Abstract
Objectives
To study the histopathological features of latanoprost-treated irides with or without darkening, compared with non–latanoprost-treated irides.
Methods
Iridectomy specimens and patient history forms were independently examined by 3 ophthalmic pathologists in a masked fashion. Specimens were evaluated for premalignant changes and for differences in level of pigmentation and degrees of cellularity, inflammation, and vascular abnormalities.
Results
The specimens consisted of 22 latanoprost-treated darkened irides, 35 latanoprost-treated irides without darkening, and 35 non–latanoprost-treated irides. There was a statistically significant decrease in the number of nuclear invaginations and prominent nucleoli in latanoprost-treated darkened irides compared with the other 2 groups (P=.004 and P=.005, respectively). The average thickness and pigmentation of the anterior border layer was greater in the latanoprost-treated darkened irides than in the other 2 groups (P=.03 and P=.02, respectively). The latanoprost-treated darkened irides had increased pigmentation of the stroma (P<.001), stromal fibroblasts (P<.001), melanocytes (P=.005), vascular endothelium (P=.02), and adventitia (P<.001) relative to the other 2 groups.
Conclusions
There is no histopathological evidence of premalignant changes in latanoprost-treated darkened irides. The latanoprost-induced iris color changes are due to a thickening of the anterior border layer and an increased amount of melanin in the anterior border layer and within the stromal melanocytes.
Prostaglandin analogues constitute one of the most effective classes of antiglaucoma drugs and include various congeners such as latanoprost, unoprostone isopropyl, travoprost, and bimatoprost.1 In various clinical studies, these drugs often have been demonstrated to cause increased pigmentation of the iris.2 A small number of studies have consisted of histopathological evaluations to determine the mechanism of iris darkening.3–5 In 2004, we performed the first large study to evaluate, in a systematic manner, key histopathological findings in iris specimens obtained from patients with (n=449) or without (n=142) a history of latanoprost therapy.3 That study included latanoprost-treated and control or nontreated irides but did not specifically look at irides with darkening as a separate group. The study investigated the safety aspects of treatment, including evidence of malignant change or cell proliferation, such as the presence of melanoma, the presence of mitoses, and an increased number of cells. The investigators concluded that latanoprost treatment caused increased amounts of melanin within the iris stromal melanocytes but was not associated with an increase in melanocyte number. That study found no evidence of adverse histopathological effects in latanoprost treated irides compared with controls.
A recent study by Arranz-Marquez et al4 compared iridectomy specimens from patients with latanoprost-induced darkening (n=22) and those from untreated controls (n=8). The authors found that latanoprost induced darkened irides had an increased number of melanocytes within tranuclear inclusions, nuclear invaginations, prominent nucleoli, and mitotic figures suggestive of atypia. Furthermore, they found more granules of melanin in the vascular walls and an increased number of melanocytes and free melanin granules in the stroma compared with controls. To evaluate these findings, we repeated the study and compared latanoprost treated irides with darkening, latanoprost treated irides without darkening, and non–latanoprost-treated control irides.
METHODS
Iris specimens were obtained at the time of iridectomy during glaucoma surgery. The source of the specimens has been previously cited.3 The tissue was received in 10% neutral-buffered formalin at the Latanoprost Pathology Center (LPC), University of Wisconsin, Madison, and labeled by the LPC coordinator with a unique study accession number. The formalin-fixed tissue specimens were processed overnight on an automated tissue processor, embedded in paraffin, serially sectioned at 5 µm, and mounted on treated slides. The first 3 slides were stained with hematoxylin-eosin, the next 3 slides were bleached with potassium permanganate before staining with hematoxylin-eosin, and the next 7 slides were left unstained. All slides were labeled with the unique study accession number assigned to the specimen. If any tissue remained after sectioning, the paraffin block was archived. Also included were the slides of 11 of the 22 cases with latanoprost-induced darkening from the study by Arranz-Marquez et al.4
Sets of microscopic slides (1 hematoxylin-eosin–stained slide, 1 bleached hematoxylin-eosin–stained slide, and 1 unstained slide) were sent to the 3 reviewing pathologists (D.M.A., H.E.G., and W.R.G.), who examined the sets independently and with no knowledge of therapy history. They completed the accompanying pathology grading form, which included an assessment of the quality of the specimens, their location, and their orientation. The specimens were graded by level of pigmentation as none, mild, moderate, and heavy. The thickness of the anterior border layer was ascertained by measuring the average number of flattened cells on the iris surface, oriented parallel to the iris surface, and overlying the loose connective tissue of the stroma proper. These were counted in 3 separate areas of the anterior border layer, and the average was determined by each of the 3 examiners. The number given represents the further average of these 3 determinations. These forms were returned to the LPC coordinator, who completed a composite grading form using specific rules for combining data from the 3 grading forms. Specimens were adjudicated using a protocol formulated by the 3 pathologists before the beginning of the study.
The patient history form that accompanied each specimen included iris color (blue, hazel, or brown); type of glaucoma; ocular history; type of surgery; treatment history, including the start of medical treatment; medication history, including specific medications used; duration of latanoprost therapy (<3 months, 3 months to 3 years, or >3 years); other glaucoma medications used; and presence or absence of increased pigmentation after glaucoma medication. The latanoprost group was defined by a history of latanoprost therapy. It was further divided into irides with and without latanoprost-induced darkening. The control group was defined as having no history of therapy with latanoprost or any other prostaglandin analogue.
After review by the LPC coordinator, the patient history form and the composite grading form (identified only by the unique accession number) were sent to the Statistical Data Analysis Center (Department of Biostatistics and Medical Informatics, University of Wisconsin–Madison) for data entry (Oracle database; Oracle, Redwood Shores, California) and statistical analysis. This study received institutional review board approval from the University of Wisconsin Health Sciences Human Subjects Committee.
Comparisons of groups were based on the F test from analysis of variance models for continuous and ordinal variables and Pearson χ2 test for categorical variables. Ordinal variables were converted to integer equivalents for analysis. Statistical significance was set at P<.05. No formal adjustment for multiple comparisons was used.
RESULTS
From May 1, 2005, through March 31, 2007, 92 iris specimens from 92 unique patients were evaluated by the 3 reviewing pathologists. The specimens consisted of 22 latanoprost-treated darkened irides, 35 latanoprost-treated irides without darkening, and 35 non–latanoprost-treated irides. The 22 latanoprost-treated darkened irides included 11 cases obtained from Arranz-Marquez et al4 and 11 other cases from the LPC. Evaluation of the composite pathology grading forms indicated that the latanoprost-treated groups with or without darkening and the control group were comparable in terms of quality, location, and orientation of the iridectomy specimens. All specimens were obtained from the peripheral iris.
No melanomas were reported on the pathology grading form in either of the latanoprost-treated groups or in the control group. The iris specimens in all of the groups were evaluated for melanocytic atypia (Table 1). The various features suggestive of melanocytic atypia included intranuclear inclusions, nuclear invaginations, prominent nucleoli, and mitotic figures. No mitotic figures were noted in any of the iris specimens. There were no statistically significant differences in the number of intranuclear inclusions among the 3 groups. There was a statistically significant decrease in the number of nuclear invaginations and prominent nucleoli in latanoprost-treated darkened irides compared with the other groups (P=.004 and P=.005, respectively).
Table 1.
Melanocytic Cellular Atypia
| Mean (SD) No. of Features | ||||
|---|---|---|---|---|
| Atypia | Latanoprost-Treated Irides With Darkening |
Latanoprost-Treated Irides With No Darkening |
Control Irides |
P Valuea |
| Intranuclear inclusions | 1.3 (1.6) | 1.1 (0.9) | 1.3 (1.1) | .67 |
| Nuclear invaginations | 2.5 (1.7) | 4.0 (1.6) | 3.4 (1.4) | .004 |
| Prominent nucleoli | 1.9 (1.3) | 3.1 (1.5) | 2.7 (1.1) | .005 |
Based on the F test from analysis of variance models for continuous and ordinal variables and Pearson χ2 test for categorical variables.
The 22 latanoprost-treated darkened irides were further examined in terms of the following 2 subgroups: 11 cases obtained from the series studied by Arranz-Marquez et al4 and 11 other cases from the LPC. There were more intranuclear inclusions in the cases obtained from Arranz-Marquez et al than in those from the LPC (mean, 2.2 vs 1.1; P=.01). However, there were fewer nuclear invaginations (mean, 1.7 vs 3.4; P=.01) and prominent nuclei (mean, 1.1 vs 2.7; P=.003) in the cases obtained from Arranz-Marquez et al compared with those from the LPC.
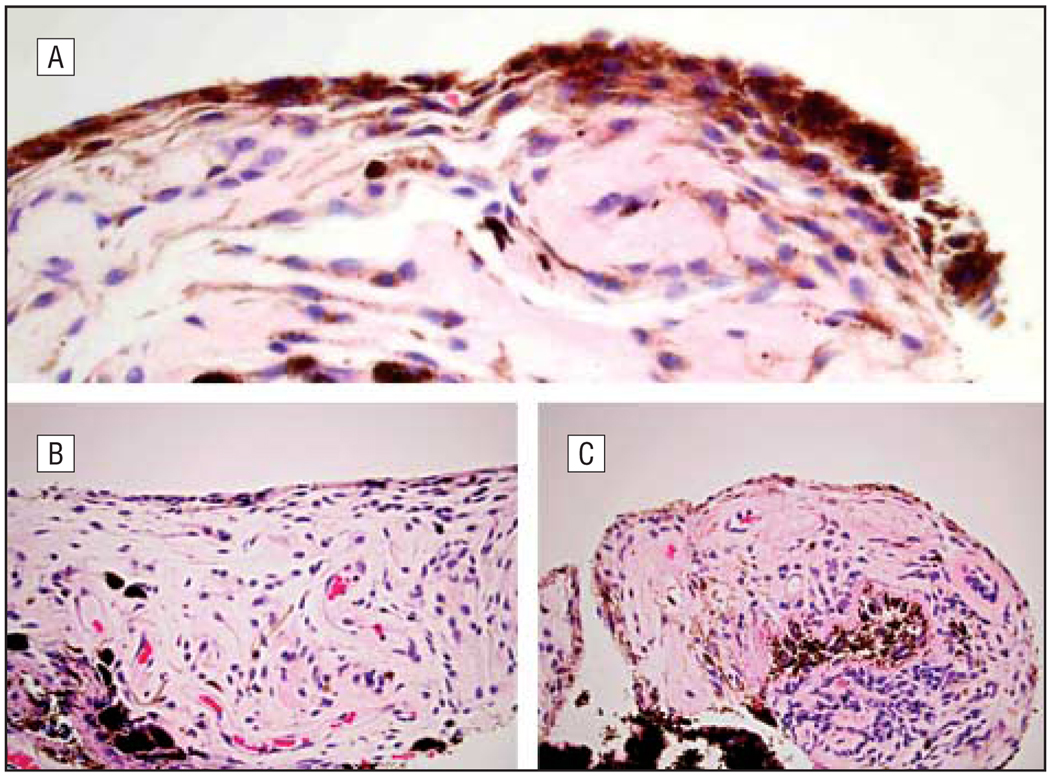
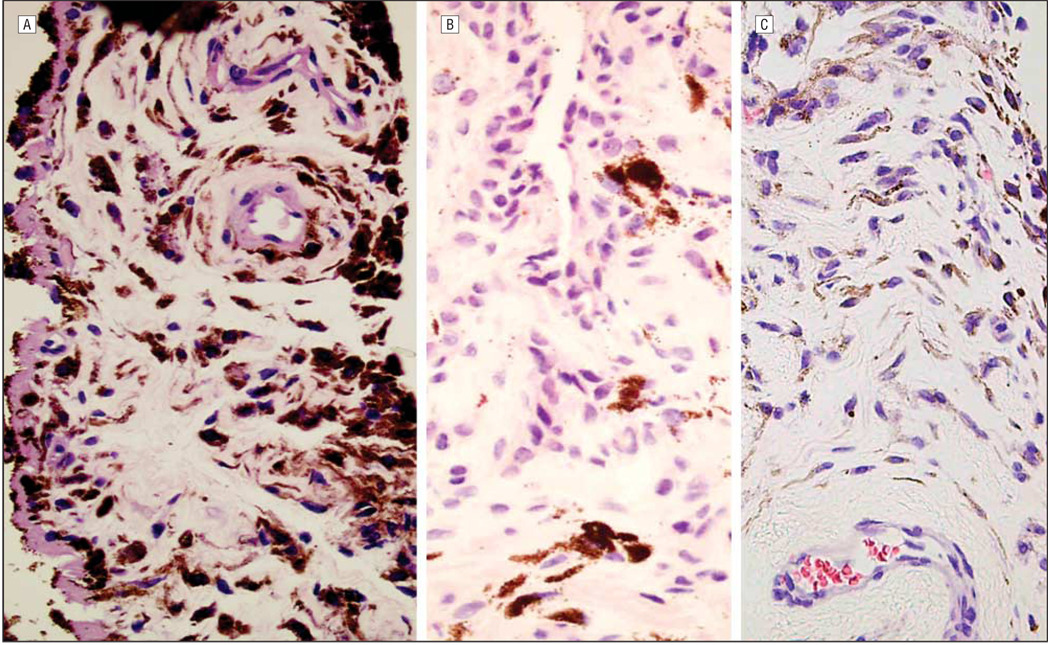
The average (SD) thickness of the anterior border layer was greater in the latanoprost-treated darkened irides (2.2 [0.9] cells) than in the latanoprost-treated irides without darkening (1.8 [0.7] cells) and the control group (1.7 [0.7] cells) (P=.03). There was also a statistically significant increase in pigmentation of the anterior border layer in the latanoprost-treated darkened irides (21 of 22 irides [95%] with moderate or heavy pigmentation) compared with the latanoprost-treated irides without darkening (20 of 35 irides [57%] with moderate or heavy pigmentation) and the controls (21 of 34 irides [62%] with moderate or heavy pigmentation) (P=.02; Figure 1). Similarly, the latanoprost-treated darkened irides had increased pigmentation of the stroma (P<.001), stromal fibroblasts (P<.001), melanocytes (P=.005), vascular endothelium (P=.02), and adventitia (P<.001) relative to the latanoprost-treated irides without darkening and the controls (Table 2 and Figure 2). In the areas of increased pigmentation, the melanocytes were differentiated from pigmented fibroblasts by the plump, fusiform cytoplasm in melanocytes, compared with fibroblasts, which have wispy cytoplasm and narrow, spindle-shaped nuclei.
Figure 1.
Hematoxylin-eosin–stained sections of iridectomy specimens showing increased thickness of the anterior border layer in latanoprost-treated darkened irides (A) compared with latanoprost-treated irides without darkening (B) and control irides (C) (original magnification ×400).
Table 2.
Constituent Cells Graded with Heavy (Maximum) Pigmentation According to Location and Treatment
| No. (%) of Casesa | ||||
|---|---|---|---|---|
| Level of Pigmentation | Latanoprost-Treated Irides With Darkening (n=22) |
Latanoprost-Treated Irides With No Darkening (n=35) |
Control Irides (n=35) |
P Valueb |
| Anterior border layerc | ||||
| None | 0 | 0 | 0 | |
| Minimal | 1 (5) | 15 (43) | 13 (38) | |
| Moderate | 14 (64) | 14 (40) | 15 (44) | |
| Heavy | 7 (32) | 6 (17) | 6 (18) | |
| Stroma | <.001 | |||
| None | 1 (5) | 8 (23) | 15 (43) | |
| Minimal | 5 (23) | 19 (54) | 10 (29) | |
| Moderate | 15 (68) | 7 (20) | 9 (26) | |
| Heavy | 1 (5) | 1 (3) | 1 (3) | |
| Fibroblasts | <.001 | |||
| None | 1 (5) | 3 (9) | 6 (17) | |
| Minimal | 2 (9) | 24 (69) | 18 (51) | |
| Moderate | 14 (64) | 6 (17) | 8 (23) | |
| Heavy | 5 (23) | 2 (6) | 3 (9) | |
| Melanocytes | .005 | |||
| None | 1 (5) | 2 (6) | 1 (3) | |
| Minimal | 1 (5) | 20 (57) | 16 (46) | |
| Moderate | 15 (68) | 10 (29) | 12 (34) | |
| Heavy | 5 (23) | 3 (9) | 6 (17) | |
| Endotheliumc | .02 | |||
| None | 12 (55) | 30 (86) | 29 (85) | |
| Minimal | 10 (45) | 5 (14) | 4 (12) | |
| Moderate | 0 | 0 | 1 (3) | |
| Heavy | 0 | 0 | 0 | |
| Adventitiac | <.001 | |||
| None | 2 (9) | 24 (69) | 21 (62) | |
| Minimal | 17 (77) | 11 (31) | 11 (32) | |
| Moderate | 3 (14) | 0 | 2 (6) | |
| Heavy | 0 | 0 | 0 | |
Percentages have been rounded and may not total 100.
Based on the F test from analysis of variance models for continuous and ordinal variables and Pearson χ2 test for categorical variables.
Only 34 samples of control irides were available for this determination.
Figure 2.
Hematoxylin-eosin–stained sections of iridectomy specimens showing increased pigmentation of the stroma, stromal fibroblasts, melanocytes, vascular endothelium, and adventitia in latanoprost-treated darkened irides (A) compared with latanoprost-treated irides without darkening (B) and control irides (C) (original magnification ×400).
A subset analysis was performed for the extent of pigmentation in the various layers of iris in the latanoprost-treated darkened irides (11 cases obtained from Arranz-Marquezet al4 and 11 cases from the LPC). Arranz-Marquez et al also noted a thickening of the anterior border layer, but this was given as a measurement in millimeters rather than in terms of the number of cells. However, in the LPC cases, there was no statistically significant difference in pigmentation of the anterior border layer (11 of 11 [100%] vs 10 of 11 [91%] with heavy or moderate pigmentation; P=.45), stroma (9[82%] vs 7[64%]; P=.51), stromal fibroblasts (11 [100%] vs 8 [73%]; P=.39), melanocytes (11 [100%] vs 9 [82%]; P=.22), vascular endothelium (0 vs 0; P>.99), and adventitia (1 [9%] vs 2 [18%]; P=.67).
A detailed evaluation of all the iris specimens also found no evidence of stromal inflammation or abnormal appearance of stromal blood vessels in any irides. The posterior iris pigment epithelium was also unremarkable in all specimens. There was no evidence of rubeosis, pseudoexfoliation material, synechiae, descemetization, or endothelialization in any of the iris specimens. Two specimens, one from the latanoprost-treated darkened irides group and the other from the control group, showed attached Descemet membrane. Three specimens, including 1 latanoprost-treated iris without darkening and 2 controls, had hemorrhage on the anterior border layer and/or the posterior iris pigment epithelium.
COMMENT
The differences in the iris color of normal eyes can be attributed to the variable amounts of melanin granules within a constant number of melanocytes in the superficial stroma of the iris.6,7 The amount of melanin is genetically predetermined and reaches its maximum concentration during early childhood.8,9 Thereafter, it usually remains constant throughout life unless affected by certain ocular disorders, which can lead to hypopigmentation or hyperpigmentation. 10 Latanoprost and various other prostaglandin analogues used in the medical management of glaucoma have been demonstrated to cause iris darkening in clinical trials in the United States.2,11,12 This iris darkening is typically noted after 6 months of drug therapy, and the degree of increased pigmentation varies from 5% to 70%.13–15 Furthermore, in a recent study, the darkening of the iris after 6 months of latanoprost treatment was noted more frequently in patients older than 75 years compared with patients younger than 60 years.16
The pathogenesis of iris darkening in latanoprost-treated eyes has been studied in various in vitro and histopathological studies.17–21 The in vitro studies have documented elevated tyrosinase activity in human uveal cell lines and iris melanocyte cultures.22–26 The light and electron microscopic studies indicate that latanoprost-induced eye color change is due to an increased amount of melanin within the iris stromal melanocytes rather than any increase in melanocyte number.27–30 As stated in the Introduction, our previous histopathological study of iris specimens obtained from patients with and without a history of latanoprost therapy3 observed no evidence of malignant or premalignant changes in either group. This conclusion was based on the absence of melanomas, atypical nevi, cells with an atypical appearance, or increased numbers of mitotic figures. There was an increased prevalence of freckles in the latanoprost-treated group. We proposed that the increased number of freckles was a manifestation of focal increased tyrosinase expression and that the same pathogenesis exists for the more diffuse darkening. We did not believe that the increase in iris freckles had malignant potential.
The study performed by Arranz-Marquez et al4 noted a statistically significant increase in atypia in melanocytes in latanoprost-treated darkened irides. There was also a thickening of the anterior border layer and a higher number of melanin granules within the stroma.4 Therefore, we designed a new study to evaluate latanoprost-treated eyes with or without iris darkening and compared those groups with non–prostaglandin-treated controls.
The anterior border layer and stroma of the latanoprost-treated darkened irides showed a statistically significant increase in melanin pigment granules compared with the other groups. There was also a thickening of the anterior border layer. These findings are consistent with the findings of other studies in the literature4,5 and may be responsible for darkening of the iris in latanoprost-treated eyes. However, in contrast to the series by Arranz-Marquez et al,4 there was no evidence of melanocyte atypia. On the contrary, we found that the numbers of nuclear invaginations and prominent nucleoli were significantly decreased in latanoprost-treated darkened irides. This unexpected finding may be related to the effects of prostaglandin analogues on cell growth and differentiation.31–33
Thus, our results indicate that the latanoprost-induced iris darkening is due to a thickening of the anterior border layer and to an increased amount of melanin in the anterior border layer. The iris stromal melanocytes also show an increase in the amount of melanin granules, but there is no evidence of melanocyte atypia. Although not entirely in agreement with the study by Arranz-Marquez et al,4 these findings are consistent with those of previous studies.5 The differences between the 2 subgroups in the latanoprost-treated darkened irides may reflect ethnic differences13,34,35 or may be related to the duration36 of latanoprost treatment and the dose37 of the medication used.
In conclusion, there is no histopathological evidence of precancerous lesions in latanoprost-treated darkened irides. This finding is consistent with the absence of documented iris melanomas in reports on patients treated with latanoprost during the past decade.
Acknowledgments
Funding/Support: This study was supported by an investigator-initiated research grant from Pfizer Inc.
Footnotes
Financial Disclosure: None reported.
Publisher's Disclaimer: Disclaimer: Dr Albert, the journal’s chief editor, was not involved in the editorial review or the decision to publish this article.
Previous Presentations: This study was presented in part at the American Association of Ophthalmic Pathologists Annual Meeting; November 10, 2006; Las Vegas, Nevada.
REFERENCES
- 1.Nguyen QH. The role of prostaglandin analogues in the treatment of glaucoma in the 21st century. Int Ophthalmol Clin. 2004;44(2):15–27. doi: 10.1097/00004397-200404420-00004. [DOI] [PubMed] [Google Scholar]
- 2.Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6(1):45–52. doi: 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Albert DM, Gangnon RE, Zimbric ML, et al. A study of iridectomy histopathologic features of latanoprost- and non–latanoprost-treated patients. Arch Ophthalmol. 2004;122(11):1680–1685. doi: 10.1001/archopht.122.11.1680. [DOI] [PubMed] [Google Scholar]
- 4.Arranz-Marquez E, Teus MA, Saornil MA, Mendez MC, Gil R. Analysis of irises with a latanoprost-induced change in iris color. Am J Ophthalmol. 2004;138(4):625–630. doi: 10.1016/j.ajo.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Cracknell KP, Grierson I, Hogg P. Morphometric effects of long-term exposure to latanoprost. Ophthalmology. 2007;114(5):938–948. doi: 10.1016/j.ophtha.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Wilkerson CL, Syed NS, Fisher MR, Robinson NL, Wallow IH, Albert DM. Melanocytes and iris color: light microscopic findings. Arch Ophthalmol. 1996;114(4):437–442. doi: 10.1001/archopht.1996.01100130433014. [DOI] [PubMed] [Google Scholar]
- 7.Imesch PD, Bindley CD, Khademian Z, et al. Melanocytes and iris color: electron microscopic findings. Arch Ophthalmol. 1996;114(4):443–447. doi: 10.1001/archopht.1996.01100130439015. [DOI] [PubMed] [Google Scholar]
- 8.Albert DM, Green WR, Zimbric ML, et al. Iris melanocyte numbers in Asian, African-American, and Caucasian irides. Trans Am Ophthalmol Soc. 2003;101:217–222. [PMC free article] [PubMed] [Google Scholar]
- 9.Eagle RC., Jr Iris pigmentation and pigmented lesions: an ultrastructural study. Trans Am Ophthalmol Soc. 1988;86:581–687. [PMC free article] [PubMed] [Google Scholar]
- 10.Imesch PD, Wallow IH, Albert DM. The color of the human eye: a review of the morphologic correlates and of some conditions that affect iridial pigmentation. Surv Ophthalmol. 1997;41 suppl 2:S117–S123. doi: 10.1016/s0039-6257(97)80018-5. [DOI] [PubMed] [Google Scholar]
- 11.Grierson I, Cracknell KP, Pfeiffer N. The iris after prostanoid treatment. Curr Opin Ophthalmol. 2001;12(2):112–118. doi: 10.1097/00055735-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Yanoff M, Fine BS. Ocular Pathology: A Text and Atlas. 2nd ed. Philadelphia, PA: Harper & Row Publishers; 1982. p. 822. [Google Scholar]
- 13.Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41 suppl 2:S129–S138. doi: 10.1016/s0039-6257(97)80020-3. [DOI] [PubMed] [Google Scholar]
- 14.Camras CB, Wax MB, Ritch R, et al. United States Latanoprost Study Group. Latanoprost treatment for glaucoma: effects of treating for 1 year and of switching from timolol. Am J Ophthalmol. 1998;126(3):390–399. doi: 10.1016/s0002-9394(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 15.Teus MA, Arranz-Marquez E, Lucea-Suescun P. Incidence of iris colour change in latanoprost treated eyes. Br J Ophthalmol. 2002;86(10):1085–1088. doi: 10.1136/bjo.86.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114(7):1255–1258. doi: 10.1016/j.ophtha.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Stjernschantz JW, Albert DM, Hu D, Drago F, Wistrand PJ. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47 suppl 1:S162–S175. doi: 10.1016/s0039-6257(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 18.Grierson I, Pfeiffer N, Cracknell KP, Appleton P. Histology and fine structure of the iris and outflow system following latanoprost therapy. Surv Ophthalmol. 2002;47 suppl 1:S176–S184. doi: 10.1016/s0039-6257(02)00304-1. [DOI] [PubMed] [Google Scholar]
- 19.Grierson I, Lee WR, Albert DM. The fine structure of an iridectomy specimen from a patient with latanoprost-induced eye color change. Arch Ophthalmol. 1999;117(3):394–396. doi: 10.1001/archopht.117.3.394. [DOI] [PubMed] [Google Scholar]
- 20.Tsai JC, Sivak-Callcott JA, Haik BG, Zhang J, McLean IW. Latanoprost-induced iris heterochromia and open-angle glaucoma: a clinicopathologic report. J Glaucoma. 2001;10(5):411–413. doi: 10.1097/00061198-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer N, Grierson I, Goldsmith H, Hochgesand D, Winkgen-Bohres A, Appleton P. Histological effects in the iris after 3 months of latanoprost therapy: the Mainz 1 Study. Arch Ophthalmol. 2001;119(2):191–196. [PubMed] [Google Scholar]
- 22.Lindsey JD, Jones HL, Hewitt EG, Angert M, Weinreb RN. Induction of tyrosinase gene transcription in human iris organ cultures exposed to latanoprost. Arch Ophthalmol. 2001;119(6):853–860. doi: 10.1001/archopht.119.6.853. [DOI] [PubMed] [Google Scholar]
- 23.Drago F, Marino A, La Manna C. Alpha-methyl-p-tyrosine inhibits latanoprost-induced melanogenesis in vitro. Exp Eye Res. 1999;68(1):85–90. doi: 10.1006/exer.1998.0581. [DOI] [PubMed] [Google Scholar]
- 24.Stjernschantz J, Ocklind A, Wentzel P, Lake S, Hu DN. Latanoprost-induced increase of tyrosinase transcription in iridial melanocytes. Acta Ophthalmol Scand. 2000;78(6):618–622. doi: 10.1034/j.1600-0420.2000.078006618.x. [DOI] [PubMed] [Google Scholar]
- 25.Dutkiewicz R, Albert DM, Levin LA. Effects of latanoprost on tyrosinase activity and mitotic index of cultured melanoma lines. Exp Eye Res. 2000;70(5):563–569. doi: 10.1006/exer.1999.0819. [DOI] [PubMed] [Google Scholar]
- 26.Hu D-N, Stjernschantz J, McCormick SA. Effect of prostaglandins A2E, E1, F2α and latanoprost on cultured human iridial melanocytes. Exp Eye Res. 2000;70(1):113–120. doi: 10.1006/exer.1999.0760. [DOI] [PubMed] [Google Scholar]
- 27.Lindquist NG, Larsson BS, Stjernschantz J. Increased pigmentation of iridial melanocytes in primates induced by a prostaglandin analogue. Exp Eye Res. 1999;69(4):431–436. doi: 10.1006/exer.1999.0718. [DOI] [PubMed] [Google Scholar]
- 28.Selén G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Ophthalmol. 1997;41 suppl 2:S125–S128. doi: 10.1016/s0039-6257(97)80019-7. [DOI] [PubMed] [Google Scholar]
- 29.Prota G, Vincensi MR, Mapolitano A, Selen G, Stjernschantz J. Latanoprost stimulates eumelanogenesis in iridial melanocytes of cynomolgus monkeys. Pigment Cell Res. 2000;13(3):147–150. doi: 10.1034/j.1600-0749.2000.130305.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhan GL, Toris CB, Camras CB, Wang YL, Bito LZ. Prostaglandin-induced iris color darkening: an experimental model. Arch Ophthalmol. 1998;116(8):1065–1068. doi: 10.1001/archopht.116.8.1065. [DOI] [PubMed] [Google Scholar]
- 31.De Asua LJ, Clingan D, Rudland PS. Initiation of cell proliferation in culture mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black FM, Wakelam MJ. Activation of inositol phospholipid breakdown by prostaglandin F2 alpha without any stimulation of proliferation in quiescent NIH-3T3 fibroblasts. Biochem J. 1990;266(3):661–667. doi: 10.1042/bj2660661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conconi MT, Spinazzi R, Tommasini M, Limoli A, Parnigotto PP. Prostaglandin F2 alpha can modulate the growth and the differentiation of bovine corneal epithelial cells cultured in vitro. Ann Anat. 2001;183(6):567–573. doi: 10.1016/s0940-9602(01)80071-6. [DOI] [PubMed] [Google Scholar]
- 34.Alm A, Widengård I. Latanoprost: experience of 2-year treatment in Scandinavia. Acta Ophthalmol Scand. 2000;78(1):71–76. doi: 10.1034/j.1600-0420.2000.078001071.x. [DOI] [PubMed] [Google Scholar]
- 35.Latanoprost-Induced Iris Pigmentation Study Group. Incidence of a latanoprost-induced increase in iris pigmentation in Japanese eyes. Jpn J Ophthalmol. 2006;50(2):96–99. doi: 10.1007/s10384-005-0288-7. [DOI] [PubMed] [Google Scholar]
- 36.Alm A, Schoenfelder J, McDermott J. A 5-year, multicenter, open-label, safety study of adjunctive latanoprost therapy for glaucoma. Arch Ophthalmol. 2004;122(7):957–965. doi: 10.1001/archopht.122.7.957. [DOI] [PubMed] [Google Scholar]
- 37.Friström B, Nilsson SE. A double masked comparison of the intraocular pressure reducing effect of latanoprost 0.005% and 0.001% administered once daily in open angle glaucoma and ocular hypertension. Br J Ophthalmol. 1997;81(10):867–870. doi: 10.1136/bjo.81.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]