Abstract
The blue colouration seen in the leaves of Selaginella willdenowii is shown to be iridescent. Transmission electron microscopy studies confirm the presence of a layered lamellar structure of the upper cuticle of iridescent leaves. Modelling of these multi-layer structures suggests that they are responsible for the blue iridescence, confirming the link between the observed lamellae and the recorded optical properties. Comparison of blue and green leaves from the same plant indicates that the loss of the blue iridescence corresponds to a loss of the multi-layer structure. The results reported here do not support the idea that iridescence in plants acts to enhance light capture of photosynthetically important wavelengths. The reflectance of light in the range 600–700 nm is very similar for both iridescent and non-iridescent leaves. However, owing to the occurrence of blue colouration in a wide variety of shade dwelling plants it is probable that this iridescence has some adaptive benefit. Possible adaptive advantages of the blue iridescence in these plants are discussed.
Keywords: iridescence, multi-layer interference, plant optics, Selaginella willdenowii, tropical understorey plants
1. Introduction
The herbaceous ground flora of tropical rainforests grow under specialized conditions with high humidity and low light levels. The leaves of these plants often display unique features (Vogelmann 1993). Some plants have velvety leaves resulting from cells in the epidermis which project as papillae (Richards 1996). The leaves are usually hydrophobic, which is thought to increase photosynthetic efficiency. Other plants are seen to have red pigmentation located in the mesophyll cells immediately beneath the palisade layer (Lee et al. 1979). It was initially thought that this pigmentation acted to increase energy absorption by backscattering of transmitted light into the palisade cells. However, it has been shown that this is not the case, instead the red pigment acts to attenuate light damage during intermittent exposure to high-intensity light (Gould et al. 1995; Hughes et al. 2008). A third set of plants, which grow in deep shade, have leaves with a bluish iridescence (Lee 1986).
One species that exhibits blue iridescence is the lycophyte Selaginella willdenowii (Desv. Bak), which grows in the rainforests of Malaya (Lee & Lowry 1975). The colouration was initially thought to result from reflective granules present in the cuticle (the waxy layer overlying the epidermal walls; Stahl 1896; Gentner 1909). However, no such granules have been found (Lee & Lowry 1975) and only ordinary photosynthetic pigments found in the chloroplasts can be extracted (Lee 1997). It has also been noted that the blue colouration of the microphyllous leaves disappears when they are made wet, or when they wilt (Fox & Wells 1971), showing that the colour must result from an optical structure close to the leaf surface, rather than arising from pigment-based absorption. In S. willdenowii the iridescence is attributed to a quarter wavelength interference filter (Lee & Hébant 1984). Cross-sectional images show two 80 nm thick lamellae at the outer edge of the cell wall of the upper epidermis. These lamellae are not found in leaves which appear green. The iridescence is also seen to be dependent on the conditions under which the plant is grown. Lee et al. grew plants under light conditions with different red (R) to far-red (FR) ratios. They found that only plants grown under a minimum R : FR ratio of 0.35 produced intensely blue leaves. The treatment was reversible; when plants were moved from one growth chamber to another, new growth was seen to develop the characteristics of the new chamber. However, this treatment was also seen to change the leaves in other ways (Lee & Hébant 1984).
Iridescence is also seen in the leaves of the ferns Danaea nodosa, Trichomeanes elegans and Diplazium tomentosum and in the angiosperm Begonia pavonina. In Danaea nodosa the iridescence is attributed to light interference from helicoidal alternating electron opaque and electron transparent layers of cellulose microfibrils in the plant cell walls, where between 18 and 30 parallel lamellae are observed (Graham et al. 1993). For green leaves of Danaea nodosa these layers are either not present or of the wrong size. In Begonia pavonina and Diplazium tomentosum the blue colouration is attributed to specialized plastids termed iridoplasts adjacent to the lower periclinal wall of the adaxial epidermis (Gould & Lee 1996). These iridoplasts contain 16 alternating electron-opaque and electron-translucent bands, which are thought to form a stack of alternating high and low refractive index lamellae resulting in thin-film multi-layer interference. In Trichomeanes elegans the colouration is attributed to uniform grana stacks in specialized chloroplasts adjacent to the adaxial epidermis (Graham et al. 1993). Golden-green iridescence is also seen in the moss Schistostega (Richards 1932).
The way in which the colouration is produced strongly influences the wavelength of iridescence. For S. willdenowii, where the colouration is a result of interference effects from lamellae on the surface of the leaf, Lee et al. observed a blue peak in the reflectance at around 405 nm. However, for plants where the colour results from light interference within specialized plastids or chloroplasts, the peak in reflectance is observed at much longer wavelengths. The reflectance peak is seen at 480 nm for Danaea nodosa, at 446 nm for Diplazium tomentosum and at 530 nm in Trichomeanes elegans.
The function of iridescence in these plants is unknown. In Trichomeanes elegans the colouration does not come from surface interference filters and it has been suggested that the iridescence may be a by-product of ultrastructure with no selective advantage (Graham et al. 1993). However, Graham et al. argue that since there is a strong association with extreme shade this suggests otherwise and the iridescence may alter internal light environments in a way that is advantageous to the plants.
Lee et al. hypothesize that the lamellae observed in S. willdenowii function as an antireflective coating, reflecting light at short wavelengths and enhancing the absorption of light at longer wavelengths through destructive interference (Lee & Lowry 1975). Owing to the filtering effect of foliage in the upper layers of the rainforests (Bazzaz & Pickett 1980; Chazdon et al. 1996), the plants which grow in the extreme shade receive light enhanced in the R to FR part of the electromagnetic spectrum: 520–620 nm and 700–1100 nm (Théry 1998). Light levels at the ground in the understorey have been reported to be between 0.1 per cent and 1.9 per cent of full sunlight (Bazzaz & Pickett 1980; Chazdon et al. 1996). An interference filter which suppresses the reflection of red light would allow for enhanced light capture for photosynthesis. However, it would also reduce the amount of light absorbed in the blue part of the spectrum, which is equally important for photosynthesis (Hipkins & Baker 1986; Blakenship 2002).
This paper aims to further the understanding of the iridescence produced in leaves of S. willdenowii. The plants were grown under normal light conditions with partial shading for light intensities greater than 100 W. Iridescence was observed in juvenile leaves and disappeared in older leaves. Transmission electron microscopy (TEM) studies confirm the presence of a layered lamellar structure of the upper cuticle of the iridescent leaves. Spectroscopic measurements and modelling of the data show the iridescent nature of the filter, confirming for the first time the link between the observed lamellae and the recorded optical properties of the material. Our measurements also demonstrate that the multi-layer does not enhance the capture of red light, refuting current theories on the function of leaf iridescence in this species. Possible adaptive advantages of the blue iridescence in these plants are discussed.
2. Materials and methods
Selaginella willdenowii plant material was obtained from the Royal Botanic Gardens, Kew, accession number 1994-1186-RONI 57. The plant was grown in a glasshouse under the following conditions: night temperature 18°C, day temperature 22°C, vent temperature 25°C, humidity 70–80%. The plants were grown under normal sunlight levels, with partial shading of the greenhouse for incident light with an intensity greater than 100 W and full shading for incident light with an intensity greater than 200 W. Plant material from the same plant was also kept at the Cambridge University Botanic Garden and grown under the same conditions, with partial shading of the plants.
2.1. Spectroscopy
Spectroscopic reflection measurements of S. willdenowii were carried out using a fibre-optic-based gonio-spectroscopic set-up, with independently adjustable incidence angles. An Ocean Optics DHL2000 light source with a combined deuterium halogen lamp provided a collimated 2 mm wide beam for illumination of the samples with light in the spectral range 300–900 nm. Reflected or scattered light was detected with an Ocean Optics USB4000 spectroscope. Plant leaves were mounted on metal washers so that the leaves were flat and free standing. Both specular reflection and scattering measurements were carried out. For specular reflection measurements, the angle of light incidence θI, measured from the sample surface normal, was varied from 16° to 60° in 2° steps. The detection angle was adjusted to θD = −θI in order to detect the specularly reflected light. For scattering measurements, the angle of incidence was fixed and the angle of detection was varied from 0° to − 90° as measured from the sample normal. The reflection measurements were normalized with respect to both a Spectralon diffuse reflectance standard of 99 per cent reflectance and the angular aperture of the detector. Measurements of absolute reflectivity were carried out in a Labsphere integrating sphere with the same light source as used for the spectroscopic measurements. Spectra were measured for a mature green leaf and a semi-iridescent blue leaf.
2.2. Microscopy
For ultrastructural studies small branches were fixed in Karnovsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2) overnight at 4°C. Sections 1 × 2 mm2 were then cut, rinsed in buffer and post-fixed in a 1 per cent osmium tetroxide (Agar) phosphate buffer solution for 2 h. They were then rinsed in buffer and dehydrated in absolute ethanol solutions in steps from 30 per cent EtOH to 100 per cent EtOH. The material was embedded in optical grade LR Resin (Agar) and left for one week at 4°C to allow the resin to infiltrate. The resin was changed daily. The samples were cured under vacuum at 400 mbar for 20 h at 58°C. Thin sections were cut using a Leica Ultracut UCT microtome and stained using a Leica EM stain with uranyl acetate and lead citrate. Images were taken using a FEI Philips Tecnai 20 TEM at 200 kV.
Surface structures were obtained by taking casts of the leaves in dental wax. Each cast was allowed to set, the leaf removed and the cast then filled with epoxy. Once the epoxy had set, the dental wax was removed leaving a positive replica of the plant leaf. This was imaged using optical microscopy.
3. Results
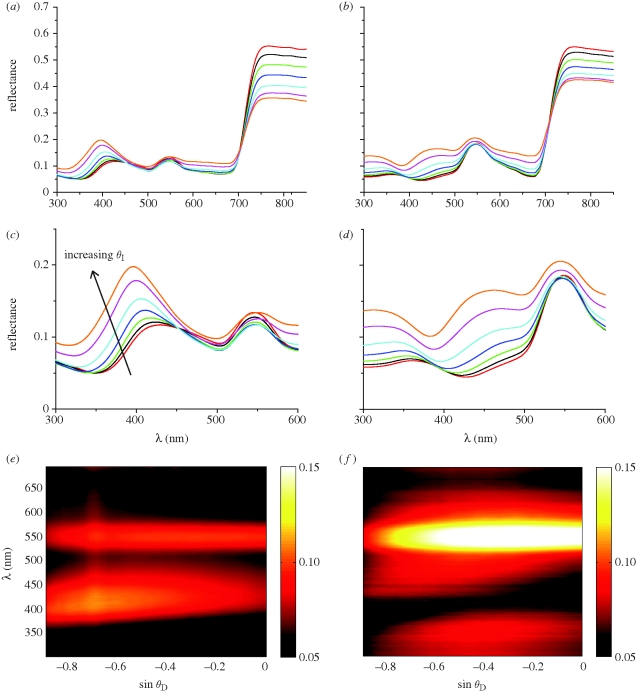
The spectroscopic measurements of the juvenile blue leaves and mature green leaves can be seen in figure 1. Both leaves were taken from the same branch of the same plant. The blue leaf was from the newest growth on the branch, while the green leaf was from older growth. Leaves in between were seen to vary in colouration from blue to green depending on age. For the juvenile blue leaves a peak in the reflectance spectra is observed at around 430 nm in specular reflection, for an angle of incidence θI = 16° (figure 2b). This peak shifts to lower wavelengths when increasing θI from 16° to 50° (figure 1a,c) and is not present in the spectra measured for the mature green leaves (figure 1b,d). In both sets of data a peak at 545 nm is observed. This peak does not change position as θI is varied. The 545 nm peak has a 50 per cent increased reflectance in the green leaf compared with the blue leaf, but the overall reflectance of both leaves is the same.
Figure 1.
Specular reflection and scattering spectra of (a,c,e) juvenile blue and (b,d,f) mature green S. willdenowii leaves. Intensity of specularly reflected light as a function of light incidence angle for (a) juvenile blue leaves and (b) mature green leaves (red line, θ1 = 16°; black line, θ1 = 22°; green line, θ1 = 28°; blue line, θ1 = 34°; light blue line, θ1 = 40°; purple line, θ1 = 46°; yellow line, θ1 = 50°). (c,d) Enlargements of the reflection peaks in the blue and green spectral ranges from (a,b), respectively. Scattered intensity as a function of detection angle for a fixed light incidence angle of 36° for (e) juvenile blue leaves and (f) mature green leaves. The intensity is colour coded, with red (dark) corresponding to a lower intensity and yellow (bright) a higher intensity.
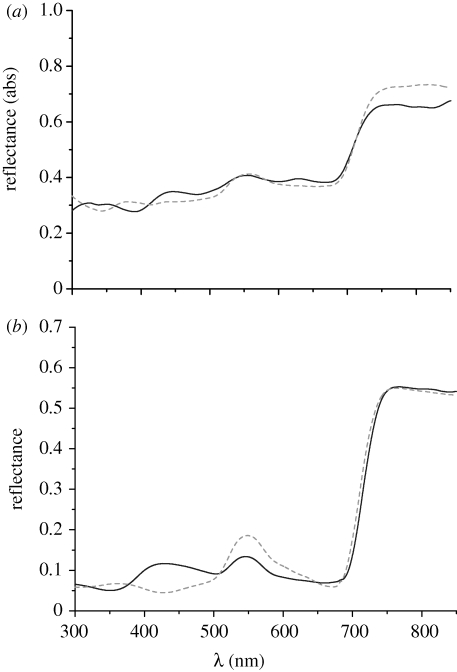
Figure 2.
(a) Integrating sphere reflection measurements of blue (solid line) and mature green (dashed line) S. willdenowii leaves. (b) Spectrally resolved specular reflection of juvenile blue (solid line) and mature green (dashed line) leaves at θI = −θD = 16°.
Figure 1e,f shows the scattering measurements for the blue and green leaves, respectively, for θI = 36°. The horizontal band at 545 nm stems from the green reflectance peak, seen for both leaves. This band is independent of the incidence and detection angles. For higher |θD| a weak peak is observed in the scattering data at around 450 nm in figure 1f, which is also present in the specular reflection measurements (figure 1b). This suggests that the multi-layer stack of the juvenile blue leaf is still partially present in the green leaf. The different position of this peak in figure 1f as compared with figure 1e suggests that the thicknesses and/or contrast between the layers has changed as the leaf has aged.
Figure 2a shows the experimental spectra for the integrating sphere measurements. The measurements were carried out on a mature green leaf and an iridescent blue leaf. The blue leaf, while being the youngest growth on the plant, was not a juvenile leaf. The results give an indication of the absolute reflectance of the leaves and show that the relative intensities of the leaves as measured in specular reflection are comparable (figure 2a,b). The spectra are averaged over three different measurements.
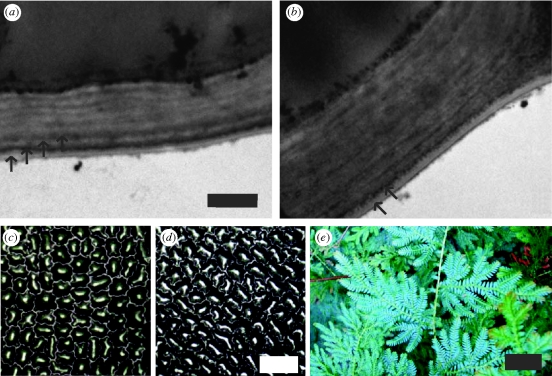
Figure 3 shows cross-sectional TEM images of the blue and green S. willdenowii leaves. The images show the outer cell wall and the cuticle from the upper epidermis of the leaves. In figure 3a four distinct layers are observed in the cuticle of the blue leaf, followed by a more disordered layer structure in the lower epidermis. These alternating light and dark bands correspond to electron-transparent and electron-opaque layers, respectively. This alternating layer structure is not visible in the cross section of the green leaf (figure 3b). Instead, only a single dark layer is discernible, followed by a more disordered lamellar structure. The cuticle of the green leaf of approximately 1.1 µm is much thicker as compared with the blue leaf of approximately 700 nm.
Figure 3.
TEM micrographs of the outer cell wall and the cuticle from the upper epidermis of (a) a juvenile blue leaf and (b) an older green leaf (scale bar, 500 nm). Arrows indicate the layers observed in the cuticle. Optical micrographs of surface morphology of plant cells for (c) a juvenile blue leaf and (d) a mature green leaf (scale bar, 50 µm). (e) Photograph of juvenile S. willdenowii leaves (scale bar, 30 mm).
The surface structures of the blue and green leaves in figure 3c,d are very similar; the epidermal cells are arranged in disordered rows.
3.1. Modelling
From the changing position of the peak wavelength seen at approximately 430 nm, it is reasonable to assume that the blue colour in the juvenile leaves comes from multi-layer interference arising from the four lamellae observed in the upper cuticle. Optical modelling of a four-layer structure was therefore used to account for the optical properties of the S. willdenowii leaves.
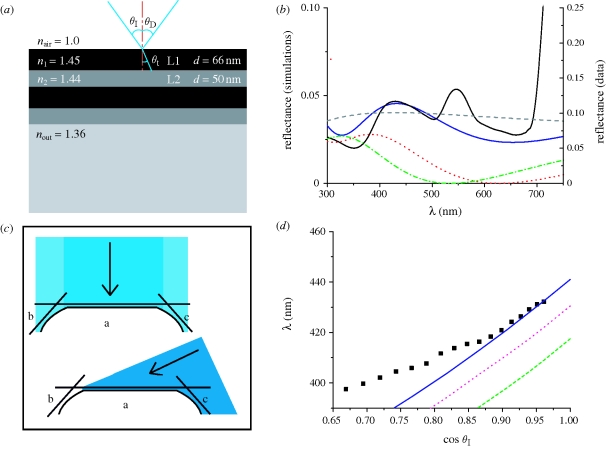
The thickness of each layer was measured from TEM cross sections (figure 3a). Layer one, the outermost layer, has a thickness of 66 ± 5 nm and layer two has a thickness of 50 ± 5 nm (as measured from cross sections of three different iridescent leaves). The refractive index of cellulose is known to vary with the degree of hydration (Woolley 1975), with a more hydrated cellulose having a lower refractive index. It is therefore probable that the outer layer L1 has a higher refractive index than the underlying layer L2, which is presumably more hydrated. The refractive index of the underlying cell is approximately 1.36 as calculated by Charney & Brackett (1961) for the refractive index of plant cell cytoplasm.
The model structure is shown schematically in figure 4a. The lamellae L1 and L2 are approximated as homogeneous, transparent dielectric films with refractive indices n1 and n2 and thicknesses d1 and d2. The reflectance of such a planar multi-layer structure can be calculated by propagating a plane wave through the stack, considering interfacial reflections and interference, using Fresnel's equations (Huxley 1968; Hecht 2002).
Figure 4.
(a) Multi-layer model showing the thickness and refractive indices of the layers. (b,d) Comparison of experimental data from the juvenile blue leaf and simulated reflectance from an optical multi-layer with d1 = 66 nm and d2 = 50 nm, assuming different combinations of n1 and n2. (b) The dashed line shows simulations for a simplified leaf structure with a single layer of n = 1.45, d = 100 nm on top of a homogeneous optical medium with n = 1.36. The dotted and dashed-dotted lines show simulated reflectance for the simplified leaf covered by an antireflective (AR) coating with n = 1.24 optimized for (dotted) 650 nm and (dashed-dotted) 550 nm, respectively (solid black line, experimental data; solid dark blue line, n1 = 1.45, n2 = 1.44, nout = 1.36; dashed grey line, simplified leaf structure; red dotted line, AR coating 650 nm, n = 1.24, d = 130 nm; green dashed-dotted line, AR coating 550 nm, n = 1.24, d = 110 nm). (c) Contributions from faces a, b and c for light with normal incidence and light with a higher incident angle. (d) Wavelength of the peak in the reflectance between 390 and 450 nm. The solid line shows the theoretical peak shift as calculated using (a). The dotted and dashed lines show the theoretical peak shift for other combinations of n1 and n2. Black points show experimental data from figure 1b. Each data point is an average of three measurements (filled black squares, experimental data; solid dark blue line, four layers n1 = 1.45, n2 = 1.44; purple dotted line, four layers n1 = 1.45, n2 = 1.43; green dashed line, four layers n1 = 1.45, n2 = 1.42).
The experimental spectra for θI = 16° and the corresponding model calculations are shown in figure 4b,d. While the model calculations allow us to confirm whether the peak in the experimental reflectance data corresponds to that of the model, the simple model does not predict the absolute intensity of the measured reflectance. While the blue colour clearly arises from multi-layer interference, the optical signal stemming from the overall structure of the leaf is complex. Its optical signature is a superposition of Bragg interference, with intensities arising from scattering off irregularities at the surface and in the multi-layer, backscattering of light from the cytoplasm and variations in the surface-normal of the multi-layer owing to the cell shape and leaf curvature (see also below). The measurements also do not take into account any polarization effects of the multi-layer; the reflected intensity varies as a function of polarization. All simulations shown here are for unpolarized light.
The model assumes that d1 = 66 nm, d2 = 50 nm, n1 = 1.45, n2 = 1.44 and nout = 1.36. Figure 4d shows the wavelength λmax of the multi-layer reflectance peak as a function of incidence angle for the juvenile blue leaves. The wavelength is averaged over spectra from three different leaves. While the data extracted from the specular reflection experiments (points) are well described by the model calculation (blue line) for small angles of incidence, the model and the data deviate for higher angles. The model assumes planar, horizontally flat homogeneous layers. However, the epidermal cells are dome-shaped (figure 3c). The cell morphology determines the local optical geometry. For normal incidence, the flat top part of the cell locally approximates the planar surface of the model in figure 4a. For larger incident angles more light impinges on the cell edges, which now play an increasing role. The local angle of light incidence at the illuminated cell edges is smaller than the global incidence angle. The reflected intensity detected at θD = −θI results from superposition of the light that is specularly reflected from the top part of the cell and light that scatters from the cell edges (figure 4c). The detected wavelength is consequently red-shifted compared with the planar multi-layer model (figure 4d), somewhat reducing the iridescent nature of the blue peak.
Other combinations of n1 and n2 were also simulated to assess whether different multi-layer sequences would result in the same reflectivity behaviour. Figure 4d shows that increasing the refractive index contrast between the two layers shifts the peak in the reflectance to lower wavelengths than those found experimentally.
The small enhancement of specular reflectance at around 450 nm for the green leaf seen at high angles (figure 1b) is consistent with a double layer interference with filter n1 = 1.45, d1 = 75 nm and n2 = 1.44, d2 = 25 nm. This corresponds to the two partial lamellae seen in figure 3b. For small θI, this maximum broadens and shifts to approximately 500 nm, almost disappearing into the peak at 550 nm that results from scattering pigments in the leaf.
4. Discussion
Our detailed reflectance measurements show that the blue colouration seen in juvenile S. willdenowii leaves is iridescent and stems from the four-layer interference filter in the cuticle on the upper surface of the leaves. The blue iridescence disappears as the leaves age. The TEM images and reflection data suggest that the loss in the blue colouration comes from a degradation or increasing disorder of the multi-layer structure, starting with the lower layers. For the older green leaves, only weak double layer interference is still present.
Modelling of the data suggests that the refractive index contrast between the two layers is of the order of 0.01. This difference is very small compared with other optical multi-layers found for example in beetles and butterflies. Here however, the layer structure consists of only one material and the refractive index variation arises from a difference in hydration. This strongly limits the achievable refractive index contrast. The comparison of model calculations with experimental spectra in figure 4b confirms that the observed blue iridescence can arise from such a weak refractive index contrast.
Figure 1e shows a blue reflection band stretching across a wide range of detection angles. The ideal planar Bragg multi-layer assumed in the model calculation would result, however, in a localized specular signal at θD = −θI (i.e. at sin θD ≈ −0.6) with little intensity at other angles. The wide blue intensity band of figure 1e stems from disorder in the surface-normal of the multi-layer. This disorder arises from cell shape and from the curvature of the leaves. As a result, parts of the leaf locally fulfil Bragg's condition for any observation angle, causing the angle-invariance of the observed colour. A similar optical response is seen for other natural multi-layer structures. For example, the blue iridescence of Morpho rhetenor has a similar angle-invariance arising from the curvature of the multi-layers on its wing-scales (Vukusic et al. 1999; Kinoshita & Yoshioka 2005). The simple model used to describe multi-layer interference therefore only approximates the optical response of the complex surface structures found in nature.
Modelling of the data shows that the multi-layer structure preferentially reflects light in the blue part of the spectrum, a photosynthetically important spectral region, which is deficient in the light present in the natural habitat of these shade-dwelling plants. In this low-light environment the light is enriched in the FR part of the spectrum. Lee et al. have suggested that the blue-reflecting multi-layer more effectively couples the long optical wavelength into the leaf, thereby enhancing photosynthesis. Our more detailed data allow the testing of this hypothesis.
A comparison of figure 1a,b shows that the reflectance in the 600–700 nm spectral range is strongly angle dependent. For the more important small angles of incidence, the average long wavelength reflectance of the blue and green leaves is comparable. This is best seen in figure 2 where the reflectance of the green leaf dips below that of the blue leaf for approximately 660 nm, the maximum for red-absorption of chlorophyll.
Owing to the complex optical signature of leaves, it is instructive to address the question of enhanced red-absorption in terms of model calculations. The dashed line in figure 4b is the prediction for a simplified leaf structure consisting only of an optically dense top 100 nm thick cuticle layer and an optically less dense lower phase. The predicted optical response of this simplified leaf structure in the 600–700 nm spectral range is very similar to the model four-layer structure of the blue leaf. While this comparison illustrates the unsuitability of the multi-layer structure to couple red light into the leaves, it is instructive to compare the measured spectra to optical coatings that are optimized for this effect.
Antireflective coatings consisting of a bilayer of low and high refractive index materials are well known. The optimization condition for such a bilayer is nout n12 = n22, with λ/4 thicknesses for each layer (Macleod 2001). Superimposing the simplified leaf (dashed line in figure 4b) with a 110–130 nm thick layer of refractive index n = 1.24 significantly reduces the reflected light not only for the optimized wavelengths of 550 (dashed-dotted line) and 650 nm (dotted line), respectively, but over most of the visible spectrum. The possibility of optical coatings consisting of porous or nanostructured cuticle becomes evident when considering the coatings found on the eyes of moths and butterflies (Stavenga et al. 2006).
Figure 2 shows that the juvenile blue leaves have a lower reflectance between 500 and 600 nm as compared with the mature green leaves and a similar reflectance between 600 and 700 nm. A similar behaviour is seen for Danaea nodosa, where the iridescence is seen in the juvenile leaves and disappears in the adult leaves owing to the loss of the multi-layer structures responsible for the iridescence (Graham et al. 1993). Graham et al. also studied the difference in the optical properties of 12 rainforest sun and 13 rainforest extreme shade plants (Lee & Graham 1986), including iridescent species. Their results show that the absorption spectra of sun and extreme shade plants are identical apart from a small increase for the shade plants at 550 nm. Our data and model calculations suggest that the role of blue iridescence in S. willdenowii is not to couple in more red light, refuting the hypothesis by Lee et al.
4.1. Possible adaptive advantages of iridescence in shade-living plants
A taxonomically diverse range of photosynthetic organisms from diatoms (Gordon et al. 2009) to algae (Abbott 1971; Gerwick & Lang 1977; Fournet et al. 1993) through to angiosperms (Graham et al. 1993) produce a vivid iridescence over the surface of their photosynthetic apparatus. An equally diverse range of methods are used to produce this iridescence. This strongly suggests that the production of iridescence has evolved multiple times and has some unknown adaptive advantage for life in deep shade.
Our results show that it is unlikely that a multi-layer structure acts to increase light capture in photosynthetically important wavelength ranges under low light conditions in this case, since no enhancement of absorption by the blue leaves is seen in the red part of the spectrum. While an interesting hypothesis, this idea is counterintuitive for a number of reasons. Firstly, it makes no sense that the plant would want to sacrifice the absorption of any incident blue light in favour of red light. Blue light is important for both photosynthesis and plant development.
Secondly, the multi-layer filter does not act as an effective antireflective coating. As shown in figure 4b, a simple strategy to implement a broad-band antireflective coating consists of the deposition of a low refractive index (n = 1.24) λ/4 layer on top of a generic leaf surface. Compared with a multi-layer arrangement, a porous or structured low refractive index surface layer has superior antireflective properties and is presumably less resource intensive for the plant. The evolutionary feasibility of this is well documented in insects (Stavenga et al. 2006). Stavenga et al. show that the papillae seen on the eyes of butterflies act to produce a graded refractive index surface layer, which acts as an antireflective surface.
Increased capture of photosynthetically important wavelengths is not the only adaptive advantage that leaf iridescence may offer. Others include a visual defence against herbivores, a photoprotective mechanism to protect shade-adapted plants against sun-flecks and other potentially damaging sudden high light levels and a polarization filter enhancing orientation of photosynthetic apparatus within the cell.
4.2. A visual defence against herbivores
Whitney et al. (2009a) have recently shown that insects can see iridescence independently of any other cue. Iridescence can act to increase detectability owing to the changing colour of the object when perceived from different angles, delivering more stimulus to the photoreceptors of the eye. However, this may come with a cost; the very changeability of iridescence that increases detectability could corrupt object identity, making it harder for insects to form a search image (Whitney et al. 2009b). In certain situations this potential corruption of identity could be a significant advantage. The constantly changing colours produced by iridescence would alternatively blend or contrast with any background, producing an effect similar to conventional disruptive colouration, like that of the zebra (Sherratt et al. 2005; Stevens & Merilaita 2009). The iridescence produced on the leaves of plants could act to confuse moving insects and herbivores by camouflaging the shape and edge of the leaf and making it difficult to form a target search image.
Alternatively, the iridescence may serve to deter herbivores by causing the leaves to look sufficiently different from all other food sources that iridescent leaves are not recognized as food, or avoided. Evidence for these hypotheses has been found in the iridescent alga Mazzaella flaccida, where non-iridescent thalli of this seaweed were seen to suffer more from herbivory than their iridescent counterparts (Gerwick & Lang 1977).
4.3. A photoprotective mechanism
All of the plants that produce blue leaf iridescence grow in extremely sheltered low-light environments and as such are thought to be strongly shade-adapted. Such plants would be extremely vulnerable to photodamage if even transiently exposed to sun-flecks (where small patches of light break through the canopy), owing to the increased sensitivity of their photosynthetic apparatus. The constructive interference produced by the blue leaf iridescence in the wavelengths of 400–485 nm may therefore function to protect against photoinhibition (the inhibition of photosynthesis owing to excessive irradiance) and damage via reduced light absorption at those wavelengths (Lee et al. 2008). Light attenuation mechanisms have been suggested for other shade dwelling plants, which have red leaves owing to the presence of anthocyanins. These pigments are seen to function in light attenuation by intercepting quanta which would otherwise be absorbed by the chlorophyll, protecting the leaves from photoinhibition when exposed to sun-flecks (Gould et al. 1995; Hughes et al. 2008).
5. Conclusions
In summary, we have shown that the blue colouration seen in the leaves of S. willdenowii is indeed iridescent. Modelling of the multi-layer structures found in the upper epidermis of the leaves, using literature values for the refractive indices of plant cell cytoplasm and cuticular wax, has shown that they are responsible for this iridescence. A comparison of blue and green leaves, from the same plant, indicates that the loss of the blue iridescence corresponds to a loss of the multi-layer structures. The results reported here do not support the idea that iridescence in plants acts to enhance light capture of photosynthetically important wavelengths. The reflectance of light in the range 600–700 nm is the same for both iridescent and non-iridescent leaves. However, owing to the occurrence of blue colouration in a wide variety of shade dwelling plants it is likely that this iridescence has some adaptive benefit.
Acknowledgement
We would like to thank Jeremy Skepper and Chrissie Prichard for their help with embedding of the plant leaves, Cambridge University Botanic Garden and the Royal Botanic Gardens, Kew for growing the plants and the German Academic Exchange Service and the EPSRC for funding.
References
- Abbott I. A. 1971. On the species of Iridaea (Rhodophyta) from the Pacific coast of North America. Syesis 4, 51–72. [Google Scholar]
- Bazzaz F. A., Pickett S. T. A. 1980. Physiological ecology of tropical succession—a comparative review. Annu. Rev. Ecol. Syst. 11, 287–310. ( 10.1146/annurev.es.11.110180.001443) [DOI] [Google Scholar]
- Blakenship R. E. 2002. Molecular mechanisms of photosynthesis. Oxford, UK: Blackwell Science Ltd. [Google Scholar]
- Charney E., Brackett F. S. 1961. Spectral dependence of scattering from a spherical alga and its implications for the state of organization of the light-accepting pigments. Arch. Biochem. Biophys. 92, 1–12. ( 10.1016/0003-9861(61)90210-7) [DOI] [PubMed] [Google Scholar]
- Chazdon R. L., Pearcy R. W., Lee D. W., Fletcher N. 1996. Photosynthetic responses of tropical forest plants to contrasting light environments. In Tropical forest plant ecophysiology (eds Mulkey S. S., Chazdon R. L., Smith A. P.). Boca Raton, FL: Chapman and Hall. [Google Scholar]
- Fournet I., Deslandes E., Floch J. Y. 1993. Iridescence—a useful criterion to sort gametophyes from sporophytes in the red alga Chondrus crispus. J. Appl. Phycol. 5, 535–537. ( 10.1007/BF02182512) [DOI] [Google Scholar]
- Fox D. L., Wells J. R. 1971. Scemocromic leaf surfaces of selaginella. Am. Fern J. 61, 137–144. ( 10.2307/1546644) [DOI] [Google Scholar]
- Gentner G. 1909. Über den blauglanz auf blättern und früchten. Biotropica 99, 337–354. [Google Scholar]
- Gerwick W. H., Lang N. J. 1977. Structural, chemical and ecological studies of iridescence in Iridaea (Rhodophyta). J. Phycol. 13, 121–127. ( 10.1111/j.0022-3646.1977.00121.x) [DOI] [Google Scholar]
- Gordon R., Losic D., Tiffany M. A., Nagy S. S. 2009. The glass menagerie: diatoms for novel applications in nanotechnology. Trends Bioechnol. 27, 116–127. ( 10.1016/j.tibtech.2008.11.003) [DOI] [PubMed] [Google Scholar]
- Gould K. S., Lee D. W. 1996. Physical and ultrastructural basis of blue leaf iridescence in four Malaysian understory plants. Am. J. Bot. 83, 45–50. ( 10.2307/2445952) [DOI] [Google Scholar]
- Gould K. S., Kuhn D. N., Lee D. W., Oberbauer S. F. 1995. Why leaves are sometimes red. Nature 378, 241–242. ( 10.1038/378241b0) [DOI] [Google Scholar]
- Graham R. M., Lee D. W., Norstog K. 1993. Physical and ultrastructural basis of blue leaf iridescence in two neotropical ferns. Am. J. Bot. 80, 198–203. ( 10.2307/2445040) [DOI] [Google Scholar]
- Hecht E. 2002. Optics. San Francisco, CA: Addison-Wesley. [Google Scholar]
- Hipkins M. F., Baker N. R. (eds) 1986. Photosynthesis energy transduction: a practical approach. Oxford, UK: IRL Press. [Google Scholar]
- Hughes N. M., Vogelmann T. C., Smith W. K. 2008. Optical effects of abaxial anthocyanin on absorption of red wavelengths by understorey species: revisiting the back-scatter hypothesis. J. Exp. Bot. 59, 3435–3442. ( 10.1093/jxb/ern193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. 1968. A theoretical treatment of the reflexion of light by multilayer structures. J. Exp. Biol. 48, 227–245. [Google Scholar]
- Kinoshita S., Yoshioka S. 2005. Structural colours in nature: the role of regularity and irregularity in the structure. ChemPhysChem 6, 1442–1459. ( 10.1002/cphc.200500007) [DOI] [PubMed] [Google Scholar]
- Lee D. W. 1986. On the economy of plant form and function, ch. 4. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lee D. W. 1997. Iridescent blue plants. Am. Sci. 85, 56–63. [Google Scholar]
- Lee D. W., Graham R. 1986. Leaf optical properties of rainforest sun and extreme shade plants. Am. J. Bot. 73, 1100–1108. ( 10.2307/2443790) [DOI] [Google Scholar]
- Lee D. W., Hébant C. 1984. Ultrastructural basis and developmental control of blue iridescence in Selaginella leaves. Am. J. Bot. 71, 216–219. ( 10.2307/2443748) [DOI] [Google Scholar]
- Lee D. W., Lowry J. B. 1975. Physical basis and ecological significance of iridescence in blue plants. Nature 254, 50–51. ( 10.1038/254050a0) [DOI] [Google Scholar]
- Lee D. W., Lowry J. B., Stone B. C. 1979. Abaxial anthocyanin layers in leaves of tropical rain-forest plants—enhancer of light capture in deep shade. Biotropica 11, 70–77. ( 10.2307/2388175) [DOI] [Google Scholar]
- Lee D. W., Kelley J., Richards J. H. 2008. Blue leaf iridescence as a by-product of photoprotection in tropical rainforest understory plants. Vancouver, Canada: Botanical Society of America. [Google Scholar]
- Macleod H. A. 2001. Thin-film optical filters, 3rd edn. London, UK: IOP Publishing. [Google Scholar]
- Richards P. W. 1932. The ecology of bryology. In Manual of bryology. The Hague, The Netherlands: Martinus Nijhoff. [Google Scholar]
- Richards P. W. 1996. The tropical rainforest, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Sherratt T. N., Rashed A., Beatty C. D. 2005. Hiding in plain sight. Trends Ecol. Evol. 20, 414–416. ( 10.1016/j.tree.2005.05.010) [DOI] [PubMed] [Google Scholar]
- Stahl E. 1896. Uber bunte Laublätter. Ann. Jard. Bot. Buitenez. 13, 137–216. [Google Scholar]
- Stavenga D. G., Foletti S., Palasantzas G., Arikawa K. 2006. Light on the moth-eye corneal nipple array of butterflies. Proc. R. Soc. B 273, 661–667. ( 10.1098/rspb.2005.3369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. 2009. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B 364, 481–488. ( 10.1098/rstb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M. 1998. Forest light and its influence on habitat selection. Plant Ecol. 153, 251–261. ( 10.1023/A:1017592631542) [DOI] [Google Scholar]
- Vogelmann T. C. 1993. Plant tissue optics. Annu. Rev. Plant Phys. 44, 231–251. ( 10.1146/annurev.pp.44.060193.001311) [DOI] [Google Scholar]
- Vukusic P., Sambles J. R., Lawrence C. R., Wootton R. J. 1999. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. Lond. B 266, 1403–1411. ( 10.1098/rspb.1999.0794) [DOI] [Google Scholar]
- Whitney H. M., Kolle M., Andrew P., Chittka L., Steiner U., Glover B. J. 2009a. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323, 130–133. ( 10.1123/science.1166256) [DOI] [PubMed] [Google Scholar]
- Whitney H. M., Kolle M., Andrew P., Chittka L., Steiner U., Glover B. J. 2009b. Response to comment on: ‘Floral irridescence, produced by diffractive optics, acts as a cue for animal pollinators’. Science 325, 1072 ( 10.1126/science.1173503) [DOI] [PubMed] [Google Scholar]
- Woolley J. T. 1975. Refractive index of soybean leaf cell-walls. Plant Physiol. 11, 172–174. ( 10.1104/pp.55.2.172) [DOI] [PMC free article] [PubMed] [Google Scholar]