Abstract
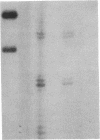
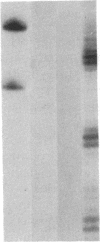
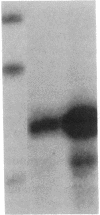
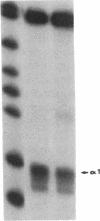
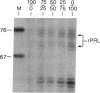
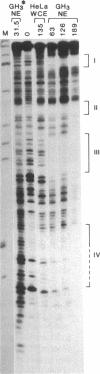
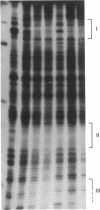
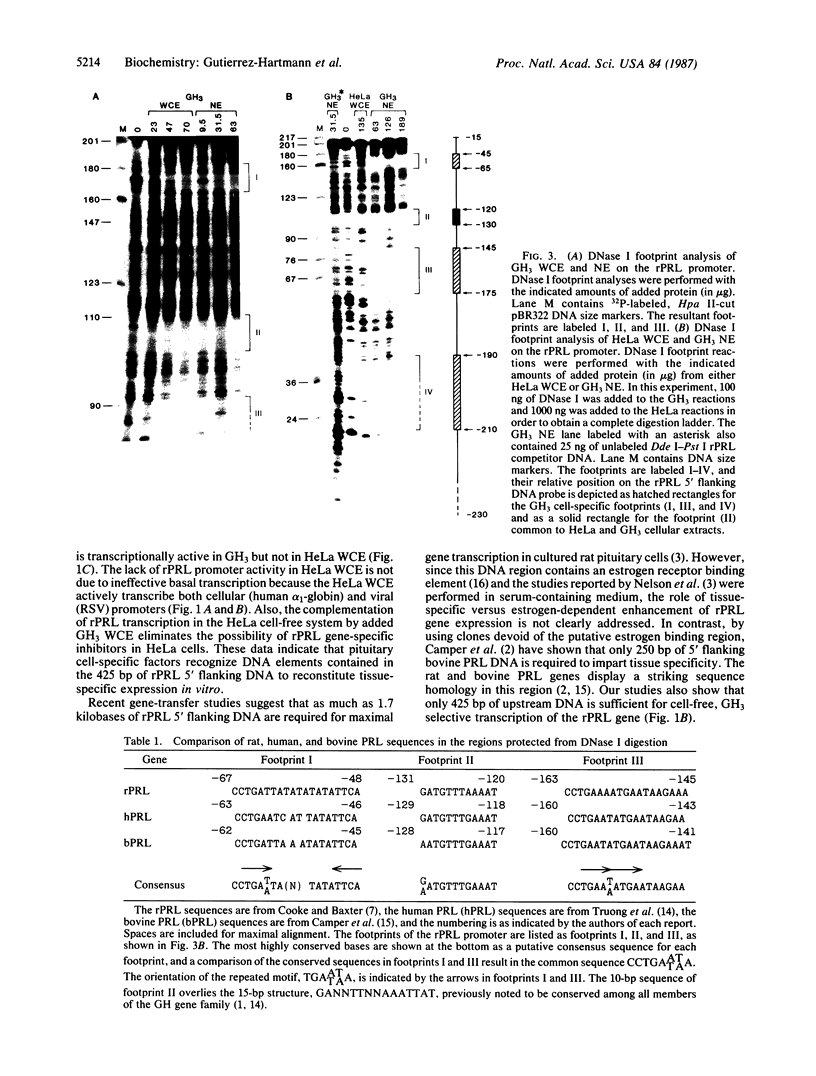
Prolactin (PRL) is a member of the growth hormone gene family that is specifically expressed in the lactotroph cells of the anterior pituitary. Whole cell extracts have been prepared from cultured GH3 rat pituitary tumor cells to study lactotroph-specific expression of the rat PRL (rPRL) gene in an in vitro transcription assay. The human alpha 1-globin and Rous sarcoma virus promoters efficiently initiate transcription in both HeLa and GH3 cell extracts, whereas the rPRL promoter containing 425 base pairs of 5' flanking DNA is active only in GH3 pituitary cell-free extracts. Transcription of the rPRL gene was reconstituted in HeLa cell extracts by the addition of GH3 cell extracts. DNase I digestion of the rPRL promoter reveals two protected regions centered at positions -55 (I) and -160 (III) that are GH3 cell-specific and rPRL promoter-selective. These "footprints" overlie a highly conserved 8-base-pair motif, CCTGATAATA. By contrast, footprint II at position -125 is common to both HeLa and GH3 cell extracts and overlies a 15-base-pair sequence found in all members of the growth hormone gene family. Thus, GH3 pituitary cell-free extracts selectively transcribe the rPRL gene and contain cell-specific factors that directly interact with the rPRL promoter. These studies provide useful assays to further identify, purify, and characterize pituitary-specific transcription factors and to address the biochemical mechanisms involved in rPRL gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camper S. A., Luck D. N., Yao Y., Woychik R. P., Goodwin R. G., Lyons R. H., Jr, Rottman F. M. Characterization of the bovine prolactin gene. DNA. 1984 Jun;3(3):237–249. doi: 10.1089/dna.1.1984.3.237. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Yao Y. A., Rottman F. M. Hormonal regulation of the bovine prolactin promoter in rat pituitary tumor cells. J Biol Chem. 1985 Oct 5;260(22):12246–12251. [PubMed] [Google Scholar]
- Catanzaro D. F., West B. L., Baxter J. D., Reudelhuber T. L. A pituitary-specific factor interacts with an upstream promotor element in the rat growth hormone gene. Mol Endocrinol. 1987 Jan;1(1):90–96. doi: 10.1210/mend-1-1-90. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Baxter J. D. Structural analysis of the prolactin gene suggests a separate origin for its 5' end. Nature. 1982 Jun 17;297(5867):603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Elsholtz H. P., Mangalam H. J., Potter E., Albert V. R., Supowit S., Evans R. M., Rosenfeld M. G. Two different cis-active elements transfer the transcriptional effects of both EGF and phorbol esters. Science. 1986 Dec 19;234(4783):1552–1557. doi: 10.1126/science.3491428. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A., Lieberburg I., Gardner D., Baxter J. D., Cathala G. G. Transcription of two classes of rat growth hormone gene-associated repetitive DNA: differences in activity and effects of tandem repeat structure. Nucleic Acids Res. 1984 Sep 25;12(18):7153–7173. doi: 10.1093/nar/12.18.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T., Turner P., Moulton A. D., Nienhuis A. W. Differences in human alpha-, beta- and delta-globin gene expression in monkey kidney cells. Cell. 1982 Aug;30(1):173–183. doi: 10.1016/0092-8674(82)90023-x. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M., Kan Y. W. Homology and concerted evolution at the alpha 1 and alpha 2 loci of human alpha-globin. Nature. 1981 Mar 5;290(5801):26–29. doi: 10.1038/290026a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., Erwin C. R., Donelson J. E. Analysis of 5' flanking sequences and intron-exon boundaries of the rat prolactin gene. J Biol Chem. 1981 Oct 25;256(20):10524–10528. [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Eberhardt N. L. Structure and evolution of the growth hormone gene family. Endocr Rev. 1983 Spring;4(2):97–130. doi: 10.1210/edrv-4-2-97. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Truong A. T., Duez C., Belayew A., Renard A., Pictet R., Bell G. I., Martial J. A. Isolation and characterization of the human prolactin gene. EMBO J. 1984 Feb;3(2):429–437. doi: 10.1002/j.1460-2075.1984.tb01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yamamoto T., Jay G., Pastan I. Unusual features in the nucleotide sequence of a cDNA clone derived from the common region of avian sarcoma virus messenger RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):176–180. doi: 10.1073/pnas.77.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]