Summary
Background
Lowering of LDL cholesterol with standard statin regimens reduces the risk of occlusive vascular events in a wide range of individuals. We aimed to assess the safety and efficacy of more intensive lowering of LDL cholesterol with statin therapy.
Methods
We undertook meta-analyses of individual participant data from randomised trials involving at least 1000 participants and at least 2 years' treatment duration of more versus less intensive statin regimens (five trials; 39 612 individuals; median follow-up 5·1 years) and of statin versus control (21 trials; 129 526 individuals; median follow-up 4·8 years). For each type of trial, we calculated not only the average risk reduction, but also the average risk reduction per 1·0 mmol/L LDL cholesterol reduction at 1 year after randomisation.
Findings
In the trials of more versus less intensive statin therapy, the weighted mean further reduction in LDL cholesterol at 1 year was 0·51 mmol/L. Compared with less intensive regimens, more intensive regimens produced a highly significant 15% (95% CI 11–18; p<0·0001) further reduction in major vascular events, consisting of separately significant reductions in coronary death or non-fatal myocardial infarction of 13% (95% CI 7–19; p<0·0001), in coronary revascularisation of 19% (95% CI 15–24; p<0·0001), and in ischaemic stroke of 16% (95% CI 5–26; p=0·005). Per 1·0 mmol/L reduction in LDL cholesterol, these further reductions in risk were similar to the proportional reductions in the trials of statin versus control. When both types of trial were combined, similar proportional reductions in major vascular events per 1·0 mmol/L LDL cholesterol reduction were found in all types of patient studied (rate ratio [RR] 0·78, 95% CI 0·76–0·80; p<0·0001), including those with LDL cholesterol lower than 2 mmol/L on the less intensive or control regimen. Across all 26 trials, all-cause mortality was reduced by 10% per 1·0 mmol/L LDL reduction (RR 0·90, 95% CI 0·87–0·93; p<0·0001), largely reflecting significant reductions in deaths due to coronary heart disease (RR 0·80, 99% CI 0·74–0·87; p<0·0001) and other cardiac causes (RR 0·89, 99% CI 0·81–0·98; p=0·002), with no significant effect on deaths due to stroke (RR 0·96, 95% CI 0·84–1·09; p=0·5) or other vascular causes (RR 0·98, 99% CI 0·81–1·18; p=0·8). No significant effects were observed on deaths due to cancer or other non-vascular causes (RR 0·97, 95% CI 0·92–1·03; p=0·3) or on cancer incidence (RR 1·00, 95% CI 0·96–1·04; p=0·9), even at low LDL cholesterol concentrations.
Interpretation
Further reductions in LDL cholesterol safely produce definite further reductions in the incidence of heart attack, of revascularisation, and of ischaemic stroke, with each 1·0 mmol/L reduction reducing the annual rate of these major vascular events by just over a fifth. There was no evidence of any threshold within the cholesterol range studied, suggesting that reduction of LDL cholesterol by 2–3 mmol/L would reduce risk by about 40–50%.
Funding
UK Medical Research Council, British Heart Foundation, European Community Biomed Programme, Australian National Health and Medical Research Council, and National Heart Foundation.
Introduction
The Cholesterol Treatment Trialists' (CTT) Collaboration previously reported a meta-analysis1 of individual data from 90 000 individuals in 14 randomised trials2–15 of statin therapy versus control. Allocation to the statin regimens in those trials resulted in a weighted mean difference of about 1·0 mmol/L in LDL cholesterol and a proportional reduction of about a fifth in major vascular events (defined as coronary death, non-fatal myocardial infarction, coronary revascularisation, or stroke). Observational studies show that there is a continuous positive relation between coronary disease risk and blood cholesterol concentrations,16–18 so larger reductions in LDL cholesterol might well produce larger reductions in risk. This hypothesis is indirectly supported by the positive association identified in the previous meta-analysis between the absolute reduction in LDL cholesterol in a trial and the proportional reduction in major vascular events in that trial.1
Standard statin regimens (eg, 20–40 mg simvastatin daily) typically reduce LDL cholesterol concentrations by about a third, but regimens involving higher doses or newer, more potent statins (eg, 40–80 mg atorvastatin or 10–20 mg rosuvastatin daily) can halve LDL cholesterol.19–22 To determine whether larger reductions in LDL cholesterol safely produce further reductions in major vascular events, several trials have compared more intensive versus standard statin regimens.23–27 Although their results tend to suggest further benefit,28 only two had significant results for their primary outcome.24,26 The present meta-analysis of individual data from all of these trials assesses the effects of more intensive statin therapy more reliably than before. Several recent trials of statin therapy in patients with renal failure29,30 or chronic heart failure31,32 have not shown clear evidence of benefit, and in our meta-analysis we also investigate those findings. Moreover, we address the question of whether lowering of LDL cholesterol to very low concentrations might have adverse consequences.18,33–35
Methods
Study eligibility and outcomes
The CTT protocol was agreed before the first trial results were available.36 In this second cycle of analyses, we aimed to include all eligible trials reported by the end of 2009. Trials were eligible for inclusion if: the main effect of the intervention was to lower LDL cholesterol; no other differences in risk factor modification were intended; and at least 1000 participants were to be recruited with at least 2 years' scheduled treatment duration.
Prespecified outcomes were cause-specific mortality, major coronary event (coronary death or non-fatal myocardial infarction), coronary revascularisation (angioplasty or bypass grafting), stroke (subdivided by type), and new cancer diagnosis (subdivided by site).36 As in the first cycle of meta-analyses,1 a major vascular event was defined as the first occurrence of any major coronary event, coronary revascularisation, or stroke. In trials in patients with acute coronary syndrome,23,24 many of the revascularisation procedures had been planned before trial entry and happened soon afterwards, so the masked treatment allocation could not affect them. Consequently, only procedures resulting from recurrent ischaemia23 or occurring more than 30 days after randomisation24 (depending on the trial) were included. For the present analyses, cardiac deaths were subdivided into those probably or definitely due to coronary disease and those that might not have been (eg, sudden deaths or deaths attributed to arrhythmia, heart failure, or unspecified cardiac causes). As a result, the 14 trials that were included in the previous report1 now involve slightly fewer major coronary and major vascular events than they did before.
Statistical analysis
Analyses were to include all randomised patients irrespective of whether they received their allocated treatment (intention to treat). The primary meta-analyses were of the effects on disease event rates in each trial calculated as the logrank (o–e) and its variance (v) for first events weighted by the absolute LDL cholesterol difference in that trial at 1 year (d mmol/L),36 and are reported as effects per 1·0 mmol/L reduction in LDL cholesterol. In a meta-analysis of several trials, the log of the rate ratio per mmol/L (log RR) is calculated as S/V with variance 1/V (and hence with 95% CI of S/V±1·96/√V), where S is the sum over all trials of d (o–e) and V is the sum over all trials of d2v. (For unweighted analyses, d is omitted from these formulae.) For most subgroup analyses, the weight for a particular subgroup was the LDL cholesterol difference observed in the whole trial, but analyses by baseline LDL cholesterol concentration used trial- and subgroup-specific LDL weights. In trials comparing more versus less intensive statin therapy, the relevant baseline lipid values would be those achieved on the less intensive regimen. In three of these trials,23–25 however, any statin therapy was stopped before randomisation, so their relevant baseline values had to be estimated by multiplying the values at the randomisation visit (ie, off statin treatment) by the mean proportional reduction observed at 1 year among those allocated the less intensive regimen. Proportional risk reductions in different subgroups were compared by standard χ2 tests for heterogeneity or, where appropriate, trend. To help to allow for multiple subdivisions, only summary rate ratios have 95% CIs; all other rate ratios have 99% CIs. Analyses were done with SAS version 9.1 (SAS Institute, Cary) and R version 2.11.1 (www.R-project.org).
Role of the funding sources
The funding sources had no involvement in study design, data collection, analysis, interpretation, report writing or publication. The writing committee had full access to all data and accepts full responsibility for the content of this report.
Results
For the meta-analyses of more versus less intensive statin therapy, individual participant data were available from all five eligible trials: two23,24 in 8659 patients with acute coronary syndrome and three in 30 953 patients with stable coronary disease25–27 (table; webappendix pp 1 and 2). Overall, among the 39 612 participants in these five trials, the weighted mean baseline LDL cholesterol concentration was estimated to be 2·53 mmol/L, the weighted mean difference at one year was 0·51 mmol/L, and the weighted median follow-up duration among survivors was 5·1 years (2·1 years for patients with acute coronary syndrome and 5·8 years for those with stable disease).
Table.
Baseline characteristics and eligibility criteria of participating trials
| Number of patients | Treatment comparison (mg per day) | Median follow–up in survivors (years)* | Baseline LDL-C (mmol/L) | LDL-C difference at 1 year (mmol/L) | Women (%) | Diabetes (%) | Prior CHD (%) | Other vascular disease (%)† | No prior vascular disease (%)‡ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| More versus less statin | |||||||||||
| PROVE–IT | 4162 | A80 vs P40 | 2·1 | 2·62§ | −0·65 | 911 (22%) | 734 (18%) | 4162 (100%) | 328 (8%) | 0 | |
| A to Z | 4497 | S40 then S80 vs placebo then S20 | 2·0 | 2·09§ | −0·30 | 1100 (24%) | 1059 (24%) | 4497 (100%) | 479 (11%) | 0 | |
| TNT | 10 001 | A80 vs A10 | 5·0 | 2·52 | −0·62 | 1902 (19%) | 1501 (15%) | 10 001 (100%) | 1537 (15%) | 0 | |
| IDEAL | 8888 | A40–80 vs S20–40 | 4·8 | 2·64§ | −0·55 | 1702 (19%) | 1069 (12%) | 8888 (100%) | 971 (11%) | 0 | |
| SEARCH | 12 064 | S80 vs S20 | 7·0 | 2·50 | −0·39 | 2052 (17%) | 1267 (11%) | 12 064 (100%) | 1062 (9%) | 0 | |
| Subtotal (5 trials) | 39 612 | NA | 5·1‖ | 2·53‖ | −0·51‖ | 7667 (19%) | 5630 (14%) | 39 612 (100%) | 4377 (11%) | 0 | |
| Statin versus control | |||||||||||
| SSSS | 4444 | S20–40 vs placebo | 5·4 | 4·88 | −1·77 | 827 (19%) | 202 (5%) | 4444 (100%) | 126 (3%) | 0 | |
| WOSCOPS | 6595 | P40 vs placebo | 4·8 | 4·96 | −1·07 | 0 | 76 (1%) | 338 (5%) | 193 (3%) | 6096 (92%) | |
| CARE | 4159 | P40 vs placebo | 5·0 | 3·58 | −1·03 | 576 (14%) | 586 (14%) | 4159 (100%) | 0 | 0 | |
| Post–CABG | 1351 | L40–80 vs L2·5–5 | 4·3 | 4·02 | −1·07 | 102 (8%) | 116 (9%) | 1351 (100%) | 37 (3%) | 0 | |
| AFCAPS/TexCAPS | 6605 | L20–40 vs placebo | 5·2 | 3·89 | −0·94 | 997 (15%) | 155 (2%) | 10 (<1%) | 9 (<1%) | 6586 (>99%) | |
| LIPID | 9014 | P40 vs placebo | 6·0 | 3·88 | −1·03 | 1516 (17%) | 782 (9%) | 9014 (100%) | 905 (10%) | 0 | |
| GISSI–P | 4271 | P20 vs no treatment | 2·0 | 3·92 | −0·35 | 587 (14%) | 582 (14%) | 4271 (100%) | 179 (4%) | 0 | |
| LIPS | 1677 | F80 vs placebo | 3·9 | 3·42 | −0·92 | 271 (16%) | 202 (12%) | 1677 (100%) | 142 (8%) | 0 | |
| HPS | 20 536 | S40 vs placebo | 5·4 | 3·38 | −1·29 | 5082 (25%) | 5963 (29%) | 13 386 (65%) | 8865 (43%) | 3161 (15%) | |
| PROSPER | 5804 | P40 vs placebo | 3·3 | 3·79 | −1·04 | 3000 (52%) | 623 (11%) | 1881 (32%) | 1026 (18%) | 3254 (56%) | |
| ALLHAT–LLT | 10 355 | P40 vs usual care | 4·9 | 3·76 | −0·54 | 5051 (49%) | 3638 (35%) | 1188 (11%) | 1788 (17%) | 8037 (78%) | |
| ASCOT–LLA | 10 305 | A10 vs placebo | 3·3 | 3·44 | −1·07 | 1942 (19%) | 2527 (25%) | 15 (<1%) | 1435 (14%) | 8860 (86%) | |
| ALERT | 2102 | F40 vs placebo | 5·5 | 4·14 | −0·84 | 715 (34%) | 396 (19%) | 400 (19%) | 241 (11%) | 1702 (81%) | |
| CARDS | 2838 | A10 vs placebo | 4·1 | 3·03 | −1·14 | 909 (32%) | 2838 (100%) | 9 (<1%) | 97 (3%) | 2738 (96%) | |
| ALLIANCE** | 2442 | A10–80 vs usual care | 4·7 | 3·80 | −1·16 | 434 (18%) | 540 (22%) | 2442 (100%) | 162 (7%) | 0 | |
| 4D** | 1255 | A20 vs placebo | 4·0 | 3·25 | −0·89 | 578 (46%) | 1255 (100%) | 630 (50%) | 666 (53%) | 344 (27%) | |
| ASPEN** | 2410 | A10 vs placebo | 4·0 | 2·93 | −0·99 | 811 (34%) | 2410 (100%) | 578 (24%) | 302 (13%) | 1663 (69%) | |
| MEGA**†† | 8214 | P10–20 vs usual care | 5·0 | 4·05 | −0·67 | 5547 (68%) | 1686 (21%) | 42 (<1%) | 53 (<1%) | 8119 (99%) | |
| JUPITER** | 17 802 | R20 vs placebo | 2·0 | 2·70 | −1·09 | 6801 (38%) | 76 (<1%) | 0 | 0 | 17 802 (100%) | |
| GISSI–HF** | 4574 | R10 vs placebo | 4·2 | 3·06 | −0·92 | 1032 (23%) | 1196 (26%) | 1797 (39%) | 4574 (100%) | 0 | |
| AURORA** | 2773 | R10 vs placebo | 4·6 | 2·58 | −0·99 | 1050 (38%) | 731 (26%) | 659 (24%) | 743 (27%) | 1663 (60%) | |
| Subtotal (21 trials) | 129 526 | NA | 4·8‖ | 3·70‖ | −1·07‖ | 37 828 (29%) | 26 580 (21%) | 48 291 (37%) | 21 543 (17%) | 70 025 (54%) | |
| Total (26 trials) | 169 138 | NA | 4·9‖ | NA | NA | 45 495 (27%) | 32 210 (19%) | 87 903 (52%) | 25 920 (15%) | 70 025 (41%) | |
LDL-C=LDL-cholesterol. CHD=coronary heart disease. PROVE-IT=Pravastatin or Atorvastatin Evaluation and Infection Therapy.24 A=atorvastatin. P=pravastatin. A to Z=Aggrastat to Zocor.23 S=simvastatin. TNT=Treating to New Targets.26 IDEAL=Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group.25 SEARCH=Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine.27 SSSS=Scandinavian Simvastatin Survival Study.2 WOSCOPS=West of Scotland Coronary Prevention Study.3 CARE=Cholesterol And Recurrent Events.4 Post-CABG=Post-Coronary Artery Bypass Graft.5 L=lovastatin. AFCAPS/TexCAPS=Air Force/Texas Coronary Atherosclerosis Prevention Study.6 LIPID=Long–term Intervention with Pravastatin in Ischaemic Disease.7 GISSI–P=Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico.8 LIPS=Lescol Intervention Prevention Study.9 F=fluvastatin. HPS=Heart Protection Study.10 PROSPER=PROspective Study of Pravastatin in the Elderly at Risk.11 ALLHAT-LLT=Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial.12 ASCOT-LLA=Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm.13 ALERT=Assessment of Lescol in Renal Transplantation.14 CARDS=Collaborative Atorvastatin Diabetes Study.15 ALLIANCE=Aggressive Lipid-Lowering Initiation Abates New Cardiac Events.39 4D=Die Deutsche Diabetes Dialyse Studie.29 ASPEN=Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus.40 MEGA=Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group.37 JUPITER=Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin study group.38 R=rosuvastatin. GISSI-HF=Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza cardiac.31 AURORA=A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events.30
Estimated with standard Kaplan-Meier methods, with patients censored at their date of death.
History of intracerebral bleed, transient ischaemic attack, ischaemic stroke, unknown stroke, peripheral artery disease, or heart failure (if known).
No known history of CHD or other vascular disease.
These three trials did not have active run–in periods; the values shown are the estimated on-treatment LDL cholesterol levels in the standard statin group.
Median follow–up, baseline LDL-C, and LDL-C difference at 1 year weighted by trial–specific variances of observed logrank (o–e) for major vascular events.
Additional statin versus control trials included in this second cycle of analyses.
Includes 382 randomised patients who were excluded from the original publication.37
The previous CTT meta-analysis of statin therapy versus control involved 14 trials in 90 056 participants.1 For this second cycle, individual participant data were available from seven more trials29–31,37–40 of statin versus control among 39 470 participants: two in primary prevention,37,38 two in haemodialysis patients,29,30 and one each in patients with coronary disease,39 diabetes,40 and heart failure31 (table; webappendix pp 1 and 2). Overall, among the 129 526 participants in these 21 trials, the weighted mean baseline LDL cholesterol concentration was 3·70 mmol/L, the weighted mean difference at 1 year was 1·07 mmol/L, and the weighted median follow-up duration in survivors was 4·8 years. Individual participant data were unavailable from three eligible trials involving 11 342 patients: CORONA,32 SPARCL,33 and GREACE.41
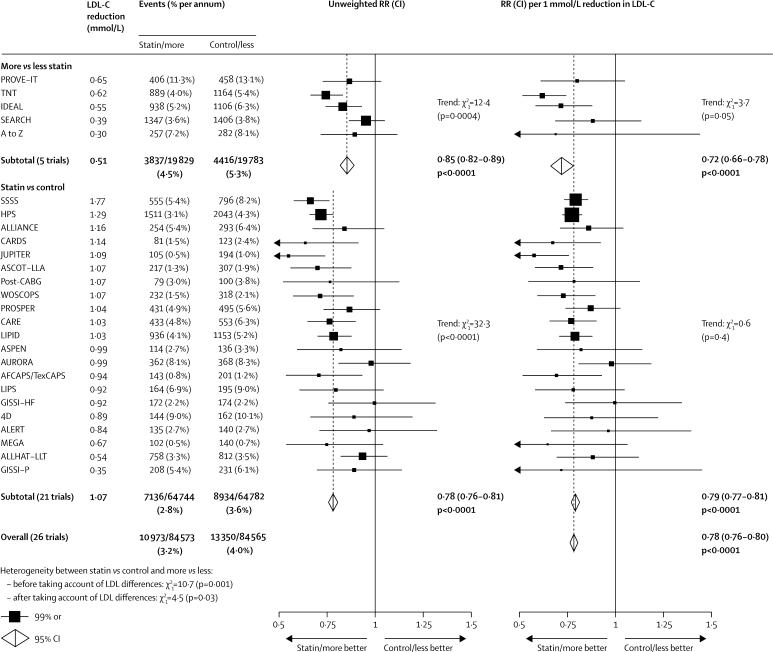
First major vascular events were recorded in the five trials of more versus less intensive statin therapy in 3837 (4·5% per annum) of 19 829 participants allocated more intensive versus 4416 (5·3% per annum) of 19 783 allocated less intensive therapy (figure 1), corresponding to a highly significant further proportional risk reduction of 15% (95% CI 11–18; p<0·0001) associated with the mean 0·51 mmol/L further LDL cholesterol reduction. In comparisons between these five trials, larger absolute reductions in LDL cholesterol were associated with larger proportional risk reductions (trend p=0·0004), but there was little residual variation after adjustment for LDL cholesterol differences (trend p=0·05). Overall, the weighted average further reduction in first major vascular events was 28% (95% CI 22–34; p<0·0001) per 1·0 mmol/L reduction in LDL cholesterol (figure 1), with separately significant reductions in each of the major components of this composite outcome (figure 2).
Figure 1.
Effects on any major vascular event in each study
In the left panel, unweighted rate ratios (RRs) for each trial of the comparison of first event rates between randomly allocated treatment groups are plotted along with 99% CIs. Trials are ordered according to the absolute reduction in LDL cholesterol (LDL-C) at 1 year within each type of trial comparison (more vs less statin and statin vs control). In the right panel, rate ratios are weighted per 1·0 mmol/L LDL cholesterol difference at 1 year. Subtotals and totals with 95% CIs are shown by open diamonds.
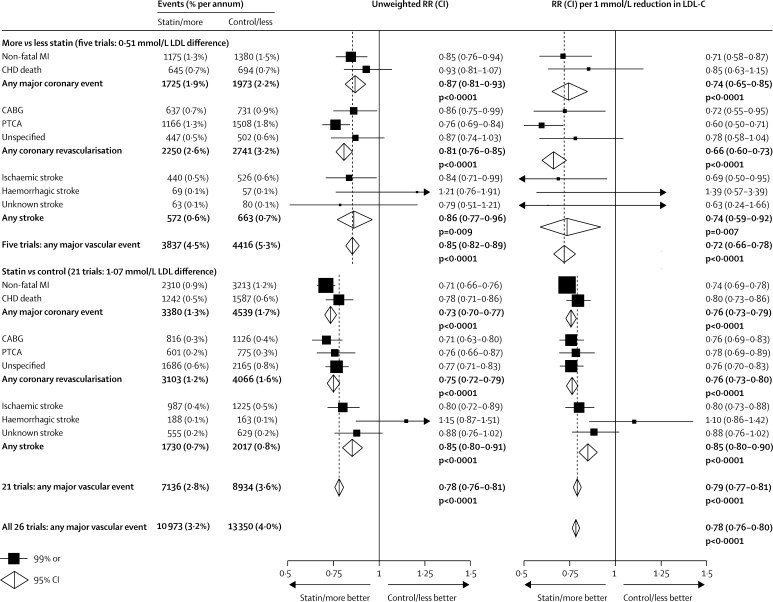
Figure 2.
Effects on each type of major vascular event
In the left panel, unweighted rate ratios (RRs) are plotted for each comparison of first event rates between randomly allocated treatment groups. In the right panel, RRs are weighted per 1·0 mmol/L LDL cholesterol (LDL-C) difference at 1 year. RRs are shown with horizontal lines denoting 99% CIs or with open diamonds denoting 95% CIs. MI=myocardial infarction. CHD=coronary heart disease. CABG=coronary artery bypass graft. PTCA=percutaneous transluminal coronary angioplasty.
In the updated meta-analysis of 21 trials of statin versus control, 7136 (2·8% per annum) of 64 744 participants allocated statin therapy had first major vascular events versus 8934 (3·6% per annum) of 64 782 allocated control (figure 1), corresponding to a highly significant 22% (95% CI 19–24; p<0·0001) risk reduction with a 1·07 mmol/L LDL cholesterol reduction. In comparisons between these 21 trials, larger absolute reductions in LDL cholesterol were associated with larger proportional reductions in risk (trend p<0·0001), but no significant residual variation remained after adjustment for LDL cholesterol differences (trend p=0·4). Overall, the weighted average reduction in major vascular events was 21% (95% CI 19–23; p<0·0001) per 1·0 mmol/L reduction in LDL cholesterol (figures 1 and 2).
After differences in the absolute reductions in LDL cholesterol were accounted for, the proportional reduction in the incidence of major vascular events per mmol/L was slightly larger (heterogeneity p=0·03; figure 1) in the trials of more versus less intensive therapy than in those of statin versus control. Taking all 26 trials together, the risk reduction was 22% (95% CI 20–24; p<0·0001) per 1·0 mmol/L reduction in LDL cholesterol at 1 year, with a significant 12% reduction during the first year after randomisation (p<0·0001) and highly significant reductions of about a quarter during each subsequent year (all p<0·0001; webappendix p 3).
First major coronary events were recorded in the five trials of more versus less intensive statin therapy in 1725 (1·9% per annum) participants allocated more intensive versus 1973 (2·2% per annum) allocated less intensive therapy (figure 2). This highly significant further risk reduction of 13% (95% CI 7–19; p<0·0001) represented a significant reduction in non-fatal myocardial infarction of 15% (99% CI 6–24; p<0·0001) and a non-significant reduction in coronary death of 7% (p=0·2). The proportional reduction in the incidence of major coronary events per 1·0 mmol/L LDL cholesterol reduction was similar (heterogeneity p=0·8; figure 2; webappendix p 4) in the trials of more versus less intensive therapy (26% reduction, 95% CI 15–35) and in those of statin versus control (24%, 95% CI 21–27). Taking all 26 trials together, the risk reduction was 24% (95% CI 22–27; p<0·0001) per 1·0 mmol/L reduction in LDL cholesterol, with highly significant reductions in non-fatal myocardial infarction of 27% (95% CI 23–30; p<0·0001; webappendix p 5) and in coronary death of 20% (95% CI 15–25; p<0·0001; webappendix p 6).
First coronary revascularisation procedures were recorded in the five trials of more versus less intensive statin therapy in 2250 (2·6% per annum) participants allocated more intensive versus 2741 (3·2% per annum) allocated less intensive therapy (figure 2). This highly significant further risk reduction of 19% (95% CI 15–24; p<0·0001) represented significant reductions in coronary artery surgery of 14% (99% CI 1–25; p=0·005) and in coronary angioplasty of 24% (99% CI 16–31; p<0·0001). The proportional reduction in the incidence of coronary revascularisation per 1·0 mmol/L reduction in LDL cholesterol was significantly larger (heterogeneity p=0·01; figure 2; webappendix p 7) in the trials of more versus less intensive therapy (34% reduction, 95% CI 27–40) than in those of statin versus control (24%, 95% CI 20–27). This significant heterogeneity reflected a larger effect on coronary angioplasty and accounted for the observed difference between these groups of trials in the proportional reduction in major vascular events. Taking all 26 trials together, the risk reduction was 25% (95% CI 22–28; p<0·0001; webappendix p 7) per 1·0 mmol/L reduction in LDL cholesterol, with similar reductions in coronary artery surgery (25%, 99% CI 18–31) and in coronary angioplasty (28%, 99% CI 20–35).
First strokes of any type were recorded in the five trials of more versus less intensive statin therapy in 572 (0·6% per annum) participants allocated more intensive versus 663 (0·7% per annum) allocated less intensive therapy (figure 2). This significant further risk reduction of 14% (95% CI 4–23; p=0·009) represented a 16% (99% CI 1–29) reduction in the risk of ischaemic stroke (440 vs 526; risk ratio [RR] 0·84, 99% CI 0·71–0·99; p=0·005) and a non-significant excess of haemorrhagic stroke (69 vs 57; RR 1·21, 99% CI 0·76–1·91; p=0·3). The proportional reduction in the incidence of stroke per 1·0 mmol/L LDL cholesterol reduction was non-significantly larger (heterogeneity p=0·2; figure 2; webappendix p 8) in the trials of more versus less intensive statin therapy (26% reduction, 95% CI 8–41) than in those of statin versus control (15% reduction, 95% CI 10–20). Taking all 26 trials together, the risk reduction was 16% (95% CI 11–21; p<0·0001; webappendix p 8) per 1·0 mmol/L LDL cholesterol reduction, with a highly significant reduction in ischaemic stroke (1427 vs 1751; RR 0·79, 95% CI 0·74–0·85; p<0·0001; webappendix p 9) and a non-significant excess of haemorrhagic stroke (257 vs 220; RR 1·12, 95% CI 0·93–1·35; p=0·2; webappendix p 10).
The outcome of first stroke after randomisation was available from 24 of the 26 trials, with 728 (15%) of 4948 first strokes classified as fatal and a further 256 stroke deaths reported (253 after non-fatal first strokes and three in a trial9 without stroke incidence data). Overall, there was no significant effect on mortality from stroke (483 statin/more statin vs 501 control/less statin; RR 0·96, 95% CI 0·84–1·09; p=0·5), on mortality from first stroke (369 vs 359), or on mortality from first ischaemic (136 vs 124) or first haemorrhagic (94 vs 75) stroke. Likewise, there was no significant effect on the incidence of first non-fatal haemorrhagic stroke (163 vs 145; RR 1·05, 99% CI 0·77–1·43; p=0·7). There was, however, a highly significant reduction in first non-fatal ischaemic stroke (1291 vs 1627), corresponding to a 23% (99% CI 15–30; p<0·0001) reduction per 1·0 mmol/L reduction in LDL cholesterol.
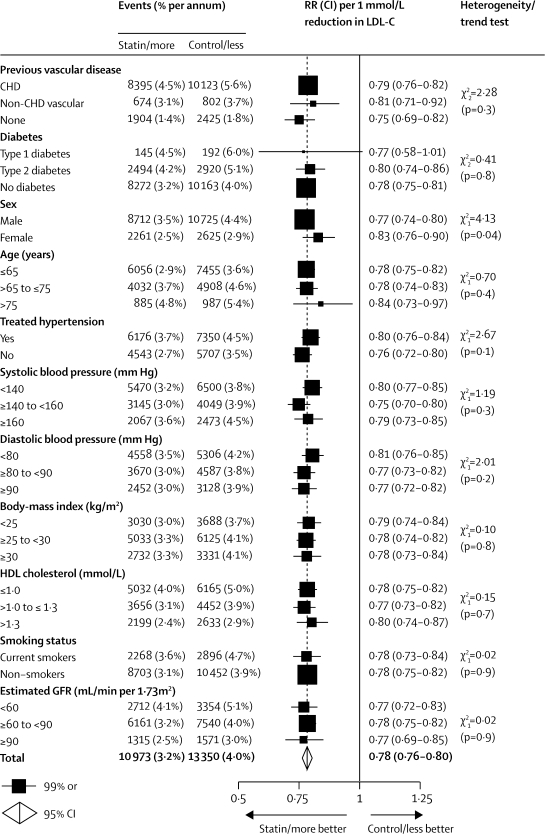
First major vascular events were reduced by about a fifth per 1·0 mmol/L LDL cholesterol reduction in each subgroup examined in the five trials of more versus less intensive statin therapy (webappendix p 11), in the 21 trials of statin versus control (webappendix p 12), and in all 26 trials combined (figure 3), even though the annual event rates in control groups differed substantially according to participants' medical history and other characteristics. In particular, there was a highly significant proportional risk reduction of 25% (99% CI 18–31; p<0·0001) per 1·0 mmol/L reduction in LDL cholesterol in participants with no previous history of vascular disease, as well as significant reductions of 17% (99% CI 10–24; p<0·0001) among women and of 16% (99% CI 3–27; p=0·002) in people older than 75 years at entry (figure 3).
Figure 3.
Effects on major vascular events per 1·0 mmol/L reduction in LDL cholesterol, by baseline prognostic factors
Rate ratios (RRs) are plotted for each comparison of first event rates between treatment groups, and are weighted per 1·0 mmol/L LDL cholesterol (LDL-C) difference at 1 year. Missing data are not plotted. RRs are shown with horizontal lines denoting 99% CIs or with open diamonds showing 95% CIs. CHD=coronary heart disease. GFR=glomerular filtration rate.
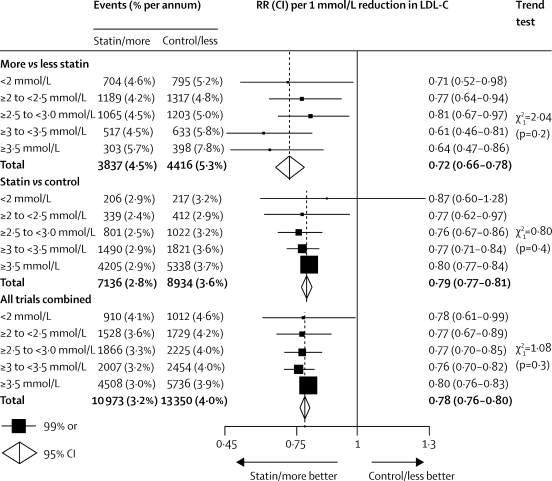
Baseline LDL cholesterol concentrations were substantially higher in the trials of statin versus control (3·70 mmol/L on no statin) than in those of more versus less intensive therapy (2·53 mmol/L on the less intensive regimen), so the latter group provides most of the information about the effects of reducing LDL cholesterol concentrations that were already low (eg, less than 2·5 mmol/L; figure 4). In these trials of more versus less statin, the RR per 1·0 mmol/L further reduction in LDL cholesterol did not depend on the baseline LDL cholesterol concentration (trend p=0·2; figure 4), with significant reductions of 23% (99% CI 6–36; p=0·0005) in participants who had LDL cholesterol of 2·0–2·5 mmol/L reduced further and of 29% (99% CI 2–48; p=0·007) in those who had LDL cholesterol lower than 2·0 mmol/L (mean 1·71 mmol/L) reduced further. Indeed, even among those reaching 1·8 mmol/L (70 mg/dL) or lower with a standard statin regimen, further reduction yielded definite benefit (RR 0·63, 99% CI 0·41–0·95; p=0·004; not shown separately in figure 4).
Figure 4.
Effects on major vascular events per 1·0 mmol/L reduction in LDL cholesterol, by baseline LDL cholesterol concentration on the less intensive or control regimen
Rate ratios (RRs) are plotted for each comparison of first event rates between treatment groups, and are weighted per 1·0 mmol/L LDL cholesterol (LDL-C) difference at 1 year. Analyses were done with trial-specific and subgroup-specific LDL weights for each baseline LDL cholesterol category. Missing data are not plotted. RRs are shown with horizontal lines denoting 99% CIs or with open diamonds showing 95% CIs.
Some have suggested that HDL cholesterol concentrations might not be inversely associated with vascular disease risk when LDL cholesterol is reduced intensively42 (which would imply that the risk reduction with statin therapy is smaller in people with higher HDL cholesterol). But, this hypothesis was not supported by comparisons of the major vascular event risks in baseline HDL cholesterol subgroups (figure 3; webappendix pp 11 and 12). In particular, after adjustment for other risk factors, the risk ratio for upper versus lower tertiles of HDL cholesterol in participants allocated more intensive statin therapy (RR 0·81, 95% CI 0·74–0·89) was similar to that in those allocated less intensive therapy (RR 0·84, 95% CI 0·77–0·92).
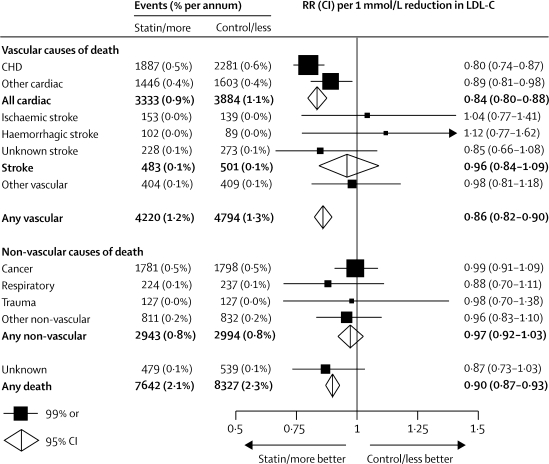
Death was recorded for 3593 participants in the five trials of more versus less intensive statin therapy and for 12 376 in the 21 trials of statin versus control, yielding a total of 15 969 deaths in all 26 trials. Overall, 9014 (56%) of these deaths were attributed to vascular causes (4168 coronary, 3049 other cardiac, 984 stroke, 813 other vascular), 5937 (37%) were attributed to non-vascular causes (3579 cancer, 461 respiratory, 254 trauma, and 1643 other), and 1018 (6%) had unknown causes (webappendix p 2). For each of these categories of death, the proportional reductions in risk per 1·0 mmol/L LDL cholesterol reduction did not differ between the two types of trial comparison (all heterogeneity p values >0·1). Taking all 26 trials together, there was a proportional reduction in all-cause mortality of 10% (95% CI 7–13; p<0·0001; figure 5) per 1·0 mmol/L reduction in LDL cholesterol, which consisted of a highly significant reduction in vascular mortality of 14% (95% CI 10–18; p<0·0001) and a marginally significant reduction in mortality from unknown causes of 13% (95% CI 1–24; p=0·04), with no apparent effect on non-vascular mortality (RR 0·97, 95% CI 0·92–1·03; p=0·3). The reduction in vascular mortality was chiefly attributable to significant reductions in deaths due to coronary disease of 20% (99% CI 13–26; p<0·0001) and other cardiac causes of 11% (99% CI 2–19; p=0·002) per 1·0 mmol/L, with no apparent effects on deaths due to stroke (RR 0·96, 95% CI 0·84–1·09; p=0·5) or other vascular causes (RR 0·98, 99% CI 0·81–1·18; p=0·8). With respect to non-vascular mortality, there were no apparent effects on deaths from cancer (RR 0·99, 99% CI 0·91–1·09), respiratory disease (RR 0·88, 99% CI 0·70–1·11), trauma (RR 0·98, 99% CI 0·70–1·38), or all other non-vascular causes (RR 0·96, 99% CI 0·83–1·10). There was no indication that reduction of LDL cholesterol in individuals with lower baseline concentrations increased non-vascular mortality (trend p=0·2).
Figure 5.
Effects on cause-specific mortality per 1·0 mmol/L reduction in LDL cholesterol
Rate ratios (RRs) are plotted for each comparison of first event rates between treatment groups and are weighted per 1·0 mmol/L LDL cholesterol (LDL-C) difference at 1 year. RRs are shown with horizontal lines denoting 99% CIs or with open diamonds showing 95% CIs. CHD=coronary heart disease.
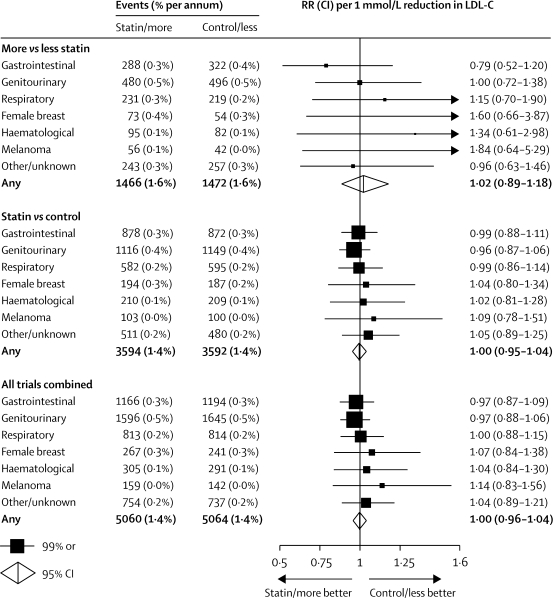
First cancers after randomisation were recorded in 2938 participants in the five trials of more versus less intensive statin therapy and in 7186 participants in the 21 trials of statin versus control, yielding a total of 10 124 first cancers in all 26 trials (excluding cancers known to be recurrences of primary tumours diagnosed before randomisation, and non-melanoma skin cancers since they were not recorded routinely). In the five trials of more versus less intensive statin therapy, reduction of LDL cholesterol to a mean of about 2 mmol/L had no significant effect on the incidence of cancer at all sites combined (RR 1·02 per 1·0 mmol/L LDL cholesterol reduction, 95% CI 0·89–1·18; p=0·8) or at any particular site (figure 6 and webappendix p 13). Similarly, there was no significant effect in the 21 trials of statin versus control and, taking all 26 trials together, there was no evidence of an excess of cancer at all sites combined (RR 1·00 per 1·0 mmol/L LDL reduction, 95% CI 0·96–1·04; p=0·9) or at any particular site. There was also no indication that reduction of LDL cholesterol in individuals with lower baseline concentrations increased cancer incidence (indeed, if anything, the opposite pattern was observed; trend p=0·1).
Figure 6.
Effects on site-specific cancer incidence per 1·0 mmol/L reduction in LDL cholesterol
Rate ratios (RRs) are plotted for each comparison of first event rates between treatment groups and are weighted per 1·0 mmol/L LDL cholesterol (LDL-C) difference at 1 year. RRs are shown with horizontal lines denoting 99% CIs or with open diamonds showing 95% CIs. Analyses are of first cancers, subdivided by site: gastrointestinal (International Classification of Disease codes version 9 140–159); genitourinary (179–189); respiratory (160–163,165); female breast (174); haematological (200–208); melanoma (172); other/unknown site (other cancers with codes 140–172, 174–209, plus deaths with codes 173, 210–239).
Only cases of myopathy that had progressed to rhabdomyolysis were sought from the individual trials. Overall, the observed excess of rhabdomyolysis was 4 (SE 2) per 10 000 in the five trials of more versus less intensive statin therapy (14 vs six cases) compared with 1 (SE 1) per 10 000 in the 21 trials of standard statin regimens versus control (14 vs nine cases). All of the excess (ten vs no cases) with more intensive therapy occurred in the two trials of 80 mg versus 20 mg simvastatin daily; these two trials have also reported definite excesses in the incidence of myopathy with 80 mg simvastatin daily.23,27
Discussion
The previous CTT meta-analysis of individual participant data from randomised trials showed that lowering of LDL cholesterol by about 1 mmol/L with standard statin regimens safely reduced the 5-year incidence of major coronary events, revascularisations, and ischaemic strokes by about a fifth.1 Several trials have since directly compared more intensive versus standard statin regimens.23–27 This updated meta-analysis has shown that additional reductions in LDL cholesterol (down to about 1–2 mmol/L) with more intensive therapy further reduce the incidence of these major vascular events, that the relation between absolute LDL cholesterol reductions and proportional risk reductions is consistent between the trials of more versus less intensive statin therapy and those of standard statin regimens versus control, and that these further reductions in vascular risk can be achieved safely even in individuals with low LDL cholesterol concentrations.
Only two24,26 of the five trials that assessed the effects of reducing LDL cholesterol more intensively23–27 produced separately significant results. But, adjustment for the absolute reduction in LDL cholesterol indicates that the results of these five trials are compatible with one another. Overall, a further reduction in LDL cholesterol of about 0·5 mmol/L was achieved, which reduced the residual risk of major vascular events by about a sixth, with separately significant reductions in coronary death or non-fatal myocardial infarction (p<0·0001), in coronary revascularisation procedures (p<0·0001), and in ischaemic stroke (p=0·005). Moreover, the proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol was similar to that observed in the updated meta-analysis of trials of statin versus control. The previous meta-analysis of statin versus control involved comparatively few major vascular events in participants with low LDL cholesterol before treatment,1 whereas the present meta-analyses provide good evidence of benefit, with no evidence of any hazard, even when LDL cholesterol concentrations lower than 2 mmol/L are reduced further. Overall, there was a 22% proportional reduction in the risk of major vascular events for each 1 mmol/L reduction in LDL cholesterol, which implies that, at least within the range of LDL cholesterol studied to date, a 2 mmol/L reduction would reduce the risk by about 40% (since the combination of risk ratios of 0·78×0·78 yields a risk ratio of about 0·6), and a 3 mmol/L reduction could reduce the risk by about 50%.
In the combined meta-analysis of trials of more versus less intensive statin therapy and of statin therapy versus control, coronary mortality was reduced by about a fifth per 1·0 mmol/L LDL cholesterol reduction, but the reduction in cardiac deaths that were not attributed to coronary disease was only about half as large. This finding may reflect a relative lack of benefit from lowering of LDL cholesterol on cardiac deaths that are mediated by non-occlusive mechanisms. For example, in the GISSI-HF31 trial of rosuvastatin versus placebo in patients with heart failure (which was included in this meta-analysis), as well as in the similar CORONA32 trial (which was not), most cardiac deaths were non-occlusive and there were no significant reductions in cardiac mortality. Nor were there significant reductions in cardiac mortality in the two statin trials among patients with renal disease,29,30 in which only about half of cardiac deaths were definitely due to coronary disease. By contrast, since most of the cardiac deaths that were coded as non-coronary in this meta-analysis occurred in patients with pre-existing coronary disease, some are likely to have been due to coronary occlusion (and, hence, reduced by statin therapy). These findings suggest that the absolute reduction in cardiac mortality produced by lowering of LDL cholesterol with statin therapy in a given population depends chiefly on the absolute risk of death due to coronary occlusion.
There was no significant evidence in the meta-analysis of trials of more versus less intensive therapy that further lowering of LDL cholesterol (weighted mean of 2·5 mmol/L reduced to 2·0 mmol/L) produced any adverse effects, even in participants with baseline LDL cholesterol lower than 2·0 mmol/L. In one of those trials, the mean LDL cholesterol was reduced from 2·5 mmol/L to 1·9 mmol/L, and there was a non-significant excess of death from non-vascular or unknown causes (158 on 80 mg atorvastatin vs 127 on 10 mg atorvastatin daily; p=0·06).25 But, that adverse trend was not supported by larger numbers of such deaths (590 [4·0%] vs 612 [4·1%]; RR 0·96, 95% CI 0·86–1·08; p=0·5) in the four other trials, or by an excess of any particular type of non-vascular mortality. Nor were there any adverse effects on cancer incidence in the meta-analyses of more versus less intensive therapy or of statin versus control. If lowering of LDL cholesterol with statin therapy was carcinogenic then it might be expected to increase the incidence of cancer at some particular site, and previous reports from individual trials had raised such concerns about breast4 and gastrointestinal cancers;11 there was, however, no evidence in our analyses of an increase in cancer at these or any other sites.
Previous observational studies have generated the hypothesis that low cholesterol concentrations might be associated with an increased risk of intracerebral haemorrhage.18,43,44 The present meta-analyses, which included nearly 500 confirmed haemorrhagic strokes, showed that lowering of LDL cholesterol with statin therapy was associated with a non-significant excess (257 vs 220; p=0·2: webappendix p 10). In the SPARCL trial33 of atorvastatin versus placebo in patients with previous cerebrovascular disease (which was not available for this meta-analysis), there was a significant 20% proportional reduction in major vascular events (RR 0·80, 95% CI 0·69–0·92; p=0·002). This result included a significant reduction in ischaemic stroke (218 vs 274; p=0·008), but a significant excess of haemorrhagic stroke (55 vs 33; p=0·02). In the two other trials of statin versus control that were not available for the meta-analysis, there were 15 versus nine haemorrhagic strokes in CORONA,32 but the numbers were not available for GREACE.41 If the published data for haemorrhagic stroke from SPARCL and CORONA were combined with the present meta-analysis then the rate ratio would be 1·21 (95% CI 1·05–1·41) per 1·0 mmol/L LDL cholesterol reduction. Although this result is significant (p=0·01), the absolute size of the potential hazard would be about 50 times smaller (perhaps a few extra haemorrhagic strokes annually per 10 000 treated) than the definite absolute benefits (a few hundred occlusive events avoided annually per 10 000 treated) for patients who are at high risk of occlusive vascular events.
In these meta-analyses, the size of the proportional reduction in major vascular events is directly proportional to the absolute LDL reduction that is achieved, with further benefit from more intensive statin therapy, even if LDL cholesterol is already lower than 2·0 mmol/L. Each 1 mmol/L LDL cholesterol reduction reduces the risk of occlusive vascular events by about a fifth, irrespective of baseline cholesterol concentration, which implies that a 2–3 mmol/L reduction would reduce risk by about 40–50%. These findings suggest that the primary goal for patients at high risk of occlusive vascular events should be to achieve the largest LDL cholesterol reduction possible without materially increasing myopathy risk. Current therapeutic guidelines tend to emphasise the need to reach a particular LDL cholesterol target—for example, US National Cholesterol Education Program guidelines suggest that the objective in high-risk patients should generally be to reduce LDL cholesterol to below 100 mg/dL (2·6 mmol/L) or, optionally, for very high risk patients, to below 70 mg/dL (1·8 mmol/L).45 By contrast, our results suggest that lowering of LDL cholesterol further in high-risk patients who achieve such targets would produce additional benefits, without an increased risk of cancer or non-vascular mortality. Guidelines have proposed that high doses of generic statins (eg, 80 mg simvastatin daily) be used to achieve these benefits,46 but such regimens may be associated with higher risk of myopathy.27 Instead, these benefits may be achieved more safely with newer, more potent statins (eg, 80 mg atorvastatin or 20 mg rosuvastatin daily) and, potentially, by combination of standard doses of generic statins (eg, 40 mg simvastatin or pravastatin daily) with other LDL-cholesterol-lowering therapies.47–49
Acknowledgments
Acknowledgments
This collaboration is coordinated jointly by the Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) at the University of Oxford, UK, and the National Health and Medical Research Council Clinical Trials Centre (CTC) at the University of Sydney, Australia. This work is supported at the CTSU by the UK Medical Research Council, British Heart Foundation, and, previously, the European Community Biomed Programme, and at the CTC by the Australian National Health and Medical Research Council and National Heart Foundation.
Contributors
All of the members of the writing committee contributed to collection and analysis of the data, and to the preparation of the report. All collaborators had an opportunity to contribute to the interpretation of the results and to drafting of the report.
Conflicts of interest
Most of the trials in this report were supported by research grants from the pharmaceutical industry. Some members of the writing committee have received reimbursement of costs to participate in scientific meetings from the pharmaceutical industry. AK and JS have also received honoraria from Solvay for lectures related to these studies.
Current membership of the CTT Collaboration
Writing Committee: C Baigent, L Blackwell, J Emberson, L E Holland, C Reith, N Bhala, R Peto, E H Barnes, A Keech, J Simes, R Collins.
Collaborating trialists: A to Z trial (phase Z) J de Lemos, E Braunwald, M Blazing, S Murphy; AFCAPS/TEXCAPS (AirForce/Texas Coronary Atherosclerosis Prevention Study) J R Downs, A Gotto, M Clearfield; ALERT (Assessment of Lescol in Transplantation) H Holdaas; ALLHAT (Antihypertensive Lipid Lowering Heart Attack Trial) D Gordon, B Davis; ALLIANCE (Aggressive Lipid-Lowering Initiation Abates New Cardiac Events) M Koren; ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) B Dahlof, N Poulter, P Sever; ASPEN (Atorvastatin Study for the prevention of coronary heart disease endpoints in noninsulin dependent diabetes mellitus) RH Knopp (deceased); AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events) B Fellström, H Holdaas, A Jardine, R Schmieder, F Zannad; BIP (Bezafibrate Infarction Prevention Study) U Goldbourt, E Kaplinsky; CARDS (Collaborative Atorvastatin Diabetes Study) H M Colhoun, D J Betteridge, P N Durrington, G A Hitman, J Fuller, A Neil; 4D (Die Deutsche Diabetes Dialyse study) C Wanner, V Krane; CARE (Cholesterol And Recurrent Events Study) F Sacks, L Moyé, M Pfeffer; C M Hawkins, E Braunwald; FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) P Barter, A Keech; GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico)—Heart Failure L Tavazzi, A Maggioni; GISSI–Prevention R Marchioli, G Tognoni, M G Franzosi, A Maggioni; HIT (Veteran Administration Low HDL Intervention Trial) H Bloomfield, S Robins; HPS (Heart Protection Study) R Collins, J Armitage, A Keech, S Parish, R Peto, P Sleight; IDEAL (Incremental Decrease in Endpoints through Aggressive Lipid-lowering) T R Pedersen; JUPITER (Justification for the Use of Statins in Prevention: an International Trial Evaluating Rosuvastatin) P M Ridker; LDS (Lipids in Diabetes Study) R Holman; LEADER (Lower Extremity Arterial Disease Event Reduction trial) T Meade; LIPID (Long-term Intervention with Pravastatin in Ischaemic Disease) J Simes, A Keech, S MacMahon, I Marschner, A Tonkin, J Shaw; LIPS (Lescol Intervention Prevention Study) P W Serruys; MEGA (Management of Elevated cholesterol in the primary prevention Group of Adult japanese) H Nakamura; Post-CABG (Post-Coronary Artery Bypass Graft Study) G Knatterud; PPP (Pravastatin Pooling Project) C Furberg, R Byington; PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) P Macfarlane, S Cobbe, I Ford, M Murphy, G J Blauw, C Packard, J Shepherd; 4S (Scandinavian Simvastatin Survival Study) J Kjekshus, T Pedersen, L Wilhelmsen; PROVE-IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) E Braunwald, C Cannon, S Murphy; SEARCH (Study of Effectiveness of Additional Reductions in Cholesterol and Homocysteine) R Collins, J Armitage, L Bowman, S Parish, R Peto, P Sleight; SHARP (Study of Heart and Renal Protection) C Baigent, A Baxter, R Collins, M Landray; TNT (Testing New Targets) J La Rosa; WHI (Women's Health Initiative) J Rossouw, J Probstfield; WOSCOPS (West of Scotland Coronary Prevention Study) J Shepherd, S Cobbe, P Macfarlane, I Ford.
Other members: M Flather, J Kastelein, C Newman, C Shear, J Tobert, J Varigos, H White, S Yusuf.
Observers: Bristol-Myers Squibb M Mellies, M McGovern, J Barclay, R Belder; Merck Y Mitchel, T Musliner; Laboratoires Fournier J-C Ansquer; Bayer M Llewellyn; Novartis Pharma M Bortolini; AstraZeneca G Brandrup-Wognsen, B Bryzinski, G Olsson, J Pears; Pfizer D DeMicco.
CTT secretariat: A Baxter, C Baigent, E H Barnes, N Bhala, L Blackwell, G Buck, R Collins, J Emberson, W G Herrington, L E Holland, P M Kearney, A Keech, A Kirby, D A Lewis, I Marschner, C Pollicino, C Reith, J Simes, T Sourjina.
Web Extra Material
References
- 1.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3.Shepherd J, Cobbe SM, Ford I. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Pfeffer MA, Moyé LA. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 5.The Post Coronary Artery Bypass Graft Trial Investigators The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–162. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 6.Downs JR, Clearfield M, Weis S. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 7.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 8.GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) Results of the low dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J. 2000;1:810–820. [PubMed] [Google Scholar]
- 9.Serruys PWJC, de Feyter P, Macaya C, for the Lescol Intervention Study (LIPS) Investigators Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention. JAMA. 2002;287:3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 10.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 11.Shepherd J, Blauw GJ, Murphy MB, on behalf of the PROSPER study group Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 12.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care. JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 13.Sever PS, Dahlof B, Poulter NR. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm(ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 14.Holdaas H, Fellstrom B, Jardine AG. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 15.Colhoun HM, Betteridge DJ, Durrington PN. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 16.Stamler J, Vaccaro O, Neaton JD, Werntworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MH, Stein EA, Dujovne CA. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol. 1997;79:38–42. doi: 10.1016/s0002-9149(96)00742-4. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: a reappraisal. Drug Saf. 1996;14:11–24. doi: 10.2165/00002018-199614010-00003. [DOI] [PubMed] [Google Scholar]
- 21.electronic Medicines Compendium Summary of product characteristics for atorvastatin. http://medicines.org.uk/emc/document.aspx?documentid=1424 (accessed Aug 19, 2010).
- 22.electronic Medicines Compendium Summary of product characteristics for rosuvastatin. http://medicines.org.uk/emc/document.aspx?documentid=11976 (accessed Aug 19, 2010).
- 23.de Lemos JA, Blazing MA, Wiviott SD. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Braunwald E, McCabe CH. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen TR, Faergeman O, Kastelein JJ. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 26.LaRosa JC, Grundy SM, Waters DD. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 27.Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)60310-8. published online Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 29.Wanner C, Krane V, Marz W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 30.Fellstrom BC, Jardine AG, Schmieder RE, for the AURORA Study Group Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 31.GISSI-HF investigators Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 32.Kjekshus J, Apetrei E, Barrios V, for the CORONA Group Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 33.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [Google Scholar]
- 34.Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid-lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409–418. doi: 10.1016/j.jacc.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Yehuda O, DeMaria AN. Low LDL-C levels and cancer: reassuring but still not definitive. J Am Coll Cardiol. 2008;52:1150–1151. doi: 10.1016/j.jacc.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Cholesterol Treatment Trialists' (CTT) Collaboration Protocol for a prospective collaborative overview of all current and planned randomized trials of cholesterol treatment regimens. Cholesterol Treatment Trialists' (CTT) Collaboration. Am J Cardiol. 1995;75:1130–1134. doi: 10.1016/s0002-9149(99)80744-9. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Arakawa K, Itakura H, for the MEGA Study Group Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FAH, for the JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 39.Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44:1772–1779. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 40.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 41.Athyros VG, Papageorgiou AA, Mercouris BR. Treatment with atorvastatin to the National Cholesterol Education Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Genest J, Boekholdt SM, for the JUPITER Trial Study Group HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376:333–339. doi: 10.1016/S0140-6736(10)60713-1. [DOI] [PubMed] [Google Scholar]
- 43.Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the Multiple Risk Factor Intervention Trial. N Engl J Med. 1989;320:904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein LB. Low LDL cholesterol, statins, and brain hemorrhage: should we worry? Neurology. 2007;68:719–720. doi: 10.1212/01.wnl.0000258538.06950.a1. [DOI] [PubMed] [Google Scholar]
- 45.Grundy SM, Cleeman JI, Merz CNB. Implications of recent trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 46.NICE Clinical Guideline 67 . Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. National Institute for Health and Clinical Excellence; London: 2008. [PubMed] [Google Scholar]
- 47.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 48.Davidson M, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753–1762. doi: 10.1016/j.jacc.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 49.Ara R, Pandor A, Stevens J, Rees A, Rafia R. Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–74. doi: 10.3310/hta13340. 75–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.