohl, S Nicholson; Leighton, Crewe S Mallya, M Nash, J Spruce, A Bonner, J Leather, A Searle; Macclesfield E Davies, R Egdell, B Price, A Taylor Bennett, S Horton; Manor, Walsall A Cunnington, P Giles, J Sidaway, L Tomlinson, E Walton, L Hawkins, J Long; Memorial, Darlington J Murphy, G Brennan, M Boon, S Cassidy; Monklands, Airdrie C Rodger, J Hunter, A McNeilly, G Moreland, A Radcliffe; Monkwearmouth, Sunderland M Farrer, J Bluett, L Cowell, A Farrell, S Gilroy, S Warren; Musgrove Park, Taunton T MacConnell, S Burtchaell, L Williams; New Cross, Wolverhampton P Rylance, A Hodgson, K Kertland-Hill, L Robinson, A Smallwood, S Lomas; Newton Hospital, St Helens J Ball, S Benbow, K Hardy, M Gerrard, C Langley, M Beattie, C Fagan; Ninewells, Dundee B Green, T Pringle (deceased), H Hanna, A Mackintosh, E Watson; North Manchester General J Swan, D Appleyard, D McSorland, G Thompson, C O'Neill; North Tyneside General R Curless, C Doig, P McKenna, J Martin, J Murdy, A Scott, S Martin; Northampton General J Birkhead, J O'Donnell, C Fox, S Dixon, A Hassall, E Tanqueray, D Vass, I Cosford, M Elderkin, P McKenzie; Northern General T Gray, D Appleyard, N Holmshaw, A McKinnon, A Taylor-Bennett, I Ali; Northwick Park N Stephens, A Banfield, L Chester, J Wiseman, N Harrisingh, R Patel, P Thaker; Oxford Radcliffe Hospitals J Armitage, H Watkins, S Beebe, J Fitzgerald, J Godden, A Lawson, H Lochhead, A Taylor, S Turner; Peterborough D Rowlands, A Cooper, J Graham, S Hennessy, T Rashid, C Smith; Pilgrim Hospital, Boston C Nyman, J Adams, A Hardwick, P Buck, C Pattinson, J Trigg; Poole General A McLeod, S Gardner, L Haimes, S Orr, S Johns; Princess Royal, Telford N Capps, A Cook, D Donaldson, C Keighley, C Stiles, S Asbridge; Queen Elizabeth, Birmingham N Buller, J Townend, J Hooks, C Jewkes, H Jones, R Watson, P Salt; Queen Margaret, Dunfermline M Francis, D MacLeod, P Allcoat, R Stuart; Queen's Hospital, Burton-upon-Trent T Reynolds, J Maiden, J Reynolds, D Murray; Raigmore, Inverness R MacFadyen, L Potts, A Smith, L King; Rotherham General R Muthusamy, M Jones, M Lawan, J Nixon, L Wasnidge, C Weston; Royal Bolton A Hutchesson, J Evans, K Morris, M Oultram; Royal Bournemouth M Armitage, R Skule, C Cope, M Page; Royal Cornwall S Fleming, K Andain, M Parrett, R Soper; Royal Devon and Exeter K MacLeod (deceased), K Gordon, E Green, S Havill, V Stewart, S Allen, S Henson, C Rimmer; Royal Gwent J Davies, M Javed, A Norris, M Williams; Royal Preston S Khan, G Dobie, J Fitton, S Gilbert, C Davenport, M Williamson; Royal Sussex County R Vincent, E Joyce; Royal United, Bath J Reckless, A Bishop, L Brice, R Carpenter, P Field, C Shute, S Pope, D Stacey; Russell Institute, Paisley I Findlay, C Campbell, J Hunter, M Gallacher; Russells Hall M Labib, A Hodgson, J Sidaway, L Beddoe, J Reed; St Helier J Barron, O Odemuyiwa, B Bradford, M McDonnell, L West, P Beck; St James University, Leeds S Gilbey, A Clarkson, K Drury, S Hall, D Quartey, B Whittam, D Lund, L Stott; St Luke's, Huddersfield H Griffiths, D Appleyard, J Fitzgerald, A Kudarenko; St Mary's, Portsmouth J Watkins, S Golledge, J Pottle, S Little, B Paine, C Shears; St Peter's, Chertsey M Baxter, P Wilkinson, R Chambers, C Hamper, E Hollister, H Ramsay, J Barber, T Hopkins; Sandwell General L Hughes, J Elson-Whittaker, C Verow, R Lambley-Burke, C Lloyd; Scunthorpe General J Dhawan, J John, D Bramley, A Catchpole, A Colecchia, C Gray, M LeQuelenec, D Remington, J Wiseman, C Gray, P Anderson, R Woolass; Singleton, Swansea P Thomas, C Weston, F Guy, J Lynch, R Thomas, S Coates, M Gait; Southampton University Hospitals D Waller, K Elkins, M Franklin, L Moore; Southlands M Signy, R Chilton, E Joyce, C Wrapson, C Wiltshire; Stepping Hill, Stockport P Lewis, J Curtis, J O'Toole, S Scanlon; Torbay C Carey, L Dobson, M Gould, H Mansfield, G Ranson, M Rodaway, J Germon; Univ Hospital of Wales J Cockcroft, I McDowell, R Field, J Whiting, C Dennison; Victoria Hospital, Blackpool D Roberts, M Cooper, C Davies, J Fitton, L Hutt, L Radford, L Ward, M Williamson; Victoria Infirmary, Glasgow H McAlpine, H Dougall, L Robertson, L Scott, H Young; Walton Centre P Humphrey, S Saminaden, D Watling, J Davies, L Owen; Watford General M Clements, E Walker, E Atkins; Western General, Edinburgh R Lindley, T Shaw, C Swainson, D Webb, H MacCallum, D Markie, V Melville, L Adamson, A Johnston, E Polukord, M Rudden; Whipps Cross J Hogan, F Lie, V Badger, S Duffy, C Mitchell, E MacQueen; Wishaw General R Baxter, S Campbell, L McDonald, H Wood; Worcester Royal A Munro, C Pycock, J Cadwell, A Doughty, M Harvey; Wycombe General S Price, M Aldersley, S Lock, P Pendrey;
Coordinating centre (Clinical Trial Service Unit, University of Oxford):
Administration J Barton, C Bray, R Dayanandan and E Wincott (coordinators), P Achiri, C Anderson, J Benham, H Bojowsky, I Boller, V Booker, A Brewer, G Brindley, L Cobb, A Collett, M Corbett, J Crowther, S Danesh-Pour, K Edmunds, A Fortun, T Grimsey, C Harwood, C Hope, R Jones, S Jones, K Kidney, M King, S Knight, H Lang, Z Madgwick, C Marsden, C Matthews, M Matthewson, J Miller, B Moss, Y Mostefai, K Murphy, A Naughten, S Pickworth, A Radley, S Southren, S Sutherland, R Tong, M Turakani, M Umbrath; Clinical support and adjudication J Armitage, C Baigent, L Bowman, R Bulbulia, Z Chen, R Clarke, R Collins, S Dunachie, T Dasgupta, R Haynes, M Landray, M Mafham, W Majoni, C Murray, K Naessens, T Porter, K Rahimi, C Reith, M Taylor-Clarke, C Turnbull, K Walter; Computing coordinators P Harding, M Lay, K Wallendszus; Statistics and computing C Berry, H Bettesworth, J Booth, M Bowes, Y Bu, A Charles, A Cody, J Cox, J Emberson, N Goodwin, C Hurt, E Link, P McCabe, A Munday, A Murawska, A Offer, A Palmer (deceased), S Parish, R Peto, N Prajapati, S Tochlin, A Young, A Young; Laboratory S Clark, K Kourellias and M Radley (coordinators), V Ambrose, M Bradley, E Bush, T Chavagnon, B Chukwurah, S Crowley, J Dunseath, K Emmens, L Fletcher, J Gordon, A Gordon, C Hickman, J Hill, M Ji, A Lee, N Luker, S Norris, H Priestley, J Sullivan, J Taylor, J Wintour, M Yeung, L Youngman; Nurse monitors and trainers S Beebe, J Fitzgerald, J Godden, L Haimes, A Lawson, H Lochhead, M McDonnell, M Nash, A Taylor, A Taylor-Bennett, E Walton.
Conflicts of interest
The Clinical Trial Service Unit has a staff policy of not accepting honoraria or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings. Members of the writing committee have, therefore, only had such costs reimbursed.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists' Collaboration Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 5.Cannon CP, Braunwald E, McCabe CH. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Blazing MA, Wiviott SD. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z Trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 7.LaRosa JC, Grundy SM, Waters DD. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen TR, Faergeman O, Kastelein JJP. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 10.Peto R, Emberson J, Landray M. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 11.SEARCH Study Collaborative Group Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am Heart J. 2007;154:815–823. doi: 10.1016/j.ahj.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Link E, Parish S, Armitage J. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 13.SEARCH Study Collaborative Group Randomized comparison of homocysteine-lowering with folic acid plus vitamin B12 versus placebo in 12,064 myocardial infarction survivors. JAMA. 2010;303:2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 14.Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run-in on the power of the Physicians' Health Study. Stat Med. 1991;10:1585–1593. doi: 10.1002/sim.4780101010. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 16.Davidson MH, Stein EA, Dujovne CA. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol. 1997;79:38–42. doi: 10.1016/s0002-9149(96)00742-4. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: a reappraisal. Drug Saf. 1996;14:11–24. doi: 10.2165/00002018-199614010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 19.Sacks FM, Pfeffer MA, Moye LA. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 20.Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S, Salvan A. Bias in the one-step method for pooling study results. Stat Med. 1990;9:247–252. doi: 10.1002/sim.4780090307. [DOI] [PubMed] [Google Scholar]
- 22.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357:373–380. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 23.Cholesterol Treatment Trialists' Collaboration Efficacy and safety of intensive LDL-cholesterol-lowering therapy: a meta-analysis of individual data from 170 000 participants in 26 randomised trials. Lancet. 2010 doi: 10.1016/S0140-6736(10)61350-5. published online Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Electronic Medicines Compendium Zocor 10mg, 20mg, 40mg and 80mg film-coated tablets. http://www.medicines.org.uk/EMC/medicine/1201/SPC/zocor (accessed Oct 12, 2010).
- 25.Chang NC, Yu ML, Ho KY, Ho CK. Hyperlipidemia in noise-induced hearing loss. Otolaryngol Head Neck Surg. 2007;137:603–606. doi: 10.1016/j.otohns.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol. 2003;24:382–386. doi: 10.1097/00129492-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149:1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recommended procedures for pure-tone audiometry using a manually operated instrument. Br J Audiol. 1981;15:213–216. doi: 10.3109/03005368109081440. [DOI] [PubMed] [Google Scholar]
- 29.Second Joint Task Force of European and other Societies Prevention of coronary heart disease in clinical practice: Recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Eur Heart J. 1998;19:1434–1503. doi: 10.1053/euhj.1998.1243. [DOI] [PubMed] [Google Scholar]
- 30.Ryan TJ, Antman EM, Brooks NH. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) Circulation. 1999;100:1016–1030. doi: 10.1161/01.cir.100.9.1016. [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM, Cleeman JI, Merz CN. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 32.De Backer G, Ambrosioni E, Borch-Johnsen K. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 33.JBS 2: Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(suppl 5):v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NICE clinical guideline 67 . Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. National Institute for Health and Clinical Excellence; London: 2008. [PubMed] [Google Scholar]
- 35.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]