Abstract
The dorsal-side-up body posture in standing quadrupeds is maintained by the postural system, which includes spinal and supraspinal mechanisms driven by somatosensory inputs from the limbs. A number of descending tracts can transmit supraspinal commands for postural corrections. The first aim of this study was to understand whether the rubrospinal tract participates in their transmission. We recorded activity of red nucleus neurons (RNNs) in the cat maintaining balance on the periodically tilting platform. Most neurons were identified as rubrospinal ones. It was found that many RNNs were profoundly modulated by tilts, suggesting that they transmit postural commands. The second aim of this study was to examine the contribution of sensory inputs from individual limbs to posture-related RNN modulation. Each RNN was recorded during standing on all four limbs, as well as when two or three limbs were lifted from the platform and could not signal platform displacements. By comparing RNN responses in different tests, we found that the amplitude and phase of responses in the majority of RNNs were determined primarily by sensory input from the corresponding (fore or hind) contralateral limb, whereas inputs from other limbs made a much smaller contribution to RNN modulation. These findings suggest that the rubrospinal system is primarily involved in the intralimb postural coordination, i.e., in the feedback control of the corresponding limb and, to a lesser extent, in the interlimb coordination. This study provides a new insight into the formation of supraspinal motor commands for postural corrections.
Introduction
The basic, dorsal-side-up body posture in standing quadrupeds is maintained by the postural system, which includes spinal and supraspinal mechanisms driven by somatosensory inputs from the limbs. This closed-loop control system generates a corrective motor response when the body orientation deviates from the desired one (for review, see Horak and Macpherson, 1996; Macpherson et al., 1997; Massion, 1998; Beloozerova et al., 2003; Deliagina et al., 2006b, 2008).
Supraspinal commands for postural corrections can be transmitted to the spinal cord by a number of descending tracts. Participation of one of them—the corticospinal tract—was demonstrated in our previous studies (Beloozerova et al., 2005). In those experiments, an intact cat was standing and keeping balance on a periodically tilting platform, and the activity of individual pyramidal tract neurons (PTNs) was recorded during postural corrections. It was found that almost all recorded neurons were profoundly modulated in the rhythm of tilts, and the source of modulation was the tilt-related somatosensory input from the corresponding contralateral limb. It was concluded that the PTNs are primarily involved in intralimb postural coordination and, to a lesser extent, in interlimb coordination (Karayannidou et al., 2008).
Among other possible pathways for transmitting supraspinal commands to the spinal postural mechanisms is the rubrospinal tract. This tract originates from neurons of the red nucleus, and affects mainly the spinal centers of the contralateral limbs (Pompeiano and Brodal, 1957; Massion, 1967; Robinson et al., 1987). The rubrospinal system participates in the control of a number of motor behaviors, including reaching and grasping (Gibson et al., 1985; Mewes and Cheney, 1994; van Kan and McCurdy, 2001; Horn et al., 2002), stepping (Orlovsky, 1972; Lavoie and Drew, 2002), and scratching (Arshavsky et al., 1978). However, the participation of the rubrospinal system in the maintenance of body posture and equilibrium has not been investigated.
The first aim of this study was to understand whether the rubrospinal system, like the corticospinal one, participates in the transmission of supraspinal commands for postural corrections. We used an identical task (the cat maintained balance on the tilting platform), and recorded the activity of individual red nucleus neurons (RNNs). It was found that many RNNs were profoundly modulated by tilts, suggesting that they participate in the transmission of postural commands to the spinal cord.
The second aim of this study was to reveal the contribution of sensory inputs from individual limbs to posture-related modulation of RNNs. For this purpose, we used the technique developed previously for studying the corticospinal system (Deliagina et al., 2006; Karayannidou et al., 2008). Each RNN was recorded in a number of postural tasks in which one, two, or three limbs were suspended, and thus tilt-related somatosensory input from them was abolished. By comparing RNN responses in different tests, we found that modulation of RNNs was determined mainly by the sensory input from the corresponding (fore or hind) contralateral limb, suggesting that the rubrospinal system, like the corticospinal one, is primarily involved in intralimb postural coordination and, to a lesser extent, in interlimb coordination.
Materials and Methods
Recordings were obtained from two adult female cats. Some of the methods have been described (Beloozerova et al., 2005; Prilutsky et al., 2005; Deliagina et al., 2006a) and will be reported briefly here. Experiments were conducted at Barrow Neurological Institute in accordance with National Institutes of Health guidelines and were approved by the Barrow Neurological Institute Animal Care and Use Committee.
Surgical procedures
Surgery was performed under isoflurane anesthesia using aseptic procedures. The skin and fascia were removed from the dorsal surface of the skull. At 10 points around the circumference of the head, stainless steel screws were screwed into the skull and connected together with a wire; the screw heads and the wire were then inserted into a plastic cast to form a circular base. Later, while searching for neurons before behavioral tests, awake cats were rigidly held by this base. The base was also used for fixation of connectors, a miniature micro drive, preamplifiers, contacts for stimulating electrodes, and a protective and electrically shielding cap.
An arrangement of seven 28 gauge hypodermic guide tubes was implanted vertically above the left red nucleus with the tip at the Horsley and Clarke coordinates A3.5, L2, V+ 6 (see Fig. 2E). It was fastened to the surrounding bone by orthodontic resin (Densply Caulk). A 26 gauge hypodermic guide tube was implanted vertically above the rubrospinal tract at the medulla level (with the tip at the Horsley and Clarke coordinates P8, L5, V+ 1) for insertion of a stimulating electrode into the rubrospinal tract later in the awake cat.
Figure 2.
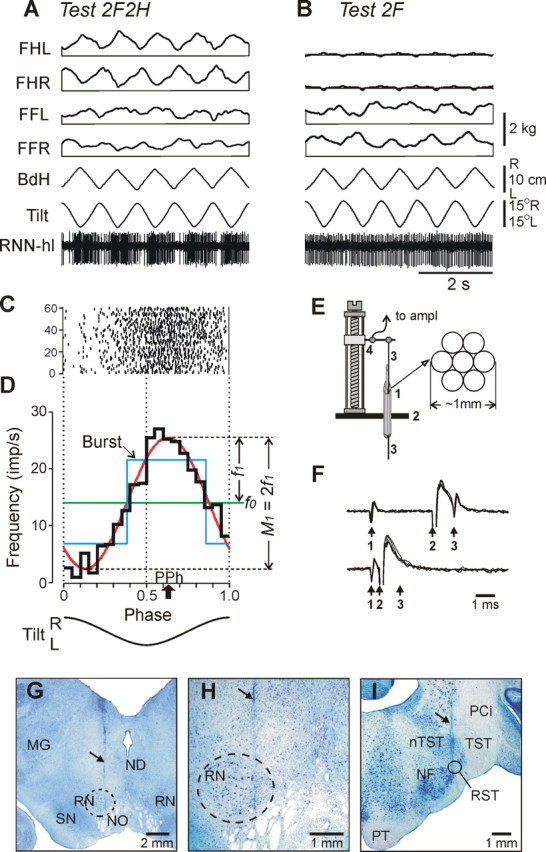
Representative example of postural and RNN responses (A–D), methods of RNN recording and identification (E, F), and histological verification (G–I). A, B, In tests 2F2H and 2F, the following traces are shown: RNN-hl—activity of a hindlimb-related RNN; Tilt—tilt of the platform; BdH—lateral displacement of the hind part of the body; FHL, FHR—contact forces under hindlimbs, left and right; FFL, FFR—contact forces under forelimbs, left and right. C, Raster of neuronal activity in 60 sequential cycles. D, Characteristics of neuronal activity: the histogram of spike activity in the tilt cycle (thick black line); first harmonic of Fourier image (red line) with its amplitude (f1) and the value of modulation (M1) indicated; mean frequency in the tilt cycle (f0, green line); preferred phase of the discharge (PPh, arrow). E, The method of inserting the microelectrode. A group of seven 28 gauge cannulas (1) are implanted through the opening in the scull (2) ∼10 mm above the red nucleus. The electrode (3) is manually inserted into one of the cannulas and soldered to an arm of a micromanipulator (4). F, Collision test determines whether RNN response is antidromic. Top trace, The RNN spontaneously discharges (arrow 1), and the rubrospinal tract is stimulated ∼3 ms later (arrow 2). The RNN responds with latency of ∼1 ms (arrow 3). Bottom trace, The RNN spontaneously discharges (arrow 1), and the rubrospinal tract is stimulated ∼0.5 ms later (arrow 2). RNN does not respond (arrow 3) because in 0.5 ms its spontaneous spike was still en route to the site of stimulation in the rubrospinal tract, and thus collision/nullification of spontaneous and evoked spikes occurred. G, H, Position of the microelectrode track within the left red nucleus (increased magnification in H). I, Position of the track of the stimulating electrode within the right rubrospinal tract. In G–I, the tracks are indicated by arrows. MG, Medial geniculate body; ND, nucleus Darkschewitsch; NF, nucleus of facial nerve; NO, n. oculomotorius; nTST, nucleus of tractus spinalis nervi trigemini; PCi, pedunculus cerebellaris inferior; PT, pyramidal tract; RST, rubrospinal tract; SN, substantia nigra; TST, tractus spinalis nervi trigemini.
Cell recording and identification
After several days of recovery, experiments were initiated by placing the animal in the head-restraining device. The cat was positioned on a table equipped with a foam rubber pad, encouraged to take a “sphinx” position, and allowed to rest for several minutes. Then the base attached to the skull during surgery was fastened to the restraining device so that the resting position of the cat's head was approximated. This procedure minimized stress on the neck when the head was temporarily immobilized, while the body was put in a comfortable position. Over several days, a number of sessions of increasing duration were used to accustom the cat to the head restrainer. After several training sessions, both cats sat quietly with their head restrained. They did not seem disturbed by the restrainer, as they frequently fell asleep. Neuronal recordings were then initiated.
Neuronal activity was recorded extracellularly from the left red nucleus using either platinum-tungsten quartz insulated microelectrodes (40 μm outer diameter) pulled to a fine tip and mechanically sharpened (Reitboeck, 1983), or commercially available tungsten varnish insulated electrodes (125 μm outer diameter, Frederick Haer). The impedance of the electrodes was 2–4 MΩ. After the electrode reached the depth of the red nucleus (where clear responses of many neurons to limb movements could be observed), a 200 μm platinum-iridium wire was inserted and slowly lowered into the rubrospinal tract through the guide tube implanted during surgery. Pulses of graded intensity (0.2 ms duration, up to 0.5 mA) were delivered through this electrode. The wire was fixed at the position that was most effective in eliciting antidromic responses in neurons of the red nucleus, and it served as the rubrospinal tract-stimulating electrode during subsequent experiments.
Before, during, and after testing in each postural task, all encountered neurons were tested for antidromic activation. The criterion for identification was the test for collision of spikes (Bishop et al., 1962; Fuller and Schlag, 1976) (see Fig. 2F). In addition, waveform analysis was used to discriminate and identify the spikes of a single neuron using the Power-1401/Spike-2 system waveform-matching algorithm. Only the neurons with a stable response latency and spike shape, which consistently satisfied the collision test, were considered as rubrospinal neurons.
Signals from the microelectrode preamplifier, as well as from the platform position and body position sensors were amplified (CyberAmp 380, Molecular Devices), digitized with sampling frequencies of 30 kHz (microelectrode) and 400 Hz (sensors), displayed on the screen, and saved to a computer disc by means of data acquisition software (Power-1401/Spike-2).
Postural tests
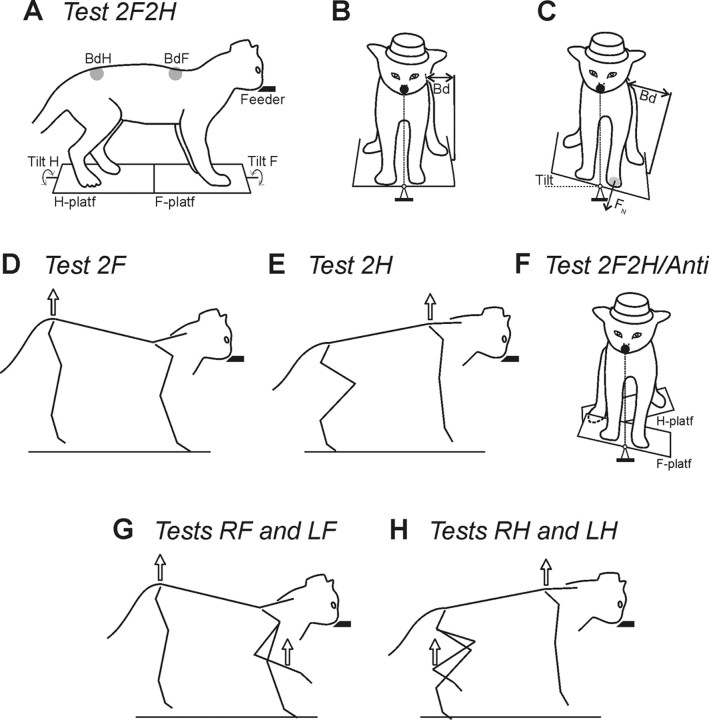
The basic experimental arrangement for postural tests was the same as in our previous studies (Beloozerova et al., 2005; Deliagina et al., 2006a; Karayannidou et al., 2008). The unrestrained cats were trained to quietly stand on a platform, which consisted of two parts—the F-platform under the forelimbs and the H-platform under the hindlimbs (Fig. 1A). They were rewarded by food paste continuously ejected from a feeder. The feeder (a plastic tube of 18 mm outer diameter and 6 mm inner diameter) was positioned in front of the cat at a height of 21–23 cm (Fig. 1A). The platforms under the cat were periodically tilted in the frontal (roll) plane of the animal. A sine-like tilt trajectory was used, with a period of 1 s and amplitude of ±15°. Cats were easily engaged in this postural task and maintained equilibrium during tilts. They tended to compensate for the platform tilts by performing lateral displacements of the body in relation to the supporting platform (postural corrections), which allowed them to hold their mouth against the feeder and keep licking food despite the tilting of the platform. Postural tests differed in the composition of the group of limbs supporting the body (Deliagina et al., 2006a). This composition is reflected in the name of each test.
Figure 1.
During postural tests, the cat was standing on two platforms, one under the forelimbs (F-platform) and one under the hindlimbs (H-platform). The platforms were tilted in the frontal (roll) plane. The cat was continuously licking food from a feeder (feeder position is indicated by the filled bars in the side views and by the filled circles in the front views). A–C, In-phase tilts of the two platforms. A, The body outline (view from the right side) is shown for the horizontal position of the platforms. B, C, The body outline (view from the front) is shown for two positions of the platforms, horizontal and 15°L. Postural corrections were characterized by measuring the lateral displacement of the upper point of the fore and hind parts of the trunk in relation to the corresponding platform (body displacements, BdF and BdH, in A–C). The normal component of the contact force produced by each limb was measured by means of a force plate (shown only for the left forelimb, FN in C). D, Lifting the hindquarters. E, Lifting the forequarters. F, Antiphase tilt of the two platforms. G, Lifting the hindquarters and one forelimb. H, Lifting the forequarters and one hindlimb.
Contribution of afferent input from individual girdles (shoulder or hip) to the periodic modulation of RNNs
Test 2F2H (control).
The cat was standing on the platform with two forelimbs and two hindlimbs, and compensated for the platform tilts by producing corrective movements with all four limbs (Fig. 1A–C).
Test 2F.
The hindquarters of the cat were suspended in a hammock and slightly lifted, so that the hindlimbs were hanging freely and did not touch the platform, and thus were largely deprived of the ability to signal displacements of the platform. In this test, compensation for the platform tilts was performed by the two forelimbs, which remained on the platform (Fig. 1D).
Test 2H.
The forequarters of the cat were suspended in the hammock and slightly lifted, so that the forelimbs were hanging freely and did not touch the platform, and thus did not signal displacements of the platform. In this test, compensation for the platform tilts was performed by the two hindlimbs, which remained on the platform (Fig. 1E).
Test 2F2H/Anti.
In this test, the two platforms were uncoupled, and the cat was standing with its forelimbs on the F-platform and with its hindlimbs on the H-platform, while the platforms were tilted in antiphase (Fig. 1F).
Contribution of afferent inputs from individual limbs (right or left) of the same girdle to the tilt-related modulation of RNNs
Tests RF and LF.
The hindquarters of the cat were suspended in a hammock. In addition, one of the forelimbs was lifted. For this purpose, an experimenter took the limb by hand (in the elbow or the ankle region), lifted it for a few centimeters from the platform, and kept in this position during the test (∼10 s). Thus, in this test both of the hindlimbs and one of the forelimbs were deprived from the ability to signal displacements of the platform. Corrective postural movements in these tests were performed either by the right (test RF) or left (test LF) forelimb (Fig. 1G).
Tests RH and LH.
The forequarters of the cat were suspended in the hammock. In addition, one of the hindlimbs was lifted. Thus, both of the forelimbs and one of the hindlimbs were not signaling displacements of the platform. Corrective movements were performed either by the right (test RH) or the left (test LH) hindlimb (Fig. 1H).
Data recorded and statistical analysis
The following parameters were recorded during the postural tests: (1) the tilt angle of the platform (Tilt in Fig. 1A); (2) postural corrections, that is, the lateral displacements of the body in relation to the platform, separately in the fore and hind parts of the trunk (BdF and BdH in Fig. 1A); and (3) the normal component of contact forces under each foot (FN in Fig. 1C).
A representative example of the data recording (tests 2F2H and 2F) is shown in Figure 2, A and B. For each RNN, responses to 30–60 tilt cycles were collected in each test (see Fig. 2C). Each of the cycles was divided into 20 equal bins, and the peak of the right tilt was taken as the cycle onset. For each sequential cycle (1–60) of the raster shown in Figure 2C, the firing frequency in each bin was calculated. Then the firing frequency in each bin was averaged over the identical bins in all sequential cycles of the test, and the phase histogram was generated (see Fig. 2D). The Rayleigh test for directionality (p < 0.05) was used to determine whether the activity of the RNN was modulated in relation to tilts (Batshelet, 1981; Fisher, 1993). Only RNNs whose activity in test 2F2H was modulated were selected for this study.
For each modulated RNN, the following characteristics of the activity were assessed using Fourier image of the spike sequence (see Fig. 2D): f(ϕ) = f0 + f1cos(ϕ − ϕ1) + r(ϕ), where ϕ is the phase of the tilt cycle. The constant component f0 of the image provides the average frequency during the test. The first harmonic f1cos(ϕ − ϕ1) is a sine approximation of the one-peak modulation (we found that most modulated RNNs have one dominating peak in their phasic response to periodic tilts). The phase of the peak of the first harmonic indicates the preferred phase of neuronal discharge, ϕ1. Finally, r(ϕ) is the remainder after the first two terms of the series (a sum of higher harmonics). As a measure of periodic, tilt-related RNN modulation, we used the peak-to-peak value of the first harmonic (M1 = 2f1). In addition to these characteristics, the best two-level rectangular fit for the spike distribution was used to determine the “burst” position in the tilt cycle (see Fig. 2D).
All quantitative data are presented as the mean ± SEM. Statistical comparisons were made using t test, with the significance level p = 0.05.
Histological procedures
At the termination of experiments, cats were deeply anesthetized with pentobarbital sodium. Several reference lesions were made in the region from which neurons were sampled. Cats were then perfused with isotonic saline followed by a 10% formalin solution. Frozen brain sections of 50 μm thickness were cut in the regions of recording and stimulating electrodes. The tissue was stained for Nissl substance with cresyl violet. The position of the stimulation electrode in the rubrospinal tract was verified by observation of electrode track gliosis (see Fig. 2I). Positions of recording tracks in the red nucleus (see Fig. 2G,H) were estimated in relation to reference lesions.
Results
Postural motor responses
Postural motor responses in different tests were largely similar to those described in our previous papers (Deliagina et al., 2006a; Karayannidou et al., 2008). In brief, when all limbs were standing (test 2F2H) (Fig. 1A–C), tilts of the platform evoked postural corrections, i.e., lateral displacements of the trunk in the direction opposite to tilt, with a peak-to-peak value of 6–8 cm (Fig. 2A). Corrective movements were caused by extension of the limbs on the side moving down, and flexion of the limbs on the opposite side. Due to postural corrections, cats maintained the dorsal-side-up orientation, and stabilized their head position against the feeder.
When the two parts of the platform were tilted in antiphase (test 2F2H/Anti) (Fig. 1F), cats stabilized the dorsal-side-up orientation of both the forequarters and hindquarters. Corrective movements in the forequarters and hindquarters in this test were in antiphase to each other and to the corresponding platform, with amplitudes similar to that in test 2F2H.
When only two forelimbs (test 2F) (Fig. 1D) or only two hindlimbs (test 2H) (Fig. 1E) were standing on the platform, the platform tilts evoked postural corrections—lateral displacements of the forequarters or the hindquarters, respectively, in the direction opposite to tilt. These corrective movements were caused by extension of the limb on the side moving down, and flexion of the limb on the opposite side. The amplitudes of corrective movements in these tests were similar to that in test 2F2H. In test 2F, the cat effectively stabilized its head position against the feeder. In test 2H, the cat effectively stabilized the dorsal-side-up orientation of its hindquarters, with postural corrections of 5–6 cm peak to peak. In tests 2F and 2H, the active pair of limbs was loaded by half of the body weight.
When only one limb was standing on the platform (tests RF, LF, RH, and LH), this limb was loaded by half of the body weight. Tilts of the platform evoked corrective motor responses in the standing limb—extension when the platform under the limb was moving downward, and flexion when it was moving upward. In tests RF and LF, the position of the forequarters was effectively stabilized; in tests RH and LH, the position of the hindquarters was also stabilized but less effectively.
Database
Altogether, 216 neurons were recorded during tilts. All penetrations were histologically verified to lie within the boundaries of the left red nucleus, as illustrated in Figure 2, G and H. In many of these RNNs (n = 99, 46%), the activity was phasically modulated in relation to the tilt cycle, as in the neuron in Figure 2, A, C, and D. These 99 modulated RNNs were used for the analysis.
All 99 RNNs were tested for receptive fields, and receptive fields could be identified for 89 neurons. Forty-five cells were activated by limb proprioceptors; 18 of them were activated by movement at definite joint, and 27 of them by movement at two or three joints (most often in many directions). Thirty-five cells were activated from a large area on the forelimb, on the hindlimb, or on both limbs. Nine cells responded mainly to tactile stimulation.
In the majority of neurons (75/99), the receptive field was restricted to the contralateral forelimb (n = 38) or hindlimb (n = 37), which allowed us to assign these neurons to the forelimb or hindlimb groups. However, in a small proportion of cells, the receptive fields were not restricted to only one limb, or were not identified. These neurons were assigned to the forelimb or hindlimb group based on the response to lifting forequarters or hindquarters during standing (postural tests 2F and 2H). As described in the next section, in all RNNs classified on the basis of their receptive field position, the postural responses strongly decreased either in test 2H (forelimb RNNs) (see Fig. 4) or in test 2F (hindlimb RNNs) (see Fig. 5). Thus, few neurons with large or unidentified receptive fields, which decreased discharge when forelimbs or hindlimbs were lifted, were assigned to the forelimb (n = 9) or hindlimb (n = 15) groups, so that the groups included 47 and 52 neurons, respectively.
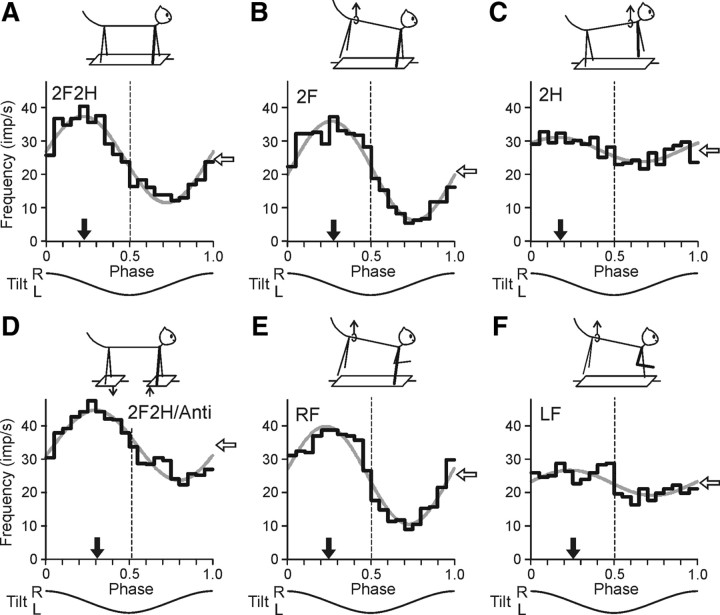
Figure 4.
Activity of a forelimb-related RRN during different postural tests. A, Control (test 2F2H). The projection limb is shown by a thick line. B, Standing on two forelimbs (test 2F). C, Standing on two hindlimbs (test 2H). D, Antiphase tilts of the F and H platforms (test 2F2H/Anti). E, Standing on the right forelimb (test RF). F, Standing on the left forelimb (test LF). For each test, the following are shown: (1) the phase histogram of spike activity in the tilt cycle (black line), (2) the first harmonic of Fourier image (gray line), (3) the mean frequency of discharge (white arrow), and (4) the preferred phase (black arrow).
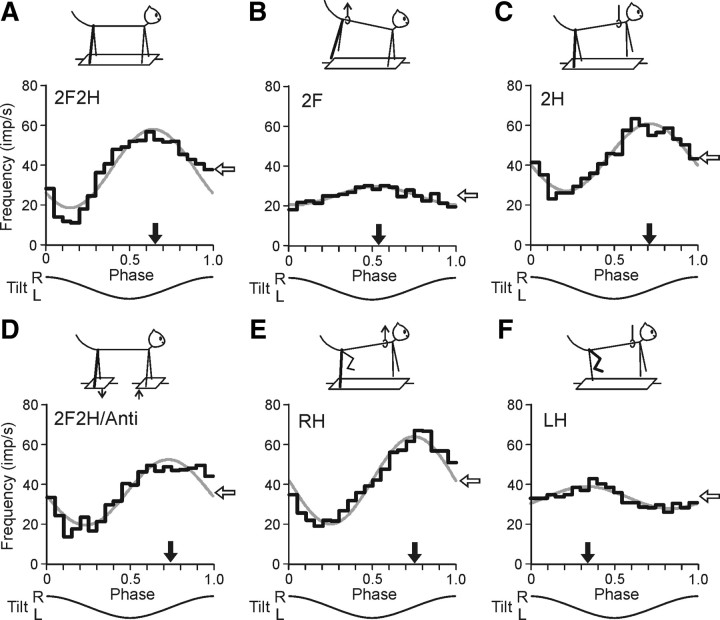
Figure 5.
Activity of a hindlimb-related RNN during different postural tests. A, Control (test 2F2H). B, Standing on two forelimbs (test 2F). C, Standing on two hindlimbs (test 2H). D, Antiphase tilts of the F and H platforms (test 2F2H/Anti). E, Standing on the right hindlimb (test RH). F, Standing on the left hindlimb (test LH). For each test, the following are shown: (1) the phase histogram of spike activity in the tilt cycle (black line), (2) the first harmonic of Fourier image (gray line), (3) the mean frequency of discharge (white arrow), and (4) the preferred phase (black arrow).
Histology confirmed that the stimulating electrode implanted into the rubrospinal tract was accurately positioned in both cats (Fig. 2I). Most of the forelimb and the hindlimb RNNs (26/47 and 31/52, respectively) were identified as rubrospinal on the basis of their responses to RS tract stimulation via the collision test (Fig. 2F). The main characteristics of rubrospinal and unidentified RNNs (mean frequency and modulation) statistically did not differ (Table 1). The phase distributions of rubrospinal and unidentified RNNs were also similar (Fig. 3A,D). Since unidentified cells were often recorded among the identified cells, one can suggest that the lack of identification was due to an inability to activate all rubrospinal axons. Therefore, we analyzed all RNNs together.
Table 1.
Characteristics of RNNs responding and not responding antidromically
| Forelimb RNNs |
Hindlimb RNNs |
|||
|---|---|---|---|---|
| Resp (n = 26) | No resp (n = 21) | Resp (n = 31) | No resp (n = 21) | |
| Freq (imp/s) | 29.6 ± 4.3 | 23.4 ± 4.1 | 36.6 ± 3.8 | 27.1 ± 3.3 |
| Modul (imp/s) | 23.6 ± 4.1 | 16.8 ± 2.3 | 40.8 ± 4.3 | 30.7 ± 4.6 |
Values are means ± SEM. No significant difference between the data for responding and not responding RNNs was found.
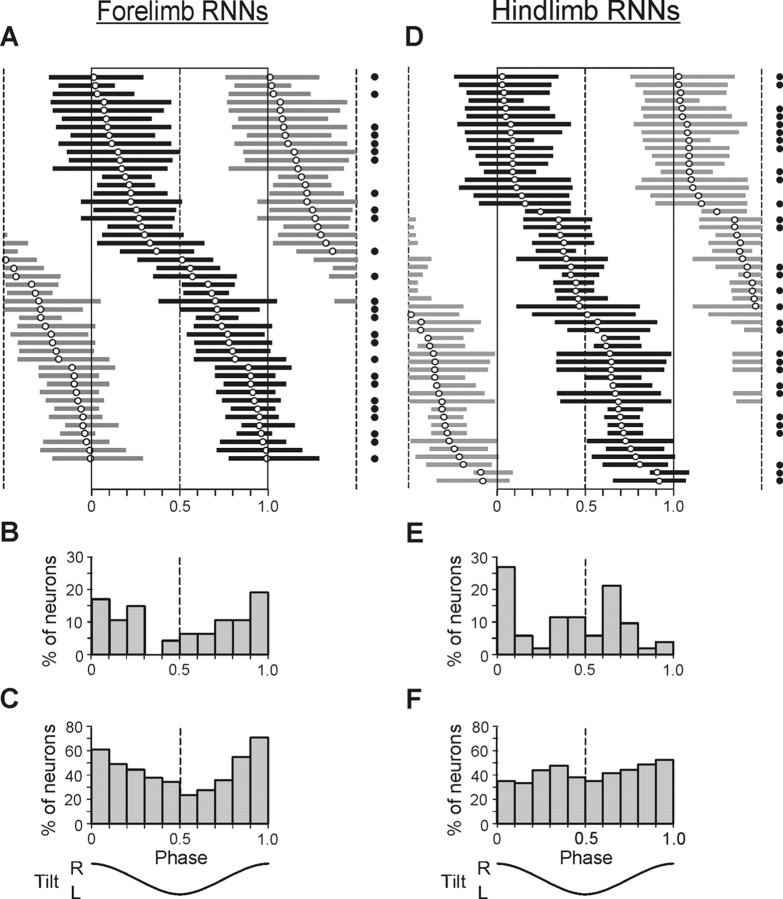
Figure 3.
Phase characteristics of forelimb-related RNNs (A–C) and hindlimb-related RNNs (D–F). A, D, Phase distribution of bursts of individual RNNs in the tilt cycle, with the preferred phase indicated (empty circles). The burst of activity of each neuron is presented in black only one time in the cycle. Neurons are rank ordered according to their preferred phase. Filled circles on the right indicate antidromically responding neurons. B, E, Percentage of RNNs with preferred phases in different parts of the cycle. C, F, Percentage of RNNs, which were active simultaneously in different phases of the cycle.
Responses of individual RNNs
Figure 3 shows phase characteristics of activity for all 99 modulated neurons, both forelimb-related (A–C) (n = 47) and hindlimb-related (D–F) (n = 52), obtained during normal, four-limb standing (test 2F2H). In both groups, the phases of activity of individual neurons (characterized by their preferred phase and burst position) were distributed rather evenly over the whole tilt cycle. However, the forelimb group reduced activity in the middle of the cycle, which is reflected in the histogram of preferred phases (Fig. 3B) and in the histogram of the number of simultaneously active neurons (Fig. 3C). The hindlimb group had two peaks of activity, in the first and in the second half of the cycle, which is reflected in the histogram of preferred phases (Fig. 3E).
Figure 4 shows a representative example of responses in different tests recorded in a forelimb RNN (#139) identified as a rubrospinal neuron. The neuron exhibited a pronounced modulation of its discharge frequency during the control test (2F2H) (Fig. 4A), with modulation value M1 = 24.1 impulses (imp)/s, and phase of peak activity (preferred phase) ϕ1 = 0.23.
When the hindquarters were lifted and only the forelimbs were standing on the platform (test 2F) (Fig. 4B), the response slightly increased (M1 = 29.5 imp/s), and the preferred phase (ϕ1 = 0.28) differed by only 0.05 from that in control. By contrast, when the forequarters were lifted and the hindlimbs were standing on the platform (test 2H) (Fig. 4C), the response decreased significantly (M1 = 7.9 imp/s), and its preferred phase (ϕ1 = 0.18) changed by 0.1. During antiphase tilts (test 2F2H/Anti) (Fig. 4D), the response slightly decreased (M1 = 20.0 imp/s), and the preferred phase (ϕ = 0.31 in the cycle of P1 platform) differed by only 0.08 from that in control.
When only the right forelimb was standing on the platform (test RF) (Fig. 4E), the modulation slightly increased (M1 = 27.6 imp/s), and the preferred phase (ϕ1 = 0.24) was similar to that in control. However, when only the left forelimb was standing on the platform (test LF), (Fig. 4F) the response was small (M1 = 7.4 imp/s), but the preferred phase (ϕ1 = 0.25) was similar to that in control. The mean frequency of the neuron (white arrow) practically did not change in tests 2H and LF (Fig. 4), while the neuronal response decreased considerably.
The results illustrated in Figure 4 demonstrated that this particular RNN received its main tilt-related sensory input from the contralateral (right) forelimb, and additional very small inputs from the ipsilateral forelimb and from the hindlimbs. The phase of additional input from the ipsilateral limb did not differ significantly from that of the main input.
Figure 5 shows a representative example of responses to tilts recorded in a hindlimb RNN (#194) identified as a rubrospinal neuron. The neuron was strongly modulated (M1 = 39.5 imp/s, ϕ1 = 0.64) during the control test (2F2H) (Fig. 5A). When only the hindlimbs were standing on the platform (test 2H) (Fig. 5C), the response slightly decreased (M1 = 33.8 imp/s), and the preferred phase (ϕ1 = 0.71) differed by only 0.07 from that in control. By contrast, when the hindquarters were lifted and the forelimbs were standing (test 2F) (Fig. 5B), the response decreased considerably (M1 = 9.2 imp/s), and its temporal pattern slightly changed (ϕ1 = 0.52). During antiphase tilts (test 2F2H/Anti) (Fig. 5D), the response slightly decreased (M1 = 32.9 imp/s), and the preferred phase (ϕ = 0.73 in the cycle of P2 platform) differed by only 0.09 from that in control.
When only the right hindlimb was standing (test RF) (Fig. 5E), the modulation slightly increased (M1 = 43.9 imp/s), and the preferred phase (ϕ1 = 0.75) was similar to that in control. However, when only the left hindlimb was standing on the platform (test LF) (Fig. 5F), the response was small (M1 = 11.1 imp/s), and the neuron was slightly more active in the first half of the cycle (ϕ1 = 0.34). From Figure 5 one can also see that the mean frequency of the neuron (white arrow) slightly decreased in tests 2F and LH.
The results illustrated in Figure 5 demonstrated that this particular RNN received its main tilt-related input from the contralateral (right) hindlimb, and additional very small inputs from the ipsilateral hindlimb and from the forelimbs. The phase of additional input from the ipsilateral limb differed considerably from that of the main input.
Results shown for RNN#179 (Fig. 4) and for RNN#194 (Fig. 5) were typical for the forelimb and the hindlimb RNNs, respectively. In the majority of them, the phase and the amplitude of tilt-related modulation were determined mainly by input from the contralateral forelimb or hindlimb. These common features were reflected in the population characteristics of RNNs.
Population characteristics of RNNs
Three characteristics were used to describe postural responses in the forelimb and in the hindlimb RNN populations: (1) The value of response (modulation, M1). (2) The mean frequency in the tilt cycle (f0). These values were averaged over the whole (forelimb or hindlimb) population of RNNs. (3) The distribution of preferred phases of response (ϕ1) of forelimb or hindlimb RNNs over the tilt cycle. To evaluate the contribution of different limbs to the generation of RNN responses to tilts, we compared these three characteristics across different tests.
Influences from shoulder and hip girdles
Forelimb RNNs.
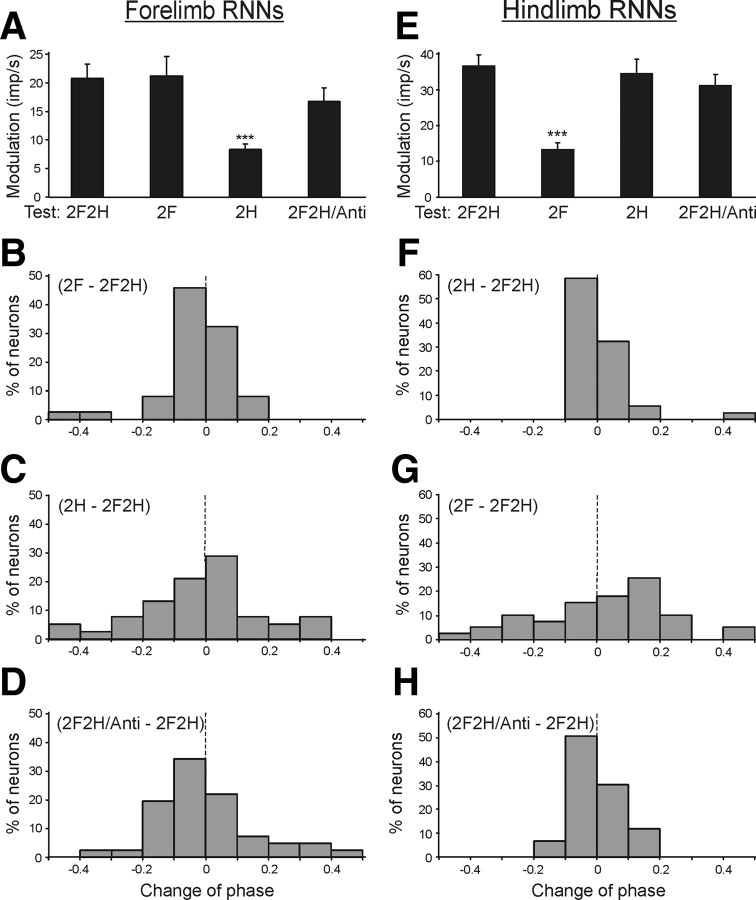
The contributions of postural mechanisms of an individual (shoulder or hip) girdle to the periodic modulation of the forelimb RNNs was examined by lifting the hindquarters or forequarters, as well as by tilting them in antiphase. Figure 6A shows that, when the cat stood on the forelimbs only (test 2F), the value of response (modulation) did not change significantly as compared to control (test 2F2H). By contrast, standing on only the hindlimbs (test 2H) led to a considerable decrease of the response. The values of responses in different tests (mean ± SEM) are given in Table 2, together with the mean value of frequency in the tilt cycle.
Figure 6.
Population characteristics of forelimb RNNs (A–D) and hindlimb RNNs (E–H) in tests revealing influences from shoulder and hip girdles. A, E, Mean value of modulation. B–D, F–H, Algebraic differences between preferred phases of individual RNNs in tests 2F and 2F2H (B, G), in tests 2H and 2F2H (C, F), and in tests 2F2H/Anti and 2F2H (D, H).
Table 2.
Characteristics of inputs to RNNs from different girdles
| Test | Forelimb RNNs |
Hindlimb RNNs |
||
|---|---|---|---|---|
| Modulation (imp/s) | Frequency (imp/s) | Modulation (imp/s) | Frequency (imp/s) | |
| 2F2H | 20.7 ± 2.6 | 26.3 ± 2.9 | 36.5 ± 3.2 | 32.5 ± 2.6 |
| 2F | 21.1 ± 3.4 | 25.2 ± 3.2 | 13.2 ± 1.8*** | 33.2 ± 3.3 |
| 2H | 8.3 ± 1.0*** | 23.6 ± 3.5 | 34.3 ± 4.0 | 31.5 ± 1.9 |
| 2F2H/Anti | 16.7 ± 2.5 | 28.3 ± 3.2 | 31.1 ± 3.2 | 32.2 ± 2.4 |
Values are means ± SEM;
***significant difference from test 2F2H (see Fig. 6A,E).
Lifting of the forequarters and lifting of the hindquarters produced also very different effects on the phases of RNN responses. A histogram in Figure 6B shows phase shift in test 2F as compared to control. In the majority of neurons (29/37, or 78%), the phase shift was <0.1. By contrast, in test 2H, phase shift in half of the neurons (19/38, or 50%) was >0.1 (Fig. 6C). Thus, lifting of the hindquarters produced a weak effect on the phases of forelimb RNNs, whereas lifting of the forequarters produced a stronger effect.
An interaction of influences from the two girdles upon the forelimb RNNs was examined in test 2F2H/Anti, with antiphase tilts of the forequarters and hindquarters. As shown in Figure 6A and Table 2, the response of RNNs to tilts (modulation) slightly decreased in test 2F2H/Anti as compared to control (p > 0.05). However, their mean frequency slightly increased (p > 0.05). We compared the phases of responses of individual RNNs in test 2F2H/Anti and test 2F2H (in test 2F2H/Anti, phase measurements were performed in relation to the tilt cycle of the F-platform). It was found that the phase shift in test 2F2H/Anti in relation to control was small (<0.1) in the majority of neurons (23/41, or 56%) (Fig. 6D).
To summarize, these results suggest that the tilt-related modulation of forelimb RNNs is primarily determined by the tilt-related sensory information coming from the forelimb afferents.
Hindlimb RNNs.
The contribution of the postural mechanisms of individual girdles to the periodic modulation of the hindlimb RNNs was examined with the same methods as the forelimb RNNs, that is, by lifting the forequarters or hindquarters, as well as by tilting them in antiphase. When the cat stood on the hindlimbs only (test 2H), the response was similar to that in control (test 2F2H). By contrast, standing on only two forelimbs (test 2F) led to a considerable decrease of the response (Fig. 6E, Table 2). The mean frequencies in these tests did not differ significantly (Table 2).
Lifting of the hindquarters and lifting of the forequarters produced different effects on the phases of RNN responses. A histogram in Figure 6F shows the phase shift in test 2H in relation to control. In the majority of neurons (34/37, or 92%), it was <0.1. By contrast, in test 2F, phase shift in the majority of neurons (26/39, or 67%) was >0.1 (Fig. 6G).
The interaction of influences from the two girdles upon the hindlimb RNNs was examined in test 2F2H/Anti. As shown in Figure 6E and in Table 2, the response of RNNs to tilts in test 2F2H/Anti was similar to that in control (p > 0.05). The mean frequency in tests 2F2H and 2F2H/Anti did not differ significantly. We also compared the phases of responses of individual RNNs in these tests (in test 2F2H/Anti, phase measurements were performed in relation to the tilt cycle of the H-platform). It was found that the phase shift in test 2F2H/Anti relative to control was small (<0.1) in the majority of neurons (42/52, or 81%) (Fig. 6H).
One can thus conclude that the tilt-related modulation of the hindlimb RNNs is primarily based on the sensory information coming from the hindlimb afferents.
Influences from ipsilateral and contralateral limb
Forelimb RNNs.
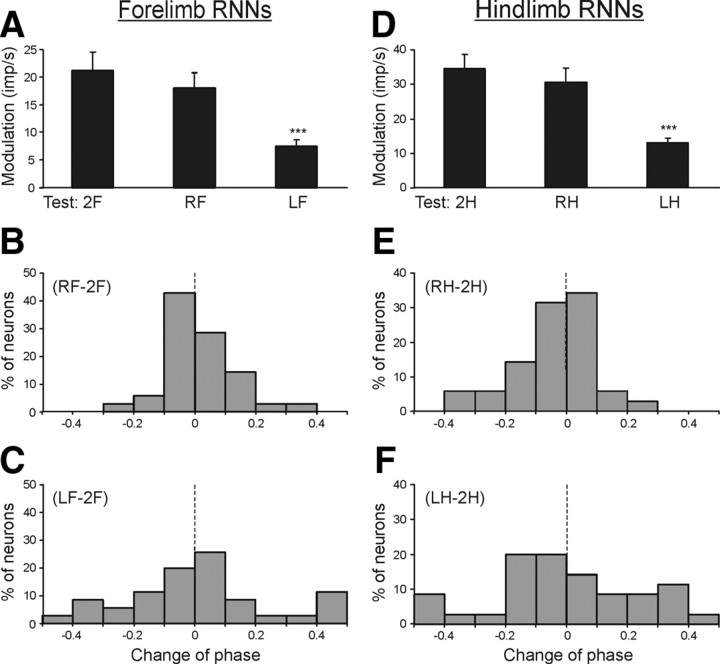
The contribution of postural mechanisms of a single forelimb to the periodic modulation of forelimb RNNs was examined by lifting one of the forelimbs in addition to lifting the hindlimbs.
Figure 7A shows that standing on the right forelimb only (test RF) caused an insignificant decrease of response (modulation) compared to test 2F (standing on both forelimbs). By contrast, standing only on the left forelimb (test LF) caused a considerable decrease of response. The values of responses in these tests (mean ± SEM) as well as the mean values of the mean frequency in the cycle are given in Table 3. The mean frequencies in tests 2F, LF, and RF did not differ significantly.
Figure 7.
Population characteristics of forelimb RNNs (A–C) and hindlimb RNNs (D–F) in tests revealing influences from individual limbs of the same girdle. A, D, Mean value of modulation. B, C, E, F, Algebraic differences between preferred phases of individual RNNs in tests RF and 2F (B); in tests RH and 2H (E); in tests LF and 2F (C); in tests LH and 2H (F).
Table 3.
Characteristics of inputs to RNNs from different limbs of the same girdle
| Test | Forelimb RNNs |
Hindlimb RNNs |
||
|---|---|---|---|---|
| Modulation (imp/s) | Frequency (imp/s) | Modulation (imp/s) | Frequency (imp/s) | |
| 2F | 21.1 ± 3.4 | 25.2 ± 3.2 | — | 33.2 ± 3.3 |
| RF | 17.9 ± 2.8 | 24.6 ± 3.0 | — | — |
| LF | 7.5 ± 1.1*** | 24.6 ± 3.1 | — | — |
| 2H | — | 23.6 ± 3.5 | 34.3 ± 4.0 | 31.5 ± 1.9 |
| RH | — | — | 30.4 ± 4.0 | 34.9 ± 2.0 |
| LH | — | — | 12.8 ± 1.5*** | 36.8 ± 2.9 |
Values are means ± SEM;
***significant difference from test 2F or 2H (see Fig. 7A,D).
Lifting of the right forelimb and lifting of the left forelimb produced different effects on the phases of RNN responses. A histogram in Figure 7B shows shift of the preferred phase in test RF in relation to test 2F. In the majority of neurons (25/35 or 71%), the shift of preferred phase was <0.1. By contrast, in test LF the shift of the preferred phase was >0.1 in the majority of neurons (19/35 or 54%) (Fig. 7C).
The results described in this section suggest that the sensory input from the contralateral limb of the shoulder girdle contributes much more strongly to the tilt-related modulation of the forelimb RNNs than the input from the ipsilateral limb.
Hindlimb RNNs.
The contribution of postural mechanisms of a single hindlimb to the periodical modulation of hindlimb RNNs was examined in the same way as for the forelimb RNNs. Figure 7D shows that standing on the right hindlimb (test RH) caused only a slight decrease of the response as compared to test 2H (standing on both hindlimbs). By contrast, standing on the left hindlimb (test LH) caused a significant decrease of the response. The values of the responses in these tests (mean ± SEM), as well as the mean values of the mean frequency are shown in Table 3. The mean frequencies in these tests did not differ significantly.
Lifting of the left or right hindlimb also produced different effects on the phases of RNN responses. A histogram in Figure 7E shows the phase shift in test RH in relation to test 2H. In the majority of neurons (23/35, or 66%), the phase shift was <0.1. By contrast, in test LH, the phase shift was >0.1 in the majority of neurons (23/35, or 66%) (Fig. 7F).
The results described in this section suggest that the sensory input from the contralateral hindlimb contributes much more strongly to the tilt-related modulation of hindlimb RNNs than the input from the ipsilateral limb.
Discussion
It is known that the rubrospinal system participates in the control of a number of motor behaviors, such as reaching and grasping (Gibson et al., 1985; Mewes and Cheney, 1994; van Kan and McCurdy, 2001; Horn et al., 2002), stepping (Orlovsky, 1972; Lavoie and Drew, 2002), and scratching (Arshavsky et al., 1978). The basic pattern of these movements is generated by central mechanisms, whereas sensory influences modulate this pattern and adapt it to environmental conditions. In contrast to these centrally generated movements, the feedback mode of the control of body posture is based on somatosensory information, which determines the timing and value of responses to postural perturbations (Horak and Macpherson, 1996; Deliagina et al., 2006b). In the present study, we have demonstrated that neurons of the red nucleus (most of which were identified as rubrospinal ones) were strongly modulated by afferent inputs signaling postural perturbations. This principal finding suggests that the rubrospinal system participates in the control of body posture, i.e., in the motor behavior based on reflex mechanisms. What is the origin and functional role of rubrospinal commands?
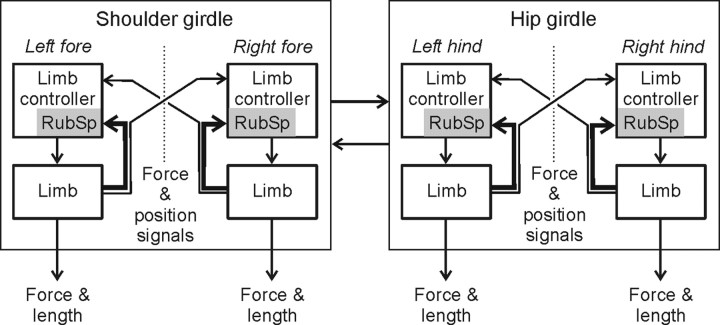
Functional organization of the postural system, responsible for the dorsal-side-up body orientation in quadrupeds, has been characterized in our previous studies (Beloozerova et al., 2003; Deliagina et al., 2006a). This system consists of two relatively independent subsystems, one for the shoulder girdle and the other for the hip girdle (Fig. 8). They compensate for tilts of the anterior and posterior parts of the body, respectively. Each subsystem includes two controllers, one for the left limb and one for the right limb (Fig. 8). Each controller contains a reflex mechanism driven by somatosensory input from its own limb. These local reflexes partly compensate for tilts by flexing or extending the limb. The controllers also receive weaker sensory inputs from the contralateral limbs. The motor responses to these crossed influences are added to the local reflexes. The forelimb and hindlimb controllers exert influences on each other promoting their mutual coordination.
Figure 8.
Role of rubrospinal mechanisms in postural control. The scheme shows sensorimotor processing in the postural system stabilizing the dorsal-side-up trunk orientation in the standing cat (adapted from Deliagina et al., 2006a). The system consists of two subsystems compensating for tilts of the shoulder and hip girdles. Each subsystem includes the controllers for the left and right limbs. Each limb controller contains a reflex mechanism driven by somatosensory input from its own limb. These local reflexes partly compensate for tilts. The limb controllers also receive somatosensory input (direct or subjected to processing) from the opposite limbs. The motor responses to these crossed influences are added to the local reflexes. The forelimb and the hindlimb controllers influence each other promoting their mutual coordination. The rubrospinal mechanisms (gray rectangles) constitute a part of each limb controller; they are primarily involved in the feedback control of their own limb (corresponding sensory inputs are shown by large arrows). The rubrospinal mechanisms are less involved in the coordination of activity between the two limbs within a girdle, as well as between the shoulder and hip girdles (corresponding sensory inputs are shown by small arrows).
In the framework of this model, the present study has demonstrated that the tilt-related modulation of the activity in a given RNN depends largely on the sensory input from its own (target) limb. This conclusion was primarily based on the finding that standing on the target limb alone did not reduce the modulation and did not change its phase, despite the fact that sensory inputs from the three other limbs were severely attenuated (Figs. 6, 7). These results strongly suggest that, in postural tasks, the rubrospinal system constitutes a part of the limb controller, and is primarily involved in the feedback control of the functional length of the target limb. The corresponding sensory influences are shown by large arrows in the scheme of the sensorimotor processing in the postural system (Fig. 8).
The input from the opposite limb and the input from the limbs of the other girdle make a much smaller contribution to the modulation of RNNs. This conclusion was based on the finding that lifting the target limb strongly reduced the tilt-related RNN modulation (as compared to the standing limb) and often caused a change in the phase of this modulation (Figs. 6, 7). These results suggest that, in postural tasks, the rubrospinal system is much less involved in the coordination of activity between the two limbs within a girdle, and between the two girdles. The corresponding sensory influences are shown in Figure 8 by small arrows.
Thus, the somatosensory input from the own limb is the primary source of the responses rubral neurons exhibit to tilts during standing. However, it remains unclear which groups of limb afferents provide the signals for driving RNNs in this motor task. It seems unlikely that the afferents that are activated by stimulation of the receptive field of a neuron at rest are responsible for the tilt-related modulation of this neuron. Less than half of RNNs were activated by proprioceptive input during stimulation of deep limb structures (muscles and joints). Even the neurons with proprioceptive input could be activated by both flexion and extension of the limb, or by movements in several planes. It was also reported that passive movements about specific joints caused only minor changes in the RNNs activity (Gibson et al., 1994; Lavoie and Drew, 2002). When an active behavior is taking place, it is possible that the input from receptive field afferents is replaced by a more specific proprioceptive input, which provides detailed information about limb movements. This hypothesis could be further supported by the view that the signals from limb mechanoreceptors are processed before they reach the rubral neurons via two main routes—cerebellum (Massion, 1967; Toyama et al., 1968) and motor cortex (Asanuma, 1989).
As shown in the present study, the mean frequencies of RNNs in different postural tests (averaged over the RNN populations) were rather similar—they ranged from 23.6 to 28.3 imp/s in forelimb RNNs and from 31.5 to 33.2 imp/s in hindlimb RNNs. This was in contrast with the tilt-related modulation of RNNs, which was considerably smaller when the target limb was lifted (Tables 2, 3). This finding suggests different origins of two components of RNN activity, i.e., the reflex origin of phasic modulation and the central origin for the background activity.
How do the rubrospinal commands (tilt-related activity of RNNs) correlate with the postural responses to tilts? As shown previously (Beloozerova et al., 2003, 2005), all limb extensors are active during standing, and platform tilts cause a deep modulation of extensor EMGs, with their peak during ipsilateral downward tilting the platform. With our definition of the tilt cycle (Fig. 2D), the extensor activity in the target (right) limbs is higher in the second half of the cycle (phases 0.7–0.8). We can compare this pattern with phasic modulation of RNNs (Fig. 3). In the forelimb RNNs, the population activity, i.e., the number of simultaneously active neurons, increased about twice by the end of cycle (phase 0.9) as compared to its middle part (Fig. 3C). In the hindlimb RNNs, this increase was much smaller (Fig. 3F). Therefore, the proportion of simultaneously active RNNs was slightly larger during the period of increasing extensor activity. This result differs from what could be expected taking into account a prevalence of flexor motor effects in the rubrospinal system (Massion, 1967; Hongo et al., 1969a,b, 1972). Thus, the role of RNNs in the postural task was difficult to assess on the basis of a simple correlation between the activity of RNNs and the motor pattern. This could be due to a variety of motor effects produced by the rubrospinal system, and their selective gating in different motor tasks (Rho et al., 1999).
The activity of rubrospinal system in the postural task has many features in common with the previously studied activity of the corticospinal system in the identical task (Beloozerova et al., 2005; Karayannidou et al., 2008). First, a considerable proportion of neurons in both systems were phasically modulated by tilts, though the proportion was smaller in rubral neurons (46%) than in cortical ones (90%). Second, in both descending systems, the key role in this modulation was played by the somatosensory input from the target limb. Third, in both systems, the phases of activity of individual neurons were distributed over the whole tilt cycle, and a simple correlation between the population activity and the motor pattern was not found. A strong resemblance in the pattern of activity of these two descending systems was also observed during locomotion, both unobstructed and voluntarily modified (for discussion, see Lavoie and Drew, 2002).
It is interesting to compare the activity of rubrospinal system during two involuntary (“automatic”) motor behaviors, i.e., unobstructed locomotion (Lavoie and Drew, 2002) and maintenance of body posture (present study). There are considerable differences between RNN activities in these two motor tasks. First, the proportion of modulated RNNs was larger during locomotion than during standing (∼90% against ∼50%). Second, both characteristics of RNN activity (mean frequency and modulation) were about two times larger in the locomotor task than in the postural task. Third, in the postural task, the preferred phases of RNN activity were distributed over the tilt cycle, slightly prevailing in its extensor part. By contrast, during locomotion, the phases of RNN activity were concentrated in the flexor (swing) phase of the step. Some of these differences can be explained by a higher motor activity during locomotion, with involvement of both flexors and extensors. By contrast, participation of flexor-related RNNs in extensor-related postural tasks is not required. It is also possible that the rubrospinal system is less involved in the task of reflex generation of motor output than in the task of its central generation.
To summarize, by recording individual rubral neurons, this study has demonstrated involvement of the rubrospinal system in the control of body posture, i.e., the motor behavior based on reflex mechanisms. Postural perturbations lead to activation of limb afferents, which cause responses of rubral neurons accompanied by postural corrections. Sensory input from the target limb plays a key role in generation of these responses, suggesting that the rubrospinal system is involved in intralimb postural coordination.
Footnotes
This work was supported by grants from the National Institutes of Health (NIH) (R01 NS-049884), Swedish Research Council (no. 11554), and Erik and Edith Fernströms Foundation to T.G.D., by NIH Grants R01 NS-39340 and R01 NS-058659 to I.N.B., and by a grant from the Swedish Research Council (no. 21076) to P.V.Z. We are grateful to Erik E. Stout for valuable comments on the manuscript and to Peter Wettenstein for excellent engineering assistance.
References
- Arshavsky YI, Orlovsky GN, Pavlova GA, Perret C. Messages conveyed by descending tracts during scratching in the cat. II. Activity of rubrospinal neurons. Brain Res. 1978;159:111–123. doi: 10.1016/0006-8993(78)90113-0. [DOI] [PubMed] [Google Scholar]
- Asanuma H. The motor cortex. New York: Raven; 1989. [Google Scholar]
- Batshelet E. Circular statistics in biology. New York: Academic; 1981. [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol. 2005;93:1831–1844. doi: 10.1152/jn.00577.2004. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The identification of single units in central visual pathways. J Physiol. 1962;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol. 2006a;573:211–224. doi: 10.1113/jphysiol.2006.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology. 2006b;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev. 2008;57:212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of circular data. Cambridge, UK: Cambridge UP; 1993. [Google Scholar]
- Fuller JH, Schlag JD. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;112:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol. 1985;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AR, Horn KM, Van Kan PLE. Grasping cerebellar function. In: Bennett KMB, Castiello U, editors. Insights into the reach to grasp movement. Amsterdam: Elsevier Science; 1994. pp. 85–108. [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurons innervating hindlimb muscles in cats. Exp Brain Res. 1969a;7:344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurons. Exp Brain Res. 1969b;7:365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. IV. Effects on interneurons. Exp Brain Res. 1972;15:54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Horak F, Macpherson J. Postural orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of physiology. Exercise: regulation and integration of multiple systems. New York: Oxford UP; 1996. pp. 255–292. [Google Scholar]
- Horn KM, Pong M, Batni SR, Levy SM, Gibson AR. Functional specialization within the cat red nucleus. J Neurophysiol. 2002;87:469–477. doi: 10.1152/jn.00949.2000. [DOI] [PubMed] [Google Scholar]
- Karayannidou A, Deliagina TG, Tamarova ZA, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Influences of sensory input from the limbs on feline corticospinal neurons during postural responses. J Physiol. 2008;586:247–263. doi: 10.1113/jphysiol.2007.144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J, Jacobs R. Postural orientation, equilibrium, and the spinal cord. In: Seil FJ, editor. Advances in neurology. Vol 72. Philadelphia: Lippincott-Raven; 1997. pp. 227–232. Neuronal regeneration, reorganization, and repair. [PubMed] [Google Scholar]
- Massion J. The mammalian red nucleus. Physiol Rev. 1967;47:383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- Mewes K, Cheney PD. Primate rubromotoneuronal cells: parametric relations and contribution to wrist movement. J Neurophysiol. 1994;72:14–30. doi: 10.1152/jn.1994.72.1.14. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of rubrospinal neurons during locomotion. Brain Res. 1972;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, Brodal A. Experimental demonstration of a somatotopical origin of rubrospinal fibers in the cat. J Comp Neurol. 1957;108:225–251. doi: 10.1002/cne.901080204. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Reitboeck HJ. Fiber microelectrodes for electrophysiological recordings. J Neurosci Methods. 1983;8:249–262. doi: 10.1016/0165-0270(83)90038-9. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol. 1999;81:2297–2315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- Toyama K, Tsukahara N, Udo M. Nature of cerebellar influences upon the red nucleus neurons. Exp Brain Res. 1968;4:292–309. doi: 10.1007/BF00235697. [DOI] [PubMed] [Google Scholar]
- van Kan PLE, McCurdy ML. Role of primate red nucleus neurons in controlling hand preshaping during reaching to grasp. J Neurophysiol. 2001;85:1461–1478. doi: 10.1152/jn.2001.85.4.1461. [DOI] [PubMed] [Google Scholar]