Abstract
In migratory passerine birds, strong magnetic pulses are thought to be diagnostic of the remagnetization of iron minerals in a putative sensory system contained in the beak. Previous evidence suggests that while such a magnetic pulse affects the orientation of migratory birds in orientation cages, no effect was present when pulse-treated birds were tested in natural migration. Here we show that two migrating passerine birds treated with a strong magnetic pulse, designed to alter the magnetic sense, migrated in a direction that differed significantly from that of controls when tested in natural conditions. The orientation of treated birds was different depending on the alignment of the pulse with respect to the magnetic field. These results can aid in advancing understanding of how the putative iron-mineral-based receptors found in birds' beaks may be used to detect and signal the intensity and/or direction of the Earth's magnetic field.
Keywords: magnetite, polarity, migration, bird navigation, magnetic field
1. Introduction
A significant body of behavioural evidence indicates that birds use the magnetic field for orientation and navigation (Wiltschko & Wiltschko 1995), both to indicate direction (Wiltschko & Wiltschko 1972; Cochran et al. 2004) and also possibly as part of a position location system or ‘map’ (Freake et al. 2006). Despite this, the way in which the magnetic field is detected and used by animals for orientation and navigation remains somewhat controversial (Mouritsen & Ritz 2005; Kirschvink et al. 2010). Three distinct sensory mechanisms have been proposed for the detection of the magnetic field. It was initially proposed that a magnetic sense could be a by-product of electroreception in animals possessing ampullary canals (Kalmijn 1981). This sense could not function in a terrestrial environment, however, and so other mechanisms are necessary to explain in particular how birds are able to sense the magnetic field. Two hypotheses currently exist: a radical-pair mechanism and an iron-mineral-based mechanism. A growing body of evidence supports both mechanisms playing a role in the navigation system of birds (see table 1 for a summary of the role of the two mechanisms in navigation based on the magnetic field).
Table 1.
Proposed mechanisms of magnetoreception in birds.
| mechanism | location | function | innervation | brain region | diagnostic |
|---|---|---|---|---|---|
| radical-pair chemical photoreceptor | eye | inclination (compass) | not known | cluster Na,b | MHz oscillating magnetic fieldsc |
| sensory cells containing iron minerals | beakd | intensity (for map?)e | trigeminal nerve (ramus ophthalmicus medius, ROM)d | not known | magnetic pulse,f conditioning to intensity anomaly and lesions of ROMe |
| sensory cells containing iron minerals | beak | polarity (compass?) | trigeminal nerve | not known | field reversal combined with local anaesthesia of beakg |
The radical-pair mechanism proposes that the Earth's magnetic field alters the unpaired electron spin state of photoreceptive chemicals in the eye, causing them to oscillate between singlet and triplet unpaired states (Schulten et al. 1978; Schulten & Windemuth 1986; Ritz et al. 2000; Wang & Ritz 2006; Rogers & Hore 2009). It follows from this that the detection of the magnetic field is thus a by-product of the visual system (Heyers et al. 2007; Zapka et al. 2009). It appears that the region of the bird's brain responsible for nocturnal vision ‘cluster N’ is necessary for magnetic compass orientation (Mouritsen et al. 2005; Zapka et al. 2009). The most likely photoreceptive chemical is the blue light receptor protein cryptochrome (Ritz et al. 2000; Liedvogel et al. 2007; Solov'yov et al. 2007; Solov'yov & Schulten 2009). Evidence for a radical-pair magnetic compass based on cryptochromes comes from the fact that migratory birds tested in orientation cages are disoriented under certain wavelengths and intensities of light (Muheim et al. 2002; Wiltschko & Wiltschko 2002; Wiltschko et al. 2008). It is also predicted that oscillating magnetic fields should disrupt the radical-pair system (Canfield et al. 1994, 1995). Experiments in which migrating birds are subjected to oscillating magnetic fields do indeed disrupt their ability to use magnetic compass orientation (Ritz et al. 2004, 2009).
A second mechanism proposes that the magnetic field is detected by iron minerals, with magnetic remanence as part of a sensory system that signals the movement of these minerals in relation to the Earth's magnetic field. This movement could potentially be used to detect the intensity, inclination or polarity of the magnetic field (Kirschvink & Gould 1981; Kirschvink et al. 2001, 2010). Initial hypotheses were based on the fact that magnetotactic bacterial cells contain magnetite chains (Blakemore 1975; Kalmijn & Blakemore 1978; Blakemore et al. 1980) and that such cells might form the basis of a ‘compass organelle’ (Kirschvink & Gould 1981). Evidence indicates that such iron-based minerals have been found in the tissue of a number of animal taxa (Wiltschko & Wiltschko 1995). Where the magnetic material has been identified within the tissue, it appears to be associated with the trigeminal nerve (Walker et al. 1997; Diebel et al. 2000; Fleissner et al. 2003, 2007). Two experimental techniques have been proposed to be diagnostic of a magnetic sense based on iron minerals. First, if the trigeminal nerve is the primary pathway of innervation of the iron based mineral magnetic sense, lesions or anaesthesia of the nerve should disrupt magnetoreception. Second, brief magnetic pulses strong enough to overcome the coercivity of the iron minerals should remagnetize them in the direction of the applied pulse and thus change the orientation of the bird. Both these techniques have been used and argue that an iron-mineral-based magnetic sense exists in birds. Trigeminal nerve section has been shown to stop pigeons from being able to detect a magnetic intensity anomaly (Mora et al. 2004), although it does not appear necessary for magnetic compass orientation in juvenile migrant robins (Zapka et al. 2009). Magnetic pulses have been shown to alter the orientation of birds (Wiltschko et al. 1994, 1998; Beason et al. 1995, 1997) as well as homing bats (Holland et al. 2008), mole rats (Marhold et al. 1997) and sea turtles (Irwin & Lohmann 2005). Magnetic pulses only appear to affect adult migratory birds, not juveniles, which indicates that iron-mineral-based magnetoreception plays a role in an experience-based mechanism, which is presumed to be the map, not the compass system of birds (Munro et al. 1997a,b; Wiltschko et al. 2006).
A recent discovery suggests that the iron-mineral-based sensory system of birds is also able to detect the polarity of the magnetic field as part of a ‘fixed direction response’ displayed by migrating birds orienting in darkness or monochromatic light (Wiltschko et al. 2005, 2008; Stapput et al. 2008). Whether this polarity-based response plays any role in naturally migrating birds during navigation in the wild is unclear as it results in a season-independent orientation that does not match with normal orientation in the tested species.
Most magnetic pulse experiments have applied the pulse at a 90° angle to the magnetic field, which would have unknown effects on the iron minerals in the sensory cells. It has been argued that the correct application of a magnetic pulse could be diagnostic of the structure of the sensory system (Kirschvink et al. 1985). This is based on the observation that the swimming direction of magnetotactic bacteria is reversed by the application of a pulse antiparallel to the magnetic biasing field owing to remagnetization, whereas a pulse parallel to it has no effect (Blakemore 1975; Blakemore et al. 1980). There is partial support for this in a bat (Eptesicus fuscus), but the response of the crucial antiparallel group was too noisy to clearly indicate that the resulting change in orientation was purely due to a reversal of polarity of the magnetic material in the sensory cell (Holland et al. 2008). In the only test of this kind on birds in the Australian silvereye (Zosterops l. lateralis), neither the parallel-treated nor antiparallel-treated group responded in a way that was suggestive of a simple ‘magnetosome-like’ sensory organelle (Wiltschko et al. 2002). Indeed, recent evidence of the structure of the iron-mineral-based sense in birds indicates that this system is far more complex than the magnetosome model and is based on two magnetic materials, magnetite bullets and maghemite platelets, in sensory dendrites arranged in a three-dimensional architecture and innervated by the trigeminal nerve (Fleissner et al. 2003, 2007; although see Winklhofer (2009) for a discussion of the diagnostic power of the X-ray technique used). Models have shown that the maghemite platelets can amplify the magnetic field such that the magnetite bullets will pull on the nerve membrane and thus transduce a signal of the intensity of the magnetic field (Solov'yov & Greiner 2009b). This structure can also, in theory, detect the polarity and inclination of the magnetic field (Solov'yov & Greiner 2009a). These structures have been shown to be present in the beaks of four bird species that have different lifestyles with regard to their movement ecology, including the European robin, which is one of the species in this study and so may be a common feature of birds (Falkenberg et al. 2010). While a magnetic pulse would be expected to affect this structure, there is no clear prediction as to how a pulse aligned either parallel/antiparallel to the magnetic field or perpendicular to it would be expected to alter the orientation of the treated animals. In fact, the pulse treatment remains something of a ‘black box’ as there have been no direct measurements of the effect of a pulse on the iron minerals in the bird magnetic sensory system, either in vivo or in vitro and so it is currently unknown precisely which aspect of the iron-mineral-based magnetoreception system the pulse affects (Fleissner et al. 2007).
A further confounding element in the iron-mineral-based magnetoreception system is that although laboratory-based tests have indicated the presence of this mechanism in birds, so far, there is less support for its use by birds in a natural setting. While pigeons have been demonstrated to be affected by a magnetic pulse (Beason et al. 1997), neither a pulse treatment on migrating birds (Holland et al. 2009) nor trigeminal nerve lesions in homing pigeons (Gagliardo et al. 2006, 2008, 2009) had an effect on the birds' orientation in the field. In the case of Holland et al. (2009), however, a significant delay between treatment and departure could have allowed the animals to recalibrate their sensory systems, as is the case in the laboratory (Wiltschko et al. 1998). The present paper thus aims to further investigate the role of the iron-mineral-based magnetoreceptor system in migratory birds using magnetic pulses. By using different alignments of the pulse to the magnetic field (perpendicular, parallel and antiparallel), the study investigates whether there are differential responses that may provide information about the structure and function of the iron-mineral-based magnetic sense in naturally migrating birds.
2. Material and methods
2.1. Subjects
European robins (Erithacus rubecula) and reed warblers (Acrocephalus scirpaceus) were caught by mist netting at the Metnau monitoring station, Radolfzell, Germany between 10 April 2009 and 21 May 2009. Birds were weighed, and scored for migratory fattening from 1 to 5 using the Kaiser scale (Kaiser 1993). A total of 19 robins were caught between 10 April 2009 and 1 May 2009 and 57 reed warblers were caught between 20 April 2009 and 21 May 2009.
2.2. Experimental treatment
After processing at the ringing station, birds were subjected to a magnetic pulse. An SCR-fired capacitive discharge unit (an SOTA magnetic pulser) was modified by the addition of a double-wrapped, 10 cm diameter Lee Whittling coil (Kirschvink 1992). The coil system produced a unidirectional magnetic pulse of approximately 0.1 ms duration, with a peak amplitude slightly over 0.1 T, and a rise time of approximately 100 ns. A pair of fine wire Helmholtz coils produced a 320 µT biasing field that could be aligned parallel or antiparallel to the pulse direction.
2.2.1. Perpendicular pulse
Robins and reed warblers both received a treatment in which a magnetic pulse aligned perpendicular to the magnetic field was applied by placing the birds into the pulse coil for a duration of one pulse. The pulse coil was aligned with the direction of the pulse west to east and the birds were placed in the coil with their heads facing the direction of the pulse, ‘south-anterior’ (figure 1), as defined by Beason et al. (1995). Controls received the same treatment but the current in the double-wrapped coils was aligned in the opposite direction, so that no pulse was administered even though the capacitor charged and fired in the same way as in the experimental group. Six robins and 13 reed warblers were treated with the perpendicular magnetic pulse, and 13 robins and 13 reed warblers received the ‘sham’ control treatment.
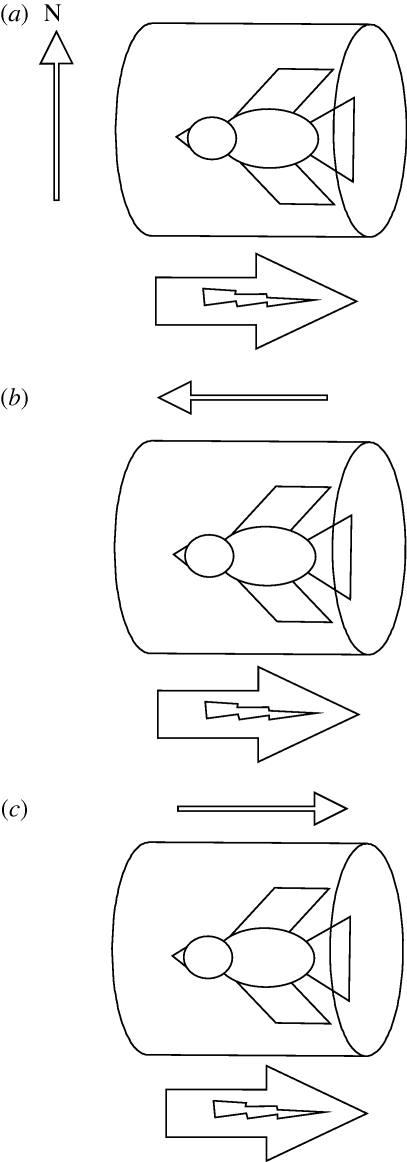
Figure 1.
A schematic of the alignment of the pulse relative to the bird and the magnetic field or biasing field. (a) Perpendicular pulse. The bird is placed in the pulse coil with its head facing the direction of the pulse and no artificial biasing field. As the pulse coil is aligned west to east, the pulse is perpendicular to the only biasing field present, that of the Earth's magnetic field. This treatment shifts the orientation of migratory birds by 90° in orientation cages (e.g. Wiltschko et al. 1994). (b) Antiparallel pulse. The bird is placed in the pulse coil with its head facing the direction of the pulse. An artificially produced biasing field is activated in the opposite direction to the pulse. This treatment reverses the swimming direction of magnetotactic bacteria (Blakemore et al. 1980), but results in a bi-modal east–west orientation in migratory birds in an orientation cage (Wiltschko et al. 2002). (c) Parallel pulse. The same as in (b), except the artificial biasing field is aligned in the same direction as the Earth's magnetic field. This treatment does not change the swimming direction of magnetotactic bacteria, but results in a bi-modal east–west orientation in birds in an orientation cage (Wiltschko et al. 2002).
2.2.2. Parallel/antiparallel pulse
Not enough robins were caught to also use the parallel/antiparallel pulse on this species, but 30 reed warblers were caught and treated with a pulse either parallel (13 birds) or antiparallel (17 birds) to the alignment of the magnetic field. The pulse was administered by aligning the solenoid west–east (direction of the pulse) and the biasing field was activated to be either parallel to the pulse (pointing east) or antiparallel to the pulse (pointing west). Birds were placed into the pulse coil south-anterior as in the perpendicular pulse experiment and received one pulse before being removed. This experiment overlapped with the perpendicular experiment and so no further controls were used (the last control departed on 17 May 2009 and the first experimental birds departed on 14 May 2009).
2.3. Data collection
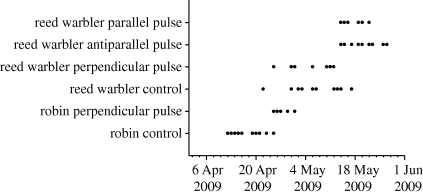
After receiving the pulse treatment birds were fitted with a 0.4 g LB2N radio transmitter (Holohil Systems Ltd). The transmitter was attached by sewing it to a square of cloth and then attaching the cloth to the bird's back after removing the feathers. Latex based eyelash glue was used to attach the cloth, which would ensure that the transmitter would not fall off. This method has been extensively tested and the transmitter was found to stay attached to birds for up to 24 days (Raim 1978), which is longer than the predicted life of the transmitter (21 days). Once the glue was dry, the bird was released back on the Metnau near where it was caught. Birds were monitored for their direction of departure from a 30 m high observation tower at the Metnau Peninsula (47.729° N, 9.002° E) by two methods. The first was to use a hand-held receiver and a three-element yagi antenna. The transmitter frequencies of the birds which could be detected at the release point were scanned. When a ‘take-off’ event occurred, this was usually characterized by an increase in signal strength with more variability, followed by a decrease in signal strength, which could be measured as vanishing in a discrete direction. The bearing of the last point at which the signal was detected was scored as the departure direction. A transmitter placed at the tower on the Metnau was detectable from the top of a hill 5.5 km north of the release point and so this would be a likely detection range for the departure bearing. During data collection, only the identifying frequencies of the birds were available to the observer (consisting of six-digit numbers), and not the treatment group of an individual bird. In the second method, an automatic receiver unit (ARU) recorded signal strength of transmitters from four four-element yagi antennae pointed north, south, east and west. The automated receiver scanned through the frequencies programmed into the memory, scanning through each antenna for a single frequency before moving to the next frequency. Departure directions were estimated from the signal strengths of the two antennae receiving the strongest signal at the last point at which signal strength was judged to be above a baseline level, indicating a signal was no longer detected (Cochran & Lord 1963; Crofoot et al. 2008). This level was estimated from observing a graphical representation of the signal trace before, during and after a departure. This was necessary because although the background noise level was recorded by the ARU, the antennae traces never precisely dropped to this level. As with hand-held bearings, only the identifying frequency number was available during analysis. The mean vector of the two direction vectors was used (Batschelet 1981), one from each antennae, with the direction of the respective antenna and length equal to the relative signal strength (signal strength−background noise) of the respective antennae. Only the signal strengths identified as being the departure point were analysed in the bearing calculator. When the observer was present, the bearing taken by hand was given priority, but comparison, where possible, between bearings taken by hand and those taken by the automatic receiver revealed no difference in the methods (all reed warblers, Watson–Williams test all cases: control, hand, n = 7, ARU, n = 6, F1,11 = 0.34; perpendicular pulse, hand, N = 5, ARU, n = 6, F1,9 = 0.74, p = 0.41; parallel pulse, hand, n = 6, ARU, n = 5, F1,9 = 0.27, p = 0.62; antiparallel pulse, hand, n = 6, ARU, n = 7, F1,11 = 1.91, p = 0.2), and so the ARU and hand-held bearings were pooled for analysis (only one bearing in the perpendicular pulsed group was taken by the ARU, so no comparison was possible for robins). In the robin group, controls were tagged before experimental birds as it was first necessary to establish that a measurable baseline-oriented response was possible. In the reed warbler group, where daily numbers caught allowed, the treatment was balanced between controls and experimental groups. However, the date of departure of each bird was the crucial factor in whether the experimental and control groups had been exposed to different conditions that would affect their orientation, as even light winds (4–5 m s−1, 10 m above ground level) have been shown to influence departure direction (Mouritsen 1998). Figure 2 shows the departure dates of each bird. No precise wind information was available for the exact departure of each bird, but wind data were obtained from www.wetteronline.de for Konstanz airport (11.5 km from the Metnau). Analysis was performed on the mean daily wind speed and mean daily wind direction measured at 10 m above ground level. Given that birds take off and rise to a height where they will be exposed to different wind strengths (Mouritsen & Larsen 1998; Bowlin et al. 2005), these data should be viewed with caution. Analysis of wind speed and direction indicates that with four exceptions all birds departed in winds of 0–0.2 m s−1 (1 on the Beaufort scale) or 0.2–0.5 m s−1 (2 on the Beaufort scale). The four exceptions took off on days when the mean daily wind speed was 0.5–1 m s−1 (3 on the Beaufort scale). Analysis of wind directions on departure dates for each bird did not indicate any differential assortment of mean daily wind directions between control and experimental groups in terms of wind exposure assigned as north, south, east or west (χ2-test, Yates correction: robins, χ2 = 1.47, p = 0.69; reed warblers, perpendicular pulse versus control, χ2 = 1.41, p = 0.70, parallel versus antiparallel pulsed, χ2 = 0.58, p = 0.47).
Figure 2.
Departure dates of birds from each experimental group.
3. Results
3.1. Perpendicular pulse
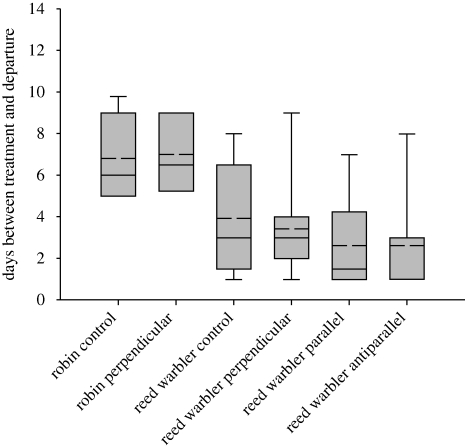
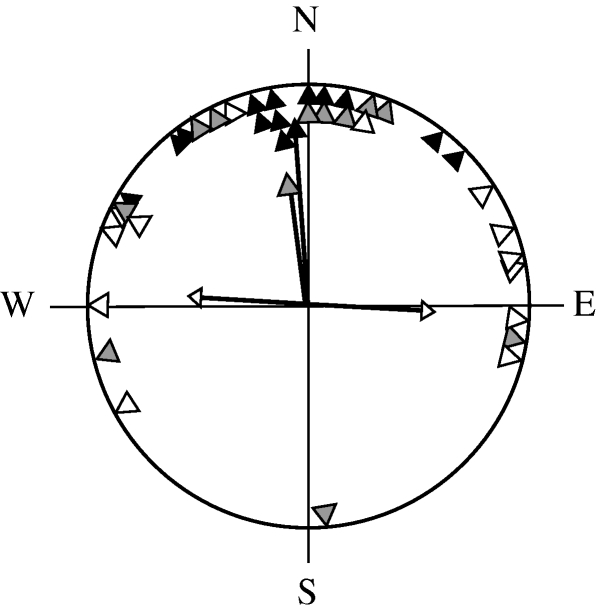
All groups displayed orientation significantly different from random (Rayleigh test: robins: control, r = 0.84, p < 0.0001, experimental, r = 0.88, p = 0.004; reed warblers: control, r = 0.89, p < 0.0001, experimental, r = 0.76, p < 0.0001). There was a significant angular difference between the orientation of the control and experimental groups in both robins (figure 3; Watson–Williams test, F = 28.689, p < 0.0001) and reed warblers (figure 4; Watson–Williams test, F = 12.182, p = 0.002) with a deflection of the experimental group east of controls in both cases. There was no significant difference in the time between tagging and departure of experimental birds and controls in either species (figure 5; ANOVA: robins: F1,15 = 0.025, p = 0.88; reed warblers: F1,23 = 0.0001, p = 0.99). Figure 2 shows the departure dates of birds in each group. There is an overlap in the departure dates of the reed warbler groups but the robin controls took off before the experimental birds.
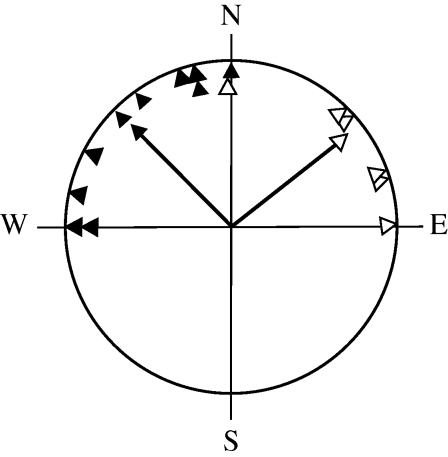
Figure 3.
Circular diagram of departure bearings of control (black triangles, n = 10) and experimental (open triangles, n = 6) robins treated with a perpendicular magnetic pulse. The arrows represent the mean bearings and vector lengths of each group.
Figure 4.
Circular diagram of departure bearings of control (black triangles, n = 13) and experimental (open triangles, n = 11) reed warblers treated with a perpendicular magnetic pulse. The arrows represent the mean bearings and vector lengths of each group.
Figure 5.
Box plots of mean times between tagging and departure. Lower and upper box limits represent the 25th and 75th percentiles and error bars represent the 10th and 90th percentiles. Dashed line represents the mean and solid line the median value.
3.2. Parallel/antiparallel pulse
Both parallel- and antiparallel-treated reed warbler groups displayed orientation significantly different from random, although the antiparallel group is axially oriented (figure 6, Rayleigh test: parallel: r = 52, p = 0.046; antiparallel: unimodal, r = 0.27, p = 0.39, bi-modal, r = 0.53 p = 0.02). There was a significant difference between the antiparallel group and the parallel group (Mardia–Watson–Wheeler test: W = 12.09, p = 0.002) and between antiparallel birds and control birds (Mardia–Watson–Wheeler test: W = 18.88, p < 0.0001), but not between the parallel and control groups (Mardia–Watson–Wheeler test: W=3.44, p = 0.179). There was a significant difference between the control and the parallel and antiparallel groups in the time between tagging and departure, with controls taking longer to depart, but not between the parallel and antiparallel groups (figure 5; ANOVA: F2,36 = 10.989, p < 0.0001; post hoc Bonferoni test: parallel versus control, p < 0.001, parallel versus antiparallel, p > 0.05, antiparallel versus control, p < 0.001). There was an overlap between the departure dates of the parallel and antiparallel groups (figure 5), but less between controls and the two experimental groups.
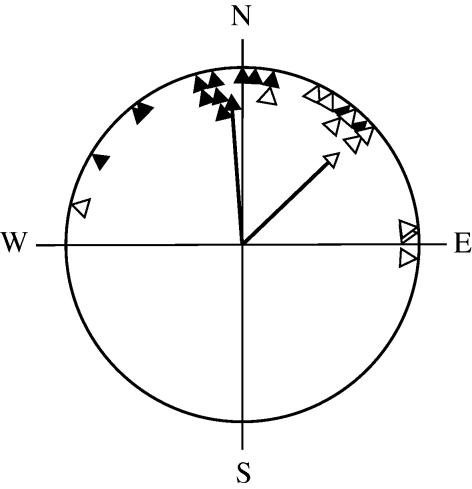
Figure 6.
Circular diagram of departure bearings of control (black triangles, n = 13), parallel pulse-treated (grey triangles, n = 11) and antiparallel pulse-treated (open triangles, n = 13) reed warblers. The arrows represent the mean bearings and vector lengths of each group.
4. Discussion
The present experiments indicate that a magnetic pulse, designed to manipulate the perception of the Earth's magnetic field by an iron-mineral-based magnetic sense, affects the behaviour of naturally migrating songbirds. The perpendicular pulse deflected the orientation of both robins and reed warblers in a clockwise direction relative to controls. This is consistent with remagnetization in the direction of the applied pulse. The parallel/antiparallel treatment produced a differential effect depending on the alignment of the pulse to the biasing field, with the parallel treatment being not significantly different from controls, and the antiparallel treatment producing orientation that was bi-modal in the east–west direction. These results are consistent with the hypothesis that an iron-mineral-based magnetoreceptor plays a role in the natural migratory navigation of these two species. The magnetic pulse is thought to be diagnostic of an iron-mineral-based sense as it should remagnetize remanence-bearing material. The fact that the pulse has led to reorientation rather than disorientation suggests that it has altered, rather than disrupted, the birds' perception of the Earth's magnetic field. While the pulse would potentially affect the radical-pair-based magnetic sense at the moment of treatment, it is not thought that this sense would be disrupted after this point. The fact that the magnetic compass orientation of juvenile passerine birds is not affected by magnetic pulses (Munro et al. 1997a,b) would seem to support this statement. The effects of such a strong electromagnetic pulse on proteins or other electrically charged processes in an organism are also not known. However, the fact that the parallel group is not significantly different from controls argues against the effect being non-specific. A similar result in bats (Holland et al. 2008) and also the lack of an effect on juvenile migrants (Munro et al. 1997a,b) also argue against the pulse causing a non-specific effect, but it is still unknown exactly how the iron minerals in the sensory cells are affected by the pulse. While the results of Fleissner et al. (2003, 2007) indicate iron mineral deposits in sensory dendrites innervated by the trigeminal nerve, it is not yet conclusively demonstrated that these structures play a role in magnetoreception. The removal of the effect of a pulse by local anaesthesia to the beak provides circumstantial evidence that these iron-mineral-based deposits may play a role in this effect (Wiltschko et al. 2009), but the crucial trigeminal nerve lesion study remains to be performed. Further ultra-structural analysis of birds that have received magnetic pulses is also required to examine exactly how or whether the pulse affects the iron minerals in the sensory dendrites.
It has been argued that the parallel/antiparallel treatment could be diagnostic of a polarity-based sense. However, if this were the case, then as well as no effect in the parallel group reversal of orientation would be expected in the antiparallel group leading to a southerly heading during northward spring migration. The bi-modal distribution of this group does not easily support this explanation. It has been proposed that it cannot be ruled out that there is an interaction of the iron-mineral-based sense with the radical-pair system to provide a magnetic compass direction in birds (Mouritsen & Ritz 2005; Kirschvink et al. 2010). However, since we do not currently know what exact effect a strong magnetic pulse would have on the iron mineral deposits found in the upper beak of European robins (Falkenberg et al. 2010) and reed warblers, it is too early to speculate exactly why these birds reacted in the way they did. Nevertheless, the reaction of the birds observed here provides new information that can help in the search for the exact functional mechanism of the iron-mineral-based receptors, and the addition of a measure of behaviour in natural conditions may be able to add insights into how the two proposed magnetic senses interact in the wild.
The finding of this experiment is in contrast to a study on the Australian silvereye (Z. l. lateralis). Application of a pulse parallel to the biasing field resulted in a change in the orientation of those birds compared with controls (Wiltschko et al. 2002). Why reed warblers and Australian silvereyes have reacted differently to the pulse with biasing field can only be speculated on at this stage. Possible differences to be explored include the ecology of the species (silvereyes are a dawn/dusk migrant, reed warblers are a night migrant), the treatment (this study administered a 0.1 T pulse, the silvereye study a 0.5 T pulse) or the methodology (silvereyes in an orientation cage versus natural migration in reed warblers).
Given that the mean time between tagging and departure in the region was 4 days, this suggests that one possible explanation for the lack of an effect in the previous study in natural conditions (Holland et al. 2009) may have been because the birds in that study departed later than this (8–10 days to departure, 14–20 days to final bearing). Birds treated with a pulse in an orientation cage have been shown to return to normal orientation after 10–14 days (Wiltschko et al. 1998). It is likely therefore that the birds treated in the present experiments would recover their magnetic sense. The site of treatment was within the known breeding range of both species.
It should also be noted that the controls of both European robins and reed warblers have a mean departure direction that does not match with data from ringing recoveries, which report a general northeasterly direction of birds from this area. This may be the result of local factors influencing the departure direction. Such effects are known in homing pigeons where so-called release site biases are known to affect the vanishing bearings of birds (Keeton 1973). Such biases have been proposed to be the result of variation in local factors causing misreading of the navigational map (Walker 1998; Mora & Walker 2009). The fact that in three of the four experimental cases the mean orientation was significantly different from controls suggests that these local factors are not masking an orientation decision. The influence of wind on orientation should also not be ruled out as a contributing factor here, given that the winds at the altitude the birds would rise up to were unknown. The lack of overlap between the control and experimental birds in the European robin group means that the difference between these groups should also be viewed with caution as while surface winds do not indicate any differential effect of wind exposure, the birds may have faced different winds at the altitude they took off to migrate.
Taken together these results are consistent with the hypothesis that an iron-mineral-based magnetic sense plays a role in the migratory navigation behaviour of naturally migrating passerine birds. The methodology used here represents a way to complement the laboratory-based studies used to investigate the behavioural aspects of the magnetic sense in migration with field-based studies in a natural setting. It should be married to further ultra-structural analysis of the putative iron-mineral-based sense of migratory birds to understand how this sense functions to provide navigational information from the magnetic field. This field-based method finally allows the study of magnetoreception in animals to span and integrate the contrasting disciplines of quantum physics, molecular biology and field-based ecology.
Acknowledgements
These experiments were approved by the animal welfare committee of the Regierungspräsidium Freiburg.
I am indebted to Heidi Schmidt and Tania Volger for help with catching birds. Adam Fudikar, Dina Dechmann, Martin Wikelski, Elena Arriero and Bart Kranstauber provided pleasant company at the top of the Metnau Tower on long dark nights. Wolfgang Fiedler provided access to data from ringing recoveries at the Max Planck Institute Metnau ringing station. Kasper Thorup aided in writing the bearing calculation spreadsheet. Henrik Mouritsen, John Phillips, Joe Kirschvink, Micheal Winklehofer and two anonymous referees provided comments that greatly improved this manuscript. This work was funded by the Max Planck Society and NSF grant IOS-0744704. The magnetic pulser was kindly modified by Joe Kirschvink.
References
- Batschelet E. 1981. Circular statistics in biology. New York, NY: Academic Press. [Google Scholar]
- Beason R. C., Dussourd N., Deutschlander M. E. 1995. Behavioral evidence for the use of magnetic material in magnetoreception by a migratory bird. J. Exp. Biol. 198, 141–146. [DOI] [PubMed] [Google Scholar]
- Beason R. C., Wiltschko R., Wiltschko W. 1997. Pigeon homing: effects of magnetic pulses on initial orientation. Auk 114, 405–415. [Google Scholar]
- Blakemore R. P. 1975. Magnetotactic bacteria. Science 190, 377–379. ( 10.1126/science.170679) [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Frankel R. B., Kalmijn A. J. 1980. South-seeking magnetotactic bacteria in the Southern Hemisphere. Nature 286, 384–385. ( 10.1038/286384a0) [DOI] [Google Scholar]
- Bowlin M., Cochran W. W., Wikelksi M. 2005. Biotelemetry of new world thrushes during migration: physiology, energetics and orientation in the wild. Integr. Comp. Biol. 45, 295–304. ( 10.1093/icb/45.2.295) [DOI] [PubMed] [Google Scholar]
- Canfield J. M., Belford R. L., Debrunner P. G., Schulten K. J. 1994. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism. Chem. Phys. 182, 1–18. ( 10.1016/0301-0104(93)E0442-X) [DOI] [Google Scholar]
- Canfield J. M., Belford R. L., Debrunner P. G., Schulten K. J. 1995. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism. Chem. Phys. 191, 347 ( 10.1016/0301-0104(94)00234-2) [DOI] [Google Scholar]
- Cochran W. W., Lord R. D. 1963. A radio tracking system for wild animals. J. Wildl. Manag. 27, 9–24. ( 10.2307/3797775) [DOI] [Google Scholar]
- Cochran W. W., Mouritsen H., Wikelski M. 2004. Migratory songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408. ( 10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- Crofoot M. C., Gilby I. C., Wikelski M. C., Kays R. W. 2008. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA 105, 577–581. ( 10.1073/pnas.0707749105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebel C. E., Proksch R., Green C. R., Neilson P., Walker M. M. 2000. Magnetite defines a vertebrate magnetoreceptor. Nature 406, 299–302. ( 10.1038/35018561) [DOI] [PubMed] [Google Scholar]
- Falkenberg G., Fleissner G., Schuchardt K., Kuehbacher M., Thalau P., Mouritsen H., Heyers D., Wellenreuther G., Fleissner G. 2010. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE 5, e9231 ( 10.1371/journal.pone.0009231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner G., Holtkamp-Rotzler E., Hanzlik M., Winklhofer M., Petersen N., Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. 2007. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 91, 632–641. ( 10.1007/s00114-007-0236-0) [DOI] [PubMed] [Google Scholar]
- Freake M. J., Muheim R., Phillips J. B. 2006. Magnetic maps in animals: a theory comes of age? Q. Rev. Biol. 81, 327–347. ( 10.1086/511528) [DOI] [PubMed] [Google Scholar]
- Gagliardo A., Ioale P., Savini M., Wild J. M. 2006. Having the nerve to home: trigeminal magnetoreceptor versus olfactory mediation of homing in pigeons. J. Exp. Biol. 209, 2888–2892. ( 10.1242/jeb.02313) [DOI] [PubMed] [Google Scholar]
- Gagliardo A., Ioale P., Savini M., Wild J. M. 2008. Navigational abilities of homing pigeons deprived of olfactory or trigeminally mediated magnetic information when young. J. Exp. Biol. 211, 2046–2051. ( 10.1242/jeb.017608) [DOI] [PubMed] [Google Scholar]
- Gagliardo A., Ioale P., Savini M., Wild J. M. 2009. Navigational abilities of adult and experienced homing pigeons deprived of olfactory or trigeminally mediated magnetic information. J. Exp. Biol. 212, 3119–3124. ( 10.1242/jeb.031864) [DOI] [PubMed] [Google Scholar]
- Heyers D., Manns M., Luksch H., Gunturkun O., Mouritsen H. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 ( 10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R. A., Kirschvink J. L., Doak T. G., Wikelski M. 2008. Bats use magnetite to detect the Earth's magnetic field. PLoS ONE 3, e1676 ( 10.1371/journal.pone.0001676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R. A., Thorup K., Gagliardo A., Bisson I., Knecht E., Mizrahi D., Wikelski M. 2009. Testing the role of sensory systems in the migratory heading of a songbird. J. Exp. Biol. 212, 4065–4071. ( 10.1242/jeb.034504) [DOI] [PubMed] [Google Scholar]
- Irwin W. P., Lohmann K. J. 2005. Disruption of magnetic orientation in hatchling loggerhead sea turtles by pulsed magnetic fields. J. Comp. Physiol. A 191, 475–480. ( 10.1007/s00359-005-0609-9) [DOI] [PubMed] [Google Scholar]
- Kaiser A. 1993. A new multi-category classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 64, 246–255. [Google Scholar]
- Kalmijn A. J. 1981. Biophysics of geomagnetic field detection. IEEE Trans. Magn. 17, 1113–1124. ( 10.1109/TMAG.1981.1061156) [DOI] [Google Scholar]
- Kalmijn A. J., Blakemore R. P. 1978. The magnetic behavior of mud bacteria. In Animal migration, navigation and homing (eds Schmidt-Koenig K., Keeton W. T.). Berlin, Germany: Springer-Verlag. [Google Scholar]
- Keeton W. T. 1973. Release-site bias as a possible guide to the ‘map’ component in pigeon homing. J. Comp. Physiol. 86, 1–16. ( 10.1007/BF00694473) [DOI] [Google Scholar]
- Kirschvink J. L. 1992. Uniform magnetic-fields and double-wrapped coil systems—improved techniques for the design of bioelectromagnetic experiments. Bioelectromagnetics 13, 401–411. ( 10.1002/bem.2250130507) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Gould J. L. 1981. Biogenetic magnetite as a basis for magnetic field detection in animals. Biosystems 13, 181–201. ( 10.1016/0303-2647(81)90060-5) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Walker M. M., Chang S. B., Dizon A. E., Peterson K. A. 1985. Chains of single domain magnetite particles in chinook salmon, Onchorhynchus tshawytscha. J. Comp. Physiol. A 157, 375–381. ( 10.1007/BF00618127) [DOI] [Google Scholar]
- Kirschvink J. L., Walker M. M., Diebel C. E. 2001. Magnetite based magnetoreception. Curr. Opin. Neurobiol. 11, 462–467. ( 10.1016/S0959-4388(00)00235-X) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Winklhofer M., Walker M. M. 2010. Biophysics of magnetic orientation: strengthening the interface between theory and experimental design. J. R. Soc. Interface 7, S179–S191. ( 10.1098/rsif.2009.0491.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Maeda K., Henbest K., Schleicher E., Simon T., Timmel C. R., Hore P. J., Mouritsen H. 2007. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhold S., Burda H., Kreilos I., Wiltschko W. 1997. Magnetic orientation in common molerats from Zambia. In Royal Institute of Navigation: birds, humans and other animals, paper no. 5. [Google Scholar]
- Mora C. V., Walker M. M. 2009. Do release-site biases reflect response to the Earth's magnetic field during position determination by homing pigeons? Proc. R. Soc. B 276, 3295–3302. ( 10.1098/rspb.2009.0872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C. V., Davison M., Wild J. M., Walker M. M. 2004. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432, 508–511. ( 10.1038/nature03077) [DOI] [PubMed] [Google Scholar]
- Mouritsen H. 1998. Redstarts, Phoenicurus phoenicurus, can orient in a true-zero magnetic field. Anim. Behav. 55, 1311–1324. ( 10.1006/anbe.1997.0696) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Larsen O. N. 1998. Migrating young pied flycatchers, Ficedula hypoleuca, did not compensate for geographical displacements. J. Exp. Biol. 204, 3855–3865. [Google Scholar]
- Mouritsen H., Ritz T. 2005. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–415. ( 10.1016/j.conb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Liedvogel M., Wada K., Jarvis E. D. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344. ( 10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheim R., Backman J., Akesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856. [DOI] [PubMed] [Google Scholar]
- Munro U., Munro J. A., Phillips J. B., Wiltschko R., Wiltschko W. 1997a Evidence for a magnetite-based navigational ‘map’ in birds. Naturwissenschaften 84, 26–28. ( 10.1007/s001140050343) [DOI] [Google Scholar]
- Munro U., Munro J. A., Phillips J. B., Wiltschko W. 1997b Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Aust. J. Zool. 45, 189–198. ( 10.1071/ZO96066) [DOI] [Google Scholar]
- Raim A. 1978. A radio transmitter attachment for small passerine birds. Bird Banding 49, 326–332. [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for vision based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Thalau P., Phillips J. B., Wiltschko R., Wiltschko W. 2004. Resonance effects indicate radical pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- Ritz T., Wiltschko R., Hore P. J., Rodgers C. T., Stapput K., Thalau P., Timmel C. R., Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. T., Hore P. J. 2009. Chemical magnetoreception in birds: the radical pair mechanism. Proc. Natl Acad. Sci. USA 106, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulten K., Windemuth A. 1986. Model for a physiological magnetic compass. In Biophysical effects of steady magnetic fields, vol. 11 (eds Maret G., Boccara N., Kiepenheuer J.), pp. 99–106. Berlin, Germany: Springer. [Google Scholar]
- Schulten K., Swenberg C. E., Weller A. 1978. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. NF111, 1–5. [Google Scholar]
- Solov'yov I. A., Greiner W. 2009a Iron-mineral-based magnetoreceptor in birds: polarity or inclination compass? Eur. Phys. J. D 51, 161–172. ( 10.1140/epjd/e2008-00118-y) [DOI] [Google Scholar]
- Solov'yov I. A., Greiner W. 2009b Micromagnetic insight into a magnetoreceptor in birds: existence of magnetic field amplifiers in the beak. Phys. Rev. E 80, 041919 ( 10.1103/PhysRevE.80.041919). [DOI] [PubMed] [Google Scholar]
- Solov'yov I. A., Schulten K. 2009. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 96, 4804–4813. ( 10.1016/j.bpj.2009.03.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solov'yov I. A., Chandler D. E., Schulten K. 2007. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys. J. 92, 2711–2726. ( 10.1529/biophysj.106.097139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapput K., Thalau P., Wiltschko R., Wiltschko W. 2008. Orientation of birds in total darkness. Curr. Biol. 18, 602–606. ( 10.1016/j.cub.2008.03.046) [DOI] [PubMed] [Google Scholar]
- Walker M. M. 1998. On a wing and a vector: a model for magnetic navigation in birds. J. Theor. Biol. 192, 341–349. ( 10.1006/jtbi.1998.0653) [DOI] [PubMed] [Google Scholar]
- Walker M. M., Diebel C. E., Haugh C. V., Pankhurst P. M., Montgomery J. C., Green C. R. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376. ( 10.1038/37057) [DOI] [PubMed] [Google Scholar]
- Wang K., Ritz T. 2006. Zeeman resonances for radical-pair reactions in weak static magnetic fields. Mol. Phys. 104, 1649–1658. ( 10.1080/00268970600564869) [DOI] [Google Scholar]
- Wiltschko R., Wiltschko W. 1995. Magnetic orientation in animals. Berlin, Germany: Springer. [Google Scholar]
- Wiltschko R., Ritz T., Stapput K., Thalau P., Wiltschko W. 2005. Two different types of light-dependent responses to magnetic fields in birds. Curr. Biol. 15, 1518–1523. ( 10.1016/j.cub.2005.07.037) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Munro U., Ford H., Stapput K., Wiltschko W. 2008. Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. J. Exp. Biol. 211, 3344–3350. ( 10.1242/jeb.020313) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2002. Magnetic compass orientation in birds and its physiological basis. Naturwissenschaften 89, 445–452. ( 10.1007/s00114-002-0356-5) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Beason R. C., Ford H., Wiltschko R. 1994. A magnetic pulse leads to a temporary deflection in the orientation of migratory birds. Experientia 50, 697–700. ( 10.1007/BF01952877) [DOI] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1998. Effect of a magnetic pulse on the orientation of silvereyes, Zosterops l. lateralis, during spring migration. J. Exp. Biol. 201, 3257–3261. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Wiltschko R., Kirschvink J. L. 2002. Magnetite-based magnetoreception in birds: the effect of a biasing field and a pulse on migratory behavior. J. Exp. Biol. 205, 3031–3037. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2006. Bird navigation: what type of information does the magnetite-based receptor provide? Proc. R. Soc. B 273, 2815–2820. ( 10.1098/rspb.2006.3651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2009. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. R. Soc. B 276, 2227–2232. ( 10.1098/rspb.2009.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer M. 2009. The physics of geomagnetic-field transduction in animals. IEEE Trans. Magn. 45, 5259–5265. ( 10.1109/TMAG.2009.2017940) [DOI] [Google Scholar]
- Zapka M., et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277. ( 10.1038/nature08528) [DOI] [PubMed] [Google Scholar]