Abstract
AIM: To investigate the presence or absence of high amplitude propagating contractions (HAPC), as well as the other measures of colonic motility, in persons with spinal cord injury (SCI).
METHODS: Prolonged colonic ambulatory manometric studies were performed on 14 male volunteers: 8 with SCI (mean age, 59 ± 13 years; mean duration of injury, 13 ± 4 years) and 6 healthy able-bodied controls (mean age, 57 ± 10 years). A solid-state manometry catheter was endoscopically clipped to the splenic flexure. Recording was performed for > 24 h after manometric catheter placement.
RESULTS: HAPC were absent in individuals with SCI during pre-sleep, sleep, and post-sleep phases. HAPC were significantly increased after awakening in non-SCI controls (0.8 ± 0.2 HAPC/h vs 10.5 ± 2.0 HAPC/h, P < 0.005). The motility index was lower in those with SCI than in controls pre- and post-sleep (SCI vs non-SCI: Pre-sleep, 2.4 ± 0.4 vs 8.8 ± 1.9, P < 0.01; Post-sleep, 4.3 ± 0.8 vs 16.5 ± 4.5, P < 0.05). However, a sleep-induced depression of colonic motility was observed in both the SCI and non-SCI groups (Pre-sleep vs Sleep, non-SCI: 8.8 ± 1.9 vs 2.1 ± 0.9, P < 0.002; SCI: 2.4 ± 0.4 vs 0.2 ± 0.03, P < 0.001), with the motility index of those with SCI during sleep not significantly different than that of the controls.
CONCLUSION: HAPC were not observed in individuals with SCI pre- or post-sleep. A sleep-induced depression in general colonic motility was evident in SCI and control subjects.
Keywords: Spinal cord injury, Neurogenic bowel, Bowel motility, High amplitude propagating contractions, Motility index
INTRODUCTION
Neurogenic bowel dysfunction (NBD) is a sequela of spinal cord injury (SCI) that leads to a disruption of normal colon physiology. This condition may be characterized by constipation, diarrhea, fecal incontinence, and/or fecal impaction, and it has a profoundly negative impact on quality of life[1]. In chronic SCI, colonic motor function is disturbed and often presents as difficulty with evacuation, associated with a delay in colonic transit, as evidenced by several colon marker transit studies[2-4]. In addition, our group has reported that colonic contractions after SCI are of lower amplitude and are decreased in frequency compared to those in healthy able-bodied controls[5].
High amplitude propagating contractions (HAPC) are specialized propagating pressure waves with high amplitude (> 105 mmHg) and prolonged duration (> 14 s) observed on manometric recordings[6]. HAPC are often associated with colonic mass movements and are thought to be a precursor of bowel evacuation; they occur mostly after arousal from sleep and after ingestion of meals[7,8]. In a study of non-SCI patients with chronic slow transit constipation, HAPC were either absent or attenuated after arousal from sleep and after ingestion of a meal, and such a finding may be indicative of neurogenic or myopathic colon dysfunction[9].
Given the above findings, it is possible that alterations in HAPC may play a role in the pathophysiology of bowel evacuation in persons with SCI. However, characteristics of HAPC and their relationship to normal physiological stimuli in persons with SCI have not been reported. Thus, our study determined HAPC in relation to sleep in individuals with chronic SCI.
MATERIALS AND METHODS
Subjects
Fourteen male volunteers were studied: 8 with chronic SCI and 6 non-SCI controls. The mean age of the patients with SCI was 59 ± 13 years with a mean duration of injury of 13 ± 4 years. Three of the 8 SCI subjects had tetraplegia (C5 or below) and the remaining 5 had paraplegia (T5 or below). The causes for SCI included trauma (6/8), cervical stenosis (1/8) and transverse myelitis (1/8). Persons with SCI who reported having < 2 spontaneous bowel movements per week were recruited for the study; they had participated in a regular bowel care program for at least 6 mo prior to enrollment in the study. None of the SCI patients enrolled in the study had autonomic dysreflexia. The non-SCI subjects had no previous history of gastrointestinal disease or surgery, had normal physical examinations, and had no electrolyte or thyroid abnormalities. Six healthy non-SCI individuals, aged 57 ± 10 years, served as the control group. Medications that may alter colonic motility and/or hemostasis (e.g. warfarin, aspirin, etc.) were withheld for 1 wk prior to the study. The Institutional Review Board of the James J Peters Veterans Affairs Medical Center approved the study protocol, and informed consent was obtained from subjects before enrollment.
Sleep phases
The pre-sleep phase was designated as the time (1 h) before the start of sleep. The reported start and end of sleep was quantified in hours and was designated as the sleep phase. The post-sleep phase was designated as the time (1 h) after the end of sleep. The information regarding sleep duration was obtained from nursing records and self-reported diaries. Subjects avoided strenuous physical activity for at least 1 h before or after sleep.
Manometry assembly and experimental design
All subjects had normal colonic examinations by colonoscopy before the study. Subjects were prescribed either polyethylene glycol (PEG) (4 L of Colyte) or oral sodium phospho soda (OSPS) (two divided doses of Fleets PhosphoSoda 45 mL each separated by 12 h). Sedation with propofol was administered when needed. Fixation of the manometric probe to the wall of the colon at the splenic flexure was accomplished, as previously described[10]. A solid state manometric catheter with four pressure transducers spaced 10 cm apart was used for obtaining intraluminal pressure data (Gaeltec Ltd, Dunvegan, Isle of Skye, UK). The colonoscope and the manometric catheter probe were advanced under direct vision to the splenic flexure. Using fluoroscopy, the last pressure sensor was placed at least 10 cm proximal to the anal verge. The distal end of the manometric probe was securely taped to the skin of the gluteal region to prevent accidental retraction; an abdominal X-ray confirmed the placement of the clipped probe at the region of the splenic flexure. An abdominal X-ray confirmed that all subjects had normal radiographic findings (i.e. none had megacolon). The manometric probe was attached to a portable recorder (type 7-MPR, Gaeltec Ltd., UK). The portable recorder was connected to a shoulder sling, permitting mobility. The subjects were provided with diaries and were instructed to record the timing of specific activities such as meals, sleep, and bowel and bladder events. Subjects were instructed that after a bowel movement care should be taken during perineal hygiene to prevent the inadvertent external displacement of the probe.
After completion of the study, the data were uploaded to a computer for storage and analysis. The probe remained securely in place after placement in the colon in all subjects. Subjects remained in the hospital overnight for the duration of the study. Because of the possibility that bowel preparation, colonic intubation, and/or sedation may have effects on colonic motility, data collection was begun 12 h after placement of the manometry catheter. Non-SCI subjects meticulously documented events of walking, strenuous activity, movement, cough, sleep and wake, bladder emptying, periods of food ingestion and talking. Because activities may alter HAPC, tracings that corresponded to periods of increased activity were excluded from the data analysis.
From the manometric recordings of the colon, three epochs were determined a priori to measure and analyze the endpoints: pre-sleep phase (i.e. 1 h before sleep), sleep phase (i.e. entire sleep duration), and post-sleep phase (i.e. 1 h after arousal from sleep). The following quantitative and qualitative endpoints were investigated in each epoch: (1) the presence and number of HAPC; and (2) other colonic motility parameters. HAPC were defined as pressure wave sequences that: (a) migrated aborad; (b) travelled across three or more consecutive channels; (c) had an amplitude of > 105 mmHg; and (d) had a duration > 14 s[9]. Other colonic motility parameters measured were the motility index (MI) (i.e. the product of the mean amplitude and percent activity) and the total number of waves. Sleep duration and periods of food ingestion were obtained from nursing records for subjects admitted to the SCI Service and from self-report diaries for non-SCI subjects.
The recordings were analyzed by observing the manometric tracings on a computer monitor and by a software analysis program (AMBB, Gaeltec Ltd, UK). Criteria for waves included in the analysis were: (1) amplitude of > 8 mmHg and; and (2) duration of > 3 s[6]. Movement artifacts (e.g. walking, transferring, coughing, etc.) were confirmed by reference to the subject’s diary, and these segments (as well as postprandial segments) were excluded from the analysis. The analyses were performed independently by investigators blinded to the subject’s identity, and any discrepancies were resolved by the senior author, who was also blinded.
All subjects tolerated the procedure well. The probe was removed after the study by gently tugging along the catheter. No complications were noted after probe removal. For the analysis of HAPC pressure wave forms, data were excluded due to technical reasons in two individuals with SCI and in two healthy controls; in these cases, malfunction of more than one sensor for a period longer than 30 min prevented the ability to observe propagation across channels, hence, preventing the identification or quantification of HAPC.
Statistical analysis
The data were expressed as mean ± SE. All the data represent values obtained per hour. The mean values of the SCI and the control groups were analyzed for differences using unpaired Student’s t test for parametric variables and Kruskal-Wallis Test for non-parametric variables. The means between phases of sleep were analyzed using paired Student’s t test in both SCI and control groups. An analysis of variance (ANOVA) was performed to determine significance among the phases of sleep and arousal (i.e. pre-sleep, sleep, and arousal). Multiple regression analysis was performed to determine the effect of level of SCI on quantitative and qualitative wave parameters.
RESULTS
Subjects
In individuals with SCI, the level of injury did not have a significant effect on the quantitative and qualitative wave parameters. Thus, the data obtained in subjects with all levels of SCI were combined for further analysis. A total of approximately 420 h of manometric recording was obtained (range of 26-32 h per patient); 112 h were analyzed for the study. The average number of hours of sleep per subject was 8.0 ± 0.2 h (range: 7 to 10 h), without a significant difference in the average number of hours of sleep between SCI and non-SCI subjects. None of the subjects reported sleep interruption.
High amplitude peristaltic contractions
There were no HAPC observed in the SCI group during pre-sleep, sleep, and post-sleep phases. In the non-SCI group, the numbers of HAPC/h were as follows: pre-sleep phase, 2.0 ± 0.4; sleep phase, 0.8 ± 0.2; and post-sleep phase, 10.5 ± 2.0; a significant increase in the number of HAPC were noted from sleep to post-sleep phases (0.8 ± 0.2 vs 10.5 ± 2.0, P < 0.005). All non-SCI subjects experienced subjective symptoms (e.g. sensation of flatus) and two had bowel movements in close proximity to having HAPC in the post-sleep phase.
Motility index
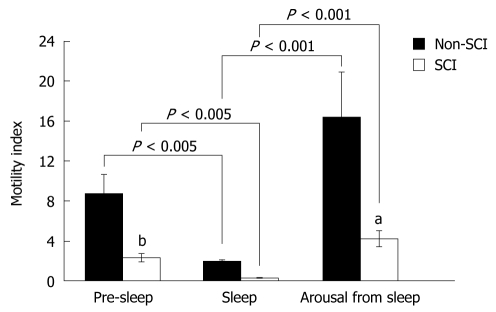
In both the non-SCI and SCI groups, the colonic motility index (MI) was significantly suppressed during sleep compared to pre-sleep (Pre-sleep vs Sleep, Control: 8.8 ± 1.9 vs 2.1 ± 0.9, P < 0.005; SCI: 2.4 ± 0.4 vs 0.2 ± 0.03, P < 0.001) and significantly increased after arousal from sleep (Sleep vs Post-sleep, non-SCI: 2.1 ± 0.9 vs 16.5 ± 4.5, P < 0.001; SCI: 0.2 ± 0.03 vs 4.3 ± 0.8, P < 0.001) (Figure 1).
Figure 1.
Colonic motility index in relation to sleep. In both the non-spinal cord injury (SCI) and SCI groups, colonic motility index (MI) was significantly suppressed during the sleep phase compared to the pre-sleep phase (Pre-sleep vs Sleep, Control: 8.8 ± 1.9 vs 2.1 ± 0.9, P < 0.005; SCI: 2.4 ± 0.4 vs 0.2 ± 0.05, P < 0.001) and significantly increased after awakening from sleep (Sleep vs Arousal, Control: 2.1 ± 0.9 vs 16.5 ± 4.5, P < 0.001; SCI: 0.2 ± 0.03 vs 4.3 ± 0.8, P < 0.001). The MI during the pre-sleep and post-sleep phases was significantly lower in SCI subjects than in the non-SCI (non-SCI vs SCI, pre-sleep: 8.8 ±1.9 vs 2.4 ± 0.4, bP < 0.01; post-sleep: 16.5 ± 4.5 vs 4.3 ± 0.8, aP < 0.05).
Furthermore, the MI during the pre-sleep and post-sleep phases was significantly lower in SCI subjects than that of the non-SCI group (SCI vs non-SCI: Pre-sleep, 2.4 ± 0.4 vs 8.8 ±1.9, P < 0.01; Post-sleep, 4.3 ± 0.8 vs 16.5 ± 4.5, P < 0.05). However, no significant difference in MI was noted during the sleep phase between SCI and non-SCI groups (SCI vs non-SCI: Sleep, 0.2 ± 0.03 vs 2.1 ± 0.9, P = 0.10).
Total number of waves
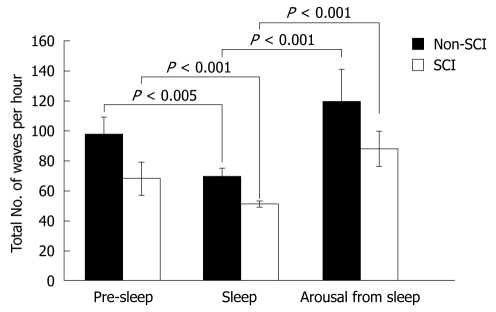
In both the control and SCI groups, the total number of waves was significantly suppressed during sleep compared to pre-sleep (Pre-sleep vs Sleep, non-SCI: 98 ± 11 vs 70 ± 5, P < 0.005; SCI: 68 ± 11 vs 51 ± 2, P < 0.001). The total number of waves was significantly increased in both groups after arousal from sleep (Sleep vs Arousal, non-SCI: 70 ± 5 vs 120 ± 21, P < 0.001; SCI: 51 ± 2 vs 88 ± 12, P < 0.001) (Figure 2).
Figure 2.
Total number of waves in relation to sleep. In both the non-spinal cord injury (SCI) and SCI groups, the total number of waves was significantly suppressed during the sleep phase compared to the pre-sleep phase (Pre-sleep vs Sleep, non-SCI: 98 ± 11 vs 70 ± 5, P < 0.005; SCI: 68 ± 11 vs 51 ± 2, P < 0.001), and was significantly increased after awakening from sleep (Sleep vs Arousal, non-SCI: 70 ± 5 vs 120 ± 21, P < 0.001; SCI: 51 ± 2 vs 88 ± 12, P < 0.001).
DISCUSSION
As was previously reported in other studies[9,11-15], HAPC are often observed after awakening or occur after ingestion of a high fat meal[12]. Previously, our group studied the effects of food on colonic motility in persons with SCI and reported that the postprandial colonic response in persons with SCI is suboptimal and confined only to the descending colon[5]. In this study, we restricted our analysis to HAPC in relation to the sleep-wake cycle in subjects with SCI compared to those in able-bodied subjects. Our study revealed that HAPC are absent in individuals with chronic SCI who had reported having fewer than 2 spontaneous bowel movements per week. The absence of HAPC in persons with SCI may be related to abnormalities in the brain-gut control over colonic motility and/or intrinsic disorder of the viscus as a result of chronic SCI. The complete absence of the HAPC after chronic SCI may be a contributing factor to difficulty with evacuation. Our findings are consistent with the study of Rao et al[9] who demonstrated that absence of HAPC may indicate colonic neuropathy.
Interestingly, in both SCI and non-SCI subjects, there appeared to be a sleep-induced depression of colonic motor waves measured, i.e. motility index and total number of all waves. Furthermore, in both SCI and non-SCI subjects, there appeared to be a significant increase of peristaltic waves measured after awakening, except for HAPC.
HAPC are thought to be the manometric equivalent of colonic mass movements[16], and they are likely to be associated with transport of intraluminal contents along colonic segments[17]. Prolonged periods of colonic recording in man have reported HAPC to occur between 4 to 10 times per 24-h period[9,11,18]. In non-SCI subjects, 60% of HAPC were associated with symptoms or events (e.g. bowel movement or passage of gas)[1]. The interest in the role of HAPC as a variable that permits discrimination between states of health and disease is evolving, as more sophisticated methodology and technology become available to allow more accurate and prolonged colonic recording under physiological conditions[6,10,11,19,20]. More recently, in a study of patients with slow-transit constipation[9], HAPC were employed to categorize patients with slow-transit constipation as having colonic neuropathy based on the absence of two of three manometric criteria, one being HAPC and the other two being lack of gastro-colonic response and lack of response to waking. The patients who were categorized as having colonic neuropathy were considered to have a refractory colonic neuromuscular disorder; after further evaluation of gastric and small bowel motility, 7 of the 10 patients were advised to have, and subsequently underwent, surgery (i.e. colectomy with ileorectal anastomosis)[9].
The current study has limitations. Initially, the frequency and number of HAPC in the non-SCI group reported in this study were significantly higher than has been previously reported[15,18,19]. The reason for this disparity is not clear but could be due to technical differences, the small number of subjects and/or the study design. Regardless of the cause, the relative differences between SCI subjects and non-SCI controls remain significant because the same procedures and manometric sensors were employed in both groups. Furthermore, studies have shown that in addition to awakening from sleep, movement (e.g. ambulation) also precipitates the generation of HAPC. Thus, activity may have been a significant factor that may serve to explain the difference in HAPC generation in the SCI compared to the non-SCI group. We have carefully excluded motion artifact, especially those related to walking in the able-bodied subjects during the analysis of our wave recordings. However, mobility as a generator of HAPC per se could not be addressed because of our study cohort (non-SCI) and our study design (e.g. non-SCI subjects were not confined to the seated position during the acquisition of data). The number of subjects studied was relatively small, and the SCI group was not uniform. Another limitation of this work was that the sleep phases were not confirmed by polysomnography; because of this limitation, we were also unable to determine if subjects had periods of arousal during the periods of reported sleep. In our study protocol, persons with SCI who reported having > 2 spontaneous bowel movements per week were excluded from study participation. Thus, another limitation of the study was that it did not address possible differences in HAPC in relation to sleep in the subjects with SCI with more normal bowel evacuation patterns (i.e. > 2 bowel movements per week) compared to those of the non-SCI subjects.
In conclusion, subjects with SCI had a complete absence of HAPC. The MI before and after sleep was depressed in persons with SCI compared to able-bodied subjects. Thus, SCI results in disruption of the colonic motility changes that are present in healthy individuals. In our subjects with SCI, a marked depression in sleep-induced colonic activity was demonstrated by the MI and the total number of waves, and this depression in activity related to sleep has also been confirmed, as expected, in healthy controls. The absence of HAPC generation is presumably an important factor in the occurrence of difficulty with evacuation observed in individuals with SCI. Prokinetic drugs, such as bisacodyl and neostigmine[21,22], that result in generation of HAPC, may improve bowel evacuation by increasing HAPC after SCI.
Footnotes
Supported by The Department of Veterans Affairs Rehabilitation Research and Development Service Center of Excellence for the Medical Consequences of Spinal Cord Injury (B4162C)
Peer reviewers: Dr. Uday C Ghoshal, MD, DNB, DM, FACG, Additional Professor, Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow 226014, India; John W Wiley, MD, Professor, Internal Medicine, Director, Michigan Clinical Research Unit, Cardiovascular Center, Rm. 1702, 1500 E. Medical Center Dr., Ann Arbor, MI 48109-5872, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
References
- 1.Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86–S102. doi: 10.1016/s0003-9993(97)90416-0. [DOI] [PubMed] [Google Scholar]
- 2.Nino-Murcia M, Stone JM, Chang PJ, Perkash I. Colonic transit in spinal cord-injured patients. Invest Radiol. 1990;25:109–112. doi: 10.1097/00004424-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Keshavarzian A, Barnes WE, Bruninga K, Nemchausky B, Mermall H, Bushnell D. Delayed colonic transit in spinal cord-injured patients measured by indium-111 Amberlite scintigraphy. Am J Gastroenterol. 1995;90:1295–1300. [PubMed] [Google Scholar]
- 4.Menardo G, Bausano G, Corazziari E, Fazio A, Marangi A, Genta V, Marenco G. Large-bowel transit in paraplegic patients. Dis Colon Rectum. 1987;30:924–928. doi: 10.1007/BF02554277. [DOI] [PubMed] [Google Scholar]
- 5.Fajardo NR, Pasiliao RV, Modeste-Duncan R, Creasey G, Bauman WA, Korsten MA. Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol. 2003;98:128–134. doi: 10.1111/j.1572-0241.2003.07157.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 7.Malcolm A, Camilleri M. Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol. 2000;95:715–719. doi: 10.1111/j.1572-0241.2000.01840.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarna SK. Colonic motor activity. Surg Clin North Am. 1993;73:1201–1223. doi: 10.1016/s0039-6109(16)46188-8. [DOI] [PubMed] [Google Scholar]
- 9.Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405–2416. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 10.Fajardo N, Hussain K, Korsten MA. Prolonged ambulatory colonic manometric studies using endoclips. Gastrointest Endosc. 2000;51:199–201. doi: 10.1016/s0016-5107(00)70418-4. [DOI] [PubMed] [Google Scholar]
- 11.Bassotti G, Crowell MD, Whitehead WE. Contractile activity of the human colon: lessons from 24 hour studies. Gut. 1993;34:129–133. doi: 10.1136/gut.34.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowell MD, Bassotti G, Cheskin LJ, Schuster MM, Whitehead WE. Method for prolonged ambulatory monitoring of high-amplitude propagated contractions from colon. Am J Physiol. 1991;261:G263–G268. doi: 10.1152/ajpgi.1991.261.2.G263. [DOI] [PubMed] [Google Scholar]
- 13.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am J Physiol. 1988;255:G660–G664. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa Y, Cook IJ, Panagopoulos V, McEvoy RD, Sharp DJ, Simula M. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology. 1994;107:1372–1381. doi: 10.1016/0016-5085(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 15.Steadman CJ, Phillips SF, Camilleri M, Haddad AC, Hanson RB. Variation of muscle tone in the human colon. Gastroenterology. 1991;101:373–381. doi: 10.1016/0016-5085(91)90014-c. [DOI] [PubMed] [Google Scholar]
- 16.Sarna SK. Physiology and pathophysiology of colonic motor activity (2) Dig Dis Sci. 1991;36:998–1018. doi: 10.1007/BF01297155. [DOI] [PubMed] [Google Scholar]
- 17.Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74–82. doi: 10.1023/a:1021734414976. [DOI] [PubMed] [Google Scholar]
- 18.Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soffer EE, Scalabrini P, Wingate DL. Prolonged ambulant monitoring of human colonic motility. Am J Physiol. 1989;257:G601–G606. doi: 10.1152/ajpgi.1989.257.4.G601. [DOI] [PubMed] [Google Scholar]
- 20.Bassotti G, Germani U, Morelli A. Human colonic motility: physiological aspects. Int J Colorectal Dis. 1995;10:173–180. doi: 10.1007/BF00298543. [DOI] [PubMed] [Google Scholar]
- 21.Hervé S, Savoye G, Behbahani A, Leroi AM, Denis P, Ducrotté P. Results of 24-h manometric recording of colonic motor activity with endoluminal instillation of bisacodyl in patients with severe chronic slow transit constipation. Neurogastroenterol Motil. 2004;16:397–402. doi: 10.1111/j.1365-2982.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 22.Korsten MA, Rosman AS, Ng A, Cavusoglu E, Spungen AM, Radulovic M, Wecht J, Bauman WA. Infusion of neostigmine-glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100:1560–1565. doi: 10.1111/j.1572-0241.2005.41587.x. [DOI] [PubMed] [Google Scholar]