Abstract
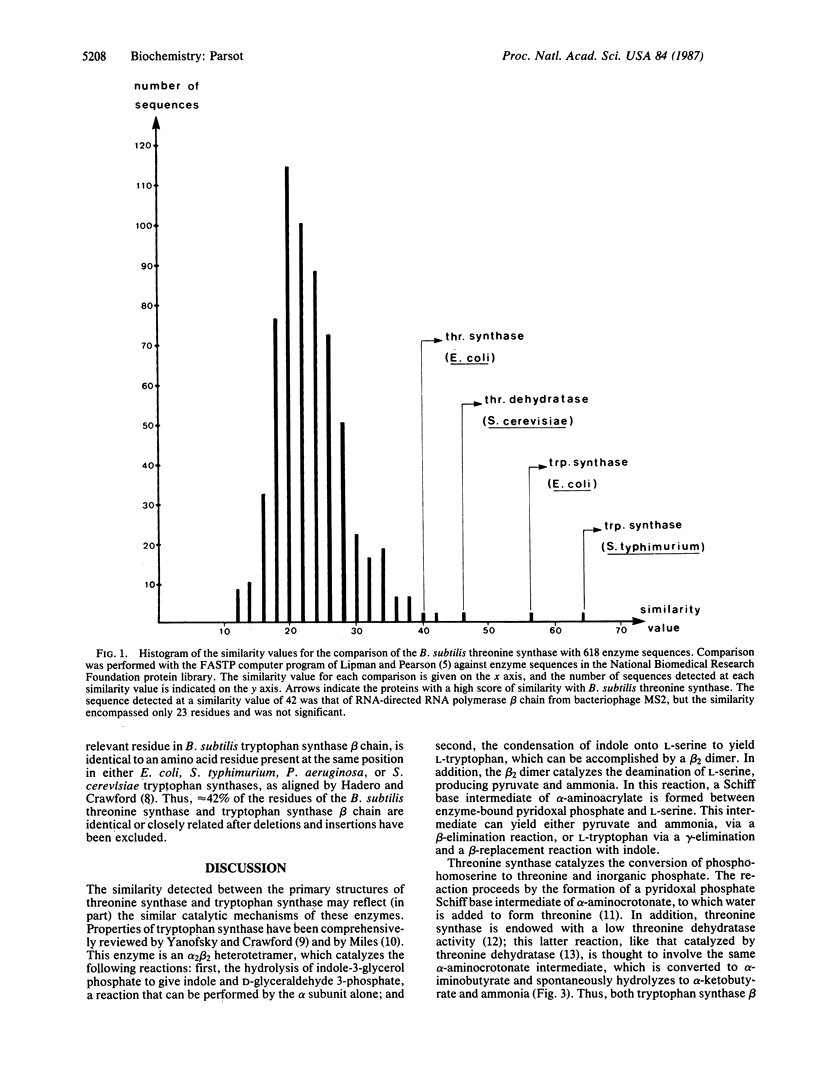
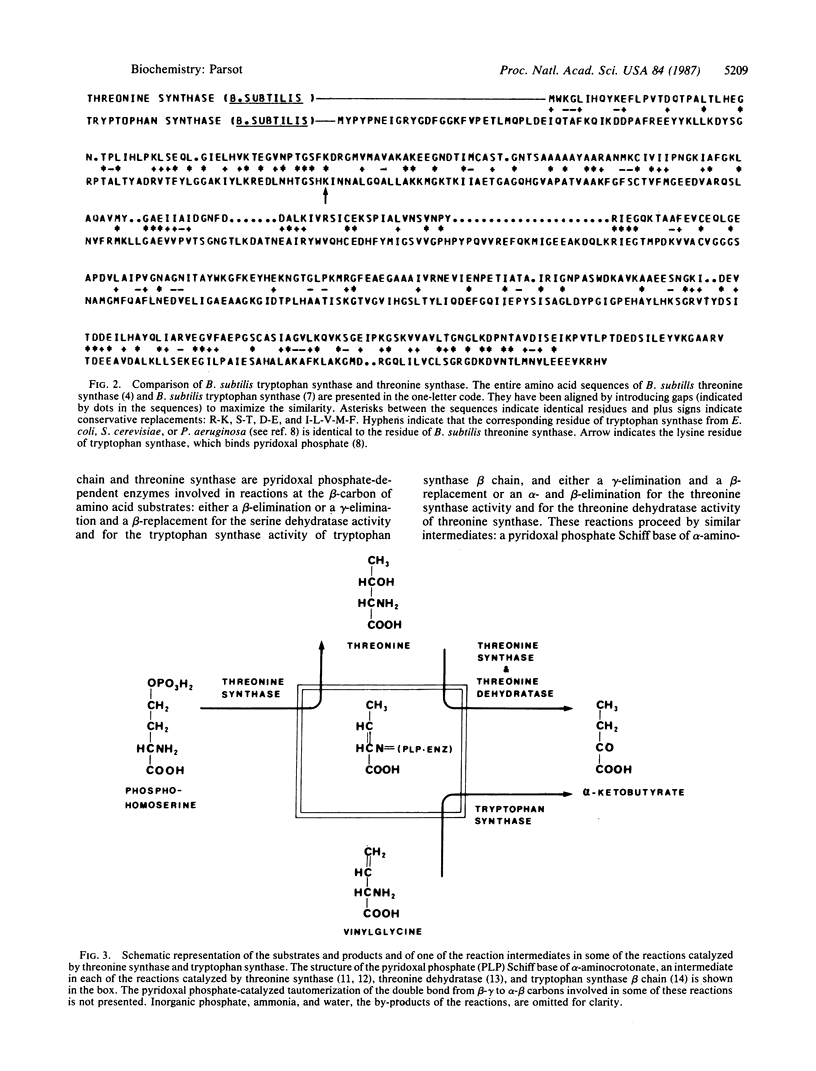
Comparison of the amino acid sequence of Bacillus subtilis threonine synthase with the National Biomedical Research Foundation protein sequence library revealed a statistically significant extent of similarity between the sequence of the tryptophan synthase beta chain from various organisms and that of threonine synthase. This homology in the primary structure of threonine synthase and tryptophan synthase beta chain, which catalyze the last step in the threonine and the tryptophan biosynthetic pathways, respectively, correlates well with some of their catalytic properties and indicates that they have evolved from a common ancestor. The evolutionary relationship between these enzymes supports the hypothesis that primitive enzymes possessed a broad substrate specificity and were active in several metabolic pathways.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Nichols B. P., Yanofsky C. Nucleotide sequence of the trpB gene in Escherichia coli and Salmonella typhimurium. J Mol Biol. 1980 Oct 5;142(4):489–502. doi: 10.1016/0022-2836(80)90259-4. [DOI] [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):787–796. doi: 10.1128/jb.147.3.787-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVIN M., SLAUGHTER C. Threonine synthetase mechanism: studies with isotopic hydrogen. J Biol Chem. 1960 Apr;235:1112–1118. [PubMed] [Google Scholar]
- Hadero A., Crawford I. P. Nucleotide sequence of the genes for tryptophan synthase in Pseudomonas aeruginosa. Mol Biol Evol. 1986 May;3(3):191–204. doi: 10.1093/oxfordjournals.molbev.a040388. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Band L., Shimotsu H. Nucleotide sequence of the Bacillus subtilis tryptophan operon. Gene. 1985;34(2-3):169–177. doi: 10.1016/0378-1119(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Miles E. W. A new type of pyridoxal-P enzyme catalyzed reaction: the conversion of beta, gamma-unsaturated amino acids to saturated alpha-keto acids by tryptophan synthase. Biochem Biophys Res Commun. 1975 Sep 2;66(1):94–102. doi: 10.1016/s0006-291x(75)80299-3. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Tryptophan synthase: structure, function, and subunit interaction. Adv Enzymol Relat Areas Mol Biol. 1979;49:127–186. doi: 10.1002/9780470122945.ch4. [DOI] [PubMed] [Google Scholar]
- Parsot C. Evolution of biosynthetic pathways: a common ancestor for threonine synthase, threonine dehydratase and D-serine dehydratase. EMBO J. 1986 Nov;5(11):3013–3019. doi: 10.1002/j.1460-2075.1986.tb04600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. T., Wood W. A. The mechanism of action of 5'-adenylic acid-activated threonine dehydrase. J Biol Chem. 1965 Dec;240(12):4703–4709. [PubMed] [Google Scholar]
- Skarstedt M. T., Greer S. B. Threonine synthetase of Bacillus subtilis. The nature of an associated dehydratase activity. J Biol Chem. 1973 Feb 10;248(3):1032–1044. [PubMed] [Google Scholar]
- Tanase S., Kojima H., Morino Y. Pyridoxal 5'-phosphate binding site of pig heart alanine aminotransferase. Biochemistry. 1979 Jul 10;18(14):3002–3007. doi: 10.1021/bi00581a015. [DOI] [PubMed] [Google Scholar]


