Abstract
AIM: To study the efficacy and safety of entecavir (ETV) as first-line therapy for hepatitis B virus (HBV) reactivation due to immunosuppression.
METHODS: Four patients that were treated with different immunosuppressive regimens for hematological malignancies, who presented with HBV reactivation were treated with ETV. Clinical outcome, biochemical and virological factors, including quantitative hepatitis B surface antigen (HBsAg) were studied.
RESULTS: In all patients, ETV induced suppression of HBV, and rapid clinical improvement without side effects. In one patient with an alanine aminotransferase (ALT) flare, tenofovir was added after 3 mo of treatment. Until death from disease progression at 6 mo after treatment initiation, this patient did not clear HBV infection. Retrospectively, it is highly probable that the patient had been non-adherent. In the other three patients, the virological responses were associated with an expeditious decrease in quantitative HBsAg titers with negativity after 2 mo, and all three had HBsAg seroconversion. In one patient, HBV DNA reached a plateau after 3 mo, before becoming undetectable after 1 year, despite early ALT normalization and undetectable quantitative HBsAg.
CONCLUSION: ETV seems to be effective and safe treatment for HBV reactivation. Monitoring of quantitative HBsAg might be an additional useful tool to monitor treatment response.
Keywords: Hepatitis B virus, Entecavir, Immunosuppression, Hepatitis B surface antigen, Seroconversion
INTRODUCTION
In patients with previous exposure to hepatitis B virus (HBV), who are hepatitis B surface antigen (HBsAg)-positive, or HBsAg-negative and hepatitis B core antibody (anti-HBc)-positive, acute exacerbation may occur when they become immunocompromised[1]. HBV reactivation (HBV-R) can be severe and is associated with high fatality rates of up to 20%-30%. The European Association for the Study of the Liver (EASL) now recommends that all patients who are planned to receive chemotherapy, immunosuppressive therapy or stem cell transplantation undergo a test for HBV serology, including anti-HBc antibody prior to treatment[2]. Prophylactic treatment is recommended for patients with positive HBsAg. For those with positive anti-HBc antibodies and undetectable HBV DNA, monitoring of serum alanine aminotransferase (ALT) and HBV DNA is recommended. Although this consensus for the prevention of HBV-R is now implemented in current guidelines, an effective management strategy for patients who are experiencing HBV-R has not yet been established.
Lamivudine is the most commonly used drug for HBV-R. It has been shown to be safe in reactivation and in severe acute or fulminant hepatitis B[3]. However, prognosis remains poor despite lamivudine therapy if hepatic decompensation occurs[4,5]. Furthermore, the efficacy of lamivudine is hampered by the high rate of drug resistance mutations within the HBV polymerase gene that are associated with treatment failure. Entecavir (ETV) was approved in 2006 for the treatment of chronic hepatitis B and offers the advantage of a higher resistance barrier than lamivudine. The EASL guidelines note that the use of tenofovir or ETV might be considered in HBV-R with high viral load, although there is very limited experience.
The aim of our study was to assess the efficacy and safety of ETV in patients with immunosuppression-associated HBV-R at our center.
MATERIALS AND METHODS
We report on four patients with hematological malignancies, who had been referred to our hepatology department because of HBV-R, after approval of ETV, in August 2006. Median age of the patients was 63 years (Table 1). Patient 1 was male, and the other three were female. Two patients had received hematopoietic stem cell transplantation (HSCT) for treatment of acute myeloid leukemia (AML) (patients 1 and 3), the other two patients suffered from follicular lymphoma (patient 2) and B-cell chronic lymphocytic leukemia (B-CLL) (patient 4, Table 1). The patient with CLL was treated with bendamustine. The patient with follicular lymphoma was managed by observation without specific anticancer treatment, but had been treated intermittently with methylprednisolone for rheumatoid arthritis and was thus, due to hematological malignancy and steroid treatment, considered immunocompromised at the time of HBV-R.
Table 1.
Baseline characteristics and clinical outcome of patients treated with entecavir for hepatitis B virus reactivation
| Parameter |
Patient No. |
|||
| 1 | 2 | 3 | 4 | |
| Age (yr) | 66 | 57 | 51 | 78 |
| Sex | M | F | F | F |
| Disease entity | AML | FL | AML | B-CLL |
| HBV serology prior to chemotherapy | ND | ND | anti-HBc pos., anti-HBs pos., HBs-Ag neg. | ND |
| Chemotherapy/treatment | HSCT | Prednisone | HSCT | Bendamustine |
| Peak ALT (IU/L) | 870 | 3290 | 2592 | 711 |
| Peak bilirubin level (mg/dL) | 20 | 10 | 4.1 | 1 |
| INR | 1.4 | 1.0 | 1.0 | 1.0 |
| Time of clinical disease presentation | 9 mo after HSCT | During prednisone treatment | 27 mo after HSCT; 8 mo after cessation of LAM prophylaxis | During current chemotherapy |
| HBV-DNA (IU/mL) at time of HBV reaction | 8.06 × 107 | 5.65 × 105 | 1.00 × 105 | 4.50 × 107 |
| HBeAg at time of reactivation | Positive | Negative | Positive | Positive |
| HBeAg loss | Yes | NA | Yes | Yes |
| HBsAg loss | Yes | Yes | Yes | No |
| Withdrawal of antiviral treatment | No | Yes | Yes | No |
| Anti-HBs (IU/L) | 42 | 361 | 277 | - |
| Clinical outcome | Alive | Alive | Alive | Malignancy related death |
AML: Acute myeloid leukemia; B-CLL: B-cell chronic lymphocytic leukemia; FL: Follicular lymphoma; LAM: Lamivudine; ND: Not determined; HBV: Hepatitis B virus; HSCT: Hematopoietic stem cell transplantation; NA: Not applicable; INR: International normalized ratio; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; ALT: Alanine aminotransferase.
In all patient, HBV DNA was measured prospectively with real-time PCR (Abbott HBV rtPCR; Abbott, Wiesbaden, Germany). On-treatment serum HBsAg kinetics were analyzed retrospectively (Abbott HBSAG). HBsAg was assessed at baseline, during treatment (1-3 mo), and during follow-up.
Only one of the patients (patient 3) had HBV screening prior to immunosuppression and was diagnosed with resolved hepatitis B with surface antigen clearance and an anti-hepatitis B surface antigen antibody (anti-HBs) level of 370 IU/L (Abbott AUSAB). During chemotherapy, quantitative HBsAg titers and HBV DNA were monitored closely and remained negative. When anti-HBs level fell below 10 IU/L, lamivudine prophylaxis was initiated and continued until 4 mo after cessation of immunosuppression with cyclosporine. At this time, HBV DNA was not detectable. HBV-R occurred 8 mo after lamivudine treatment had been stopped.
Prior to the initiation of chemotherapy or immunosuppression, all patients had normal liver values. None of them had a history of prior non-viral liver disease. Co-infection with hepatitis A, C or delta viruses or HIV was excluded serologically at the time of referral to our unit.
RESULTS
At the time of diagnosis of HBV-R, all patients presented with similar clinical, laboratory and virological findings. All patients suffered from fatigue, nausea and jaundice. They all had moderate to high HBV-DNA levels that ranged from 1 × 105 to 8.06 × 107 IU/mL. ALT levels were > 10 times the upper limit of normal in all patients. In one patient, serum bilirubin concentration was normal. In the other three patients, bilirubin levels ranged from 8 to 20 mg/dL. International normalized ratio (INR) was within the normal range in three patients. Only one patient had an impaired liver function with an INR of 1.4 (patient 1). Three of the patients were hepatitis B e antigen (HBeAg)-positive (Table 1).
When HBV-R was diagnosed, all patients were immediately treated with ETV. Three treatment-naïve patients were treated with oral 0.5 mg/d ETV (Baraclude®; Bristol-Myers-Squibb, Munich, Germany), and one patient (patient 3) received 1 mg ETV because of prior prophylactic treatment with lamivudine.
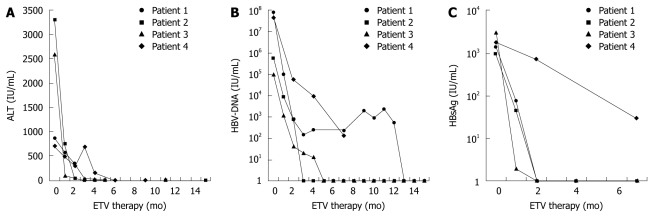
In all patients, ETV induced suppression of HBV that was associated with rapid clinical improvement and ALT normalization (Figure 1A). Three of the four patients had undetectable HBV-DNA levels after 3, 5 and 13 mo of treatment, respectively (Figure 1B).
Figure 1.
Temporal changes in laboratory values for patients 1-4 at the time of hepatitis B virus reactivation and on entecavir treatment, including: (A) alanine aminotransferase, (B) hepatitis B virus DNA and (C) quantitative hepatitis B surface antigen levels. ALT: Alanine aminotransferase; HBV: Hepatitis B virus; ETV: Entecavir.
One of the patients (patient 4) died because of disease progression of CLL after 6 mo of treatment with ETV. After 3 mo of ETV treatment, tenofovir was added because chemotherapy had to be restarted and HBV DNA showed a 4-log10 reduction but was still detectable at about 104 IU/mL, and ALT flared. When the patient died after 6 mo of ETV treatment and 3 mo of add-on tenofovir treatment, ALT had normalized and HBV DNA had declined to 136 IU/mL. The patient was tested for ETV resistance by sequencing the s-gene and pol-gene, however, no resistance-associated mutations were detected. Further investigation revealed that this patient had been non-adherent and treatment had been suspended for 8 d because she had run out of medication.
Three patients had been HBeAg-positive and all of them had HBeAg seroconversion during the course of treatment. Three of four patients (patients 1-3) even had HBsAg loss after 1, 2 and 4 mo of treatment, respectively.
On-treatment quantitative serum HBsAg levels decreased rapidly and were undetectable at 8 wk in all patients who developed HBsAg clearance (Figure 1C).
In two of the patients, ETV treatment was withdrawn after 6 mo following HBsAg seroconversion with a follow-up of 6-8 mo after discontinuation. Treatment was well tolerated in all patients and no side effects were observed.
DISCUSSION
HBV-R occurs in patients who have been exposed to HBV and who are receiving immunomodulatory drugs, after solid organ transplantation or HSCT. It has also been described to appear spontaneously[6,7] or in the presence of malignancies. HBV-R can be severe and result in acute hepatic failure and death.
Here, we report four patients with hematological malignancies, who developed HBV-R. In all of these patients, ETV induced suppression of HBV that was associated with rapid clinical improvement, without any side effects. ETV is known to have a very potent antiviral effect that has been shown to be greater than that observed with lamivudine, and has a high resistance barrier[8]. Also, almost 10 years after the introduction of lamivudine, patients are at considerable risk for primary infection with lamivudine-resistant HBV strains, against which, lamivudine treatment is useless. For these reasons, ETV was chosen as initial treatment in our patients.
Drug-induced liver injury[9] or lactic acidosis[10] that have recently been described in patients undergoing treatment with ETV were not observed in our patients. Safe and efficient use of ETV in HBV-R has also been described in one patient from Spain recently[11]. However, we have to point out that none of our patients suffered from severe hepatic failure as defined previously[3] or liver cirrhosis.
It has already been reported that patients with HBV-R tend to have a higher rate of sustained virological response and higher rates of HBeAg seroconversion than patients who are chronically infected. This was also true for our patients who cleared HBeAg in all cases, and three even experienced HBsAg seroconversion. In Japan, a patient who was receiving chemotherapy for B-cell lymphoma was also treated with ETV for HBV-R and became HBsAg-negative after 2 mo of treatment[12]. The patient from Spain also cleared HBsAg after 6 mo of treatment with ETV[11]. Another patient with B-cell lymphoma in France received ETV as first-line therapy after HBV-R. This patient also had undetectable HBV DNA after 4 mo of treatment and no side effects[11]. It is not stated whether this patient also cleared HBsAg.
In two of our patients, ETV has successfully been withdrawn after anti-HBs had reached levels > 100 IU/mL, and these patients are currently being followed up closely. The third patient has an anti-HBs level < 100 IU/mL and ETV treatment is being continued at present (Table 1).
We also evaluated quantitative HBsAg to study its behavior and possible role in the various phases of HBV-R. In acute hepatitis, the levels of HBsAg are usually > 10 000 IU/mL[13]. The highest level that we found during reactivation was 3031.5 IU/mL. All patients that achieved HBsAg clearance showed at least a 1 log10 drop of HBsAg at wk 4 of treatment with ETV, and at wk 8, quantitative HBsAg was undetectable in all patients that experienced HBsAg seroconversion during follow-up. An early serum HBsAg decline has already been shown to be predictive of sustained virological response during treatment with nucleosid(t)e analogs or pegylated interferon[14-17]. Patient 1 showed a rapid decline in quantitative HBsAg with negativity at wk 8, and normalization of ALT and HBsAg seroconversion during follow-up. Despite this response, HBV DNA reached a plateau around 103 IU/mL for 9 mo after an initial decline for 3 mo, and became negative only after 1 year of treatment. This finding implies that quantitative HBsAg might be an additional useful tool to optimize the clinical management of patients with HBV-R.
In one patient, tenofovir was added after 3 mo of ETV treatment. This patient had a high viral load prior to treatment (4.5 × 107 IU/mL). After 3 mo, she had a 4 log10 reduction in viral load and ALT decreased from 711 to 313 IU/mL. As a result of progression of CLL, chemotherapy had to be re-started. When ALT levels under chemotherapy rose to 752 IU/mL, tenofovir was added. However, there was no increase in viral load under chemotherapy, and the presence of known ETV resistance mutations was excluded retrospectively. At this time, she had already cleared HBeAg. ETV treatment was suspended for 8 d because the patient ran out of medication. Thus, incomplete viral suppression was possibly not due to lack of efficacy of ETV but resulted from non-adherence. However, we cannot completely rule out that viral suppression by ETV in this patient was insufficient, as even fulminant hepatic failure despite ETV treatment following HBV-R has been described in a single case[18].
It is important to point out that only one of our patients had HBV screening prior to initiation of immunosuppressive treatment. We cannot prove if the other patients really had true reactivation of hepatitis B. However, all of these patients had normal liver values before the start of immunosuppression. De novo infection during immunosuppressive therapy is rather unlikely, therefore, we believe that these patients had either resolved or inactive hepatitis B prior to the initiation of immunosuppression. The fact that these data are not available is a weakness of our study, but reflects the real-life situation. Although it is widely recognized that HBV-R can occur with conventional immunosuppression and newer drugs, in particular rituximab, HBV screening often is still not performed in these patients. Taking into consideration the possibly fatal outcome of HBV-R and the fact that it is preventable, HBV screening in all of these patients is mandatory, especially as it has been described that HBV is more prevalent among patients with hematological malignancies of B lineage, and has been hypothesized to even play a pathogenic role in these patients[19,20].
In the patient that was screened prior to treatment and received lamivudine prophylaxis, prophylaxis had retrospectively been stopped too early. At that time, there was no clear consensus about the duration of prophylactic therapy after cessation of chemotherapy. The new EASL guidelines now recommend that prophylaxis should be continued for at least 1 year after withdrawal of immunosuppressive drugs[2]. Furthermore, we and others[5] have experienced HBV-R in HBsAg-negative/anti-HBc-positive patients. Therefore, it might be considered to extend prophylaxis for HBV-R to this subpopulation.
We believe that our results justify further controlled prospective studies to confirm the efficacy and safety of ETV in a larger number of patients with HBV-R.
COMMENTS
Background
Resolved or inactive hepatitis B virus (HBV) infection usually does not harm the patient. However, if for other medical conditions immunosuppressive therapies need to be initiated in these patients, HBV might reactivate with potentially fatal outcome.
Research frontiers
It is recognized that HBV reactivation (HBV-R) might occur under immunosuppressive therapy. Therefore, screening for HBV is recommended before the initiation of these therapies. However, despite these recommendations, screening often is not performed and significant liver disease might develop. The optimal treatment for these patients in order to avoid death or the need for liver transplantation is currently not known, and no systematic studies have addressed this issue.
Innovations and breakthroughs
The authors have successfully treated patients suffering from HBV-R with entecavir (ETV). In all patients, ETV therapy induced suppression of HBV and rapid clinical improvement without side effects.
Applications
As the optimal treatment strategy for HBV-R is currently unknown, the data presented in this article provide a rationale for prospectively studying the use of ETV for the treatment of liver disease due to HBV-R under immunosuppressive therapies.
Terminology
Immunosuppressive therapy most frequently refers to chemotherapy for malignant tumors or agents used for treatment of autoimmune-mediated diseases such as rheumatic diseases.
Peer review
The authors have explained their experience with ETV in immunosuppressed patients with HBV-R. This paper adds important information to the literature, especially the usefulness of ETV in this field, in which data are sparse.
Footnotes
Peer reviewers: Carla W Brady, MD, MHS, Duke University Medical Center, Division of Gastroenterology, DUMC Box 3913, Durham, NC 27705, United States; Can Gonen, MD, Department of Gastroenterology, Kutahya State Hospital, 43100 Kutahya, Turkey
S- Editor Wang JL L- Editor Kerr C E- Editor Ma WH
References
- 1.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 2.EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, Graziadei I, Encke J, Schmidt H, Vogel W, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13:256–263. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 4.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 5.Borentain P, Colson P, Coso D, Bories E, Charbonnier A, Stoppa AM, Auran T, Loundou A, Motte A, Ressiot E, et al. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg-negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem cell transplantation for cancer. J Viral Hepat. 2009:Epub ahead of print. doi: 10.1111/j.1365-2893.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Hoofnagle JH, Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984;86:230–235. [PubMed] [Google Scholar]
- 7.Fattovich G, Brollo L, Alberti A, Realdi G, Pontisso P, Giustina G, Ruol A. Spontaneous reactivation of hepatitis B virus infection in patients with chronic type B hepatitis. Liver. 1990;10:141–146. doi: 10.1111/j.1600-0676.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 8.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 9.Kondo M, Kitada N, Kobayashi M, Morita S, Yoshioka M, Tsuji T, Mori A, Okamoto T, Watari M. [A case of drug-induced liver injury caused by entecavir for treatment of hepatitis B virus reactivation during RCHOP in a patient with non-Hodgkin lymphoma] Gan To Kagaku Ryoho. 2009;36:1199–1201. [PubMed] [Google Scholar]
- 10.Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, Sarrazin C. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001–2006. doi: 10.1002/hep.23346. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez MJ, Buti M, Homs M, Palacios A, Rodriguez-Frias F, Esteban R. Successful use of entecavir for a severe case of reactivation of hepatitis B virus following polychemotherapy containing rituximab. J Hepatol. 2009;51:1091–1096. doi: 10.1016/j.jhep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Ueda Y, Marusawa H, Ichinohe T, Kadowaki N, Uchiyama T, Chiba T. Effective treatment for de novo hepatitis B with nucleotide analogue in patients with hematological malignancies. Am J Hematol. 2009;84:315–316. doi: 10.1002/ajh.21388. [DOI] [PubMed] [Google Scholar]
- 13.Rodella A, Galli C, Terlenghi L, Perandin F, Bonfanti C, Manca N. Quantitative analysis of HBsAg, IgM anti-HBc and anti-HBc avidity in acute and chronic hepatitis B. J Clin Virol. 2006;37:206–212. doi: 10.1016/j.jcv.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 15.Wursthorn K, Jung M, Manns MP, Lopez PM, Wedemeyer H, Naoumov NV. Different Kinetics of serum HBsAg decline in HBeAg-positive vs HBeAg-negative patients during 3 years of telbivudine treatment in chronic hepatitis B (CHB) Hepatology. 2009;50(S4):A536. doi: 10.1002/hep.23905. [DOI] [PubMed] [Google Scholar]
- 16.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Jung YK, Kim JH, Lee YS, Lee HJ, Yoon E, Jung ES, Hong SK, Joo MK, Yeon JE, Park JJ, et al. Change in serum hepatitis B surface antigen level and its clinical significance in treatment-naïve, hepatitis B e antigen-positive patients receiving entecavir. J Clin Gastroenterol. 2010;44:653–657. doi: 10.1097/MCG.0b013e3181d52946. [DOI] [PubMed] [Google Scholar]
- 18.Stange MA, Tutarel O, Pischke S, Schneider A, Strassburg CP, Becker T, Barg-Hock H, Bastürk M, Wursthorn K, Cornberg M, et al. Fulminant hepatic failure due to chemotherapy-induced hepatitis B reactivation: role of rituximab. Z Gastroenterol. 2010;48:258–263. doi: 10.1055/s-0028-1109782. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Xu RH, Han B, Shi YX, Luo HY, Jiang WQ, Lin TY, Huang HQ, Xia ZJ, Guan ZZ. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109:1360–1364. doi: 10.1002/cncr.22549. [DOI] [PubMed] [Google Scholar]
- 20.Rossi D, Sala L, Minisini R, Fabris C, Falleti E, Cerri M, Burlone ME, Toniutto P, Gaidano G, Pirisi M. Occult hepatitis B virus infection of peripheral blood mononuclear cells among treatment-naive patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:604–611. doi: 10.1080/10428190902777673. [DOI] [PubMed] [Google Scholar]