Abstract
Atrial fibrillation (AF), a chaotic and irregular contraction of the atria, remains the most common cardiac arrhythmia affecting up to 1.5 per cent of the world population. It has significant economic and personal implications primarily owing to the associated fivefold increase in risk of thromboembolic stroke. The mainstay of risk reduction therapy remains warfarin, use of which can be limited owing to a multitude of issues ranging from drug and food interactions to under-treatment reflected in sub-therapeutic blood levels despite adequate compliance. Pursuit of novel drug alternatives have led to the licence of a new contender (Dabigatran) with a more attractive pharmacotherapeutic profile, some 50 years after warfarin was introduced for human use. A recent non-pharmacological alternative is the Watchman device which has received licence for use. Tested in the PROTECT-AF study, the Watchman device was found to be non-inferior to warfarin in the occurrence of stroke, cardiovascular or unexplained death, or systemic emboli for up to 3 years with less intracranial haemorrhages. The events in the watchman group occurred early and were related to the procedure. These peri-procedural complications are likely to diminish with improved operator experience and ongoing development of the technology. For now, patients with AF who would benefit tremendously from but cannot be treated with warfarin owing to contraindication to, or intolerance of, anticoagulation are considered for device implantation. Despite promising new pharmacotherapeutic advances in the prevention of strokes related to AF, it has taken 50 years for alternative non-pharmacological approaches to become available for clinical use.
Keywords: stroke, atrial fibrillation, Watchman
1. Introduction
The prevalence of non-valvular atrial fibrillation (AF) increases with age affecting 0.5 per cent in those aged 50–59 to almost 9 per cent in those aged 80–89 (National Institute for Health and Clinical Excellence 2006; Scottish Intercollegiate Guidelines Network 2007). The Framingham study, later supported by the Rotterdam study, reported a one in four lifetime risk of developing AF for those over the age of 40 years in both sexes (Lloyd-Jones et al. 2004; Heerimga et al. 2006).
Due to more aggressive efforts in primary and secondary prevention of events related primarily to atherosclerotic coronary disease and improved socioeconomic prosperity, the proportion of patients above the age of 70 is increasing rapidly. This is associated with a progressive increase in the prevalence of AF (Lip et al. 2007). Currently, the consequences and treatment of AF account for nearly 1 per cent of the total National Health Service expenditure in the UK and, in light of the increasing prevalence of AF, this figure is expected to increase significantly.
Several studies have shown the negative impact of AF on quality of life; however, the most catastrophic consequence remains stroke, accounting for approximately 15 per cent of all thromboembolic strokes.
According to the American Heart Association, strokes are the leading cause of disability and account for 1 in every 17 deaths in the US, ranking among the top three in all causes of death. Therefore, given its associated stroke risk, AF has a clear and significant impact on quality of life and mortality.
Cardioembolic strokes are more frequently associated with severe and persistent disabilities compared with ischaemic events from vascular disease (Wolf et al. 1991; Falk 2001; National Institute for Health and Clinical Excellence 2006). With an associated mortality of 37 per cent in 12 months and a recurrence of 17 per cent during this period, the consequences to the individual and socioeconomic implications of this complication cannot be overstated (Heuschmann et al. 1998).
2. Reducing stroke risk
In more than 90 per cent of patients with AF, stroke is owing to thrombotic embolization from the left atrial appendage (LAA) (Aberg 1969; Tsai et al. 1990; Manning et al. 1995; Blackshear & Odel 1996). An increase in atrial pressure, atrial stretch and dilatation along with abnormalities of haemostasis, endothelial dysfunction, platelet activation factors and elevated levels of coagulation parameters all aid thrombus formation within this blind-ended distensible pouch (Al-Saady et al. 1999; Muller et al. 2002). This likelihood is higher in patients with increasing age, rheumatic heart disease, poor left ventricular function, diabetes, hypertension and a previous history of thromboembolism.
Several stroke risk stratification models for AF exist but the most commonly used is the CHADS2 index (table 1; Gage et al. 2001). Individual factors are scored with one point (congestive cardiac failure, ejection fraction less than 35%; hypertension; age more than 75 years; diabetes mellitus) or 2 points (previous thromboembolism, e.g. stroke).
Table 1.
CHADS2 system which relies on scoring individual risk factors to assess overall thromboembolic risk.
| congestive cardiac failure | +1 |
| hypertension | +1 |
| age 75 or greater than 75 yrs old | +1 |
| diabetes mellitus | +1 |
| previous history of arterial thromboembolism, e.g. stroke | +2 |
Those with a score of more than or equal to 1 are recommended treatment with anticoagulation therapy (table 2). The stroke prevention in AF studies documented clear benefits of continuous anticoagulation therapy in reducing the risk of stroke by 70 per cent and is supported by published guidelines. Those with a low risk (CHADS2 score of 1 or less) should be on aspirin.
Table 2.
CHADS2 scores to guide thromboembolic prevention treatment.
| adjusted annual stroke rate | stroke risk level | warfarin recommended | |
|---|---|---|---|
| 0 | 1.9 | low | no |
| 1 | 2.8 | moderate | yes |
| 2 | 4.0 | moderate | yes |
| 3 | 5.9 | high | yes |
| 4 | 8.5 | high | yes |
| 5 | 12.5 | high | yes |
| 6 | 18.2 | high | yes |
3. Preventive pharmacologic therapy
Standard treatment of patients with AF involves use of antithrombotic medication to manage the increased risk of thromboembolism. Warfarin is the ‘cornerstone’ of pharmacotherapy for AF in patients with moderate to high thromboembolic risk, but it has limitations owing to drug and food interactions, frequent monitoring and dose adjustment, difficulty in maintaining the therapeutic range and risks of bleeding complications (Lip et al. 2007). Although the risk of intracranial haemorrhage in patients with controlled anticoagulation is small, several meta-analyses have shown a major bleeding risk varying from 40 to 80 per cent higher than compared with aspirin or no treatment (Hylek et al. 2003). Aside from this risk there remain significant relative contraindications to warfarin use such as the elderly with a high risk of falling. Other issues to consider are annoyances of frequent phlebotomy and its associated impact on the healthcare system. Due to these inconveniences warfarin is under-prescribed to those eligible for treatment with some population-based studies reporting only 50 per cent in receipt of therapy (Go et al. 1999; Waldo et al. 2005; Birman-Deych et al. 2006; Hylek et al. 2006; Niewlaat et al. 2006). Others are exposed to a significant risk of stroke owing to persistent sub-therapeutic anticoagulant levels despite appropriate compliance with therapy. A study reviewing the prevalence and quality of warfarin use in patients with AF found INR levels were within therapeutic range for 62 per cent of days during a 1 year follow-up period and were found to be below or above the therapeutic range for 25 and 13 per cent of days, respectively (McCormick et al. 2001).
Due to these limitations several alternative anticoagulation agents with novel mechanisms of action have been developed and investigated. Of these, the oral direct thrombin inhibitor, Dabigatran, has proved the most promising with the RE-LY study showing non-inferiority to warfarin for stroke prevention in AF and a clear reduction in major haemorrhage. Currently available in the European Union for venous thromboembolic, Dabigatran proved to be a powerful contender to warfarin therapy at both doses tested in the study (110 and 150 mg twice daily), although the higher dose was noted to have a slight but significantly higher risk of myocardial infarction. With a rapid onset of action, few drug–drug interactions and no monitoring requirements. Dabigatran is a sensible alternative for patients who are intolerant to warfarin for varying reasons (Connolly et al. 2009).
4. Preventive device therapy
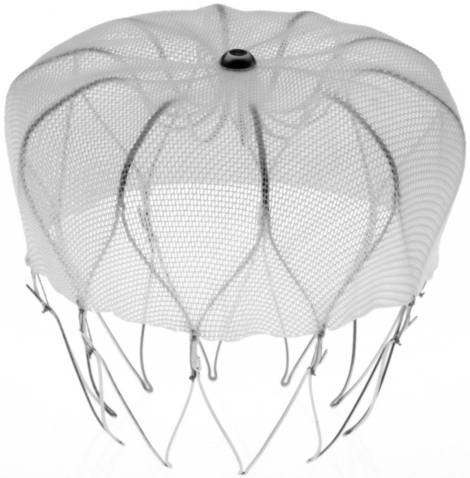
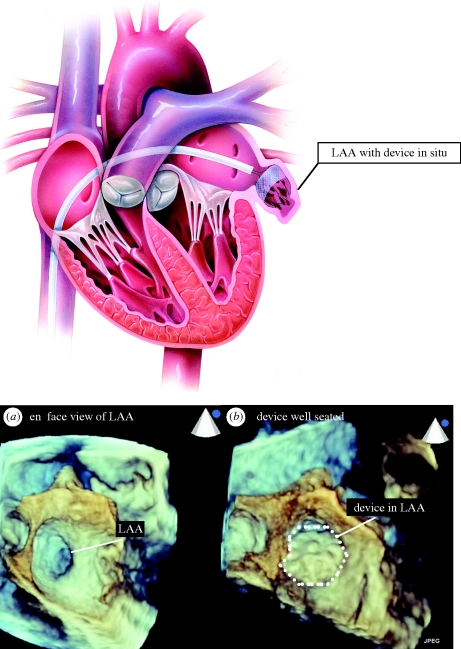
Mechanical prevention of cardioembolic stroke by non-surgical closure of the LAA has become a reality with the advent of left atrial appendage closure devices (Sievert et al. 2002). The non-randomized PLAATO (percutaneous left atrial appendage transcatheter Occlusion) study paved the way for PROTECT-AF (Ostermayer et al. 2005; Holmes et al. 2009). This recent randomized trial confirmed non-inferiority of the Watchman (Atritech) LAA closure device to warfarin. This is a self-expanding nitinol frame structure with fixation barbs on the LAA side and a permeable polyester fabric covering the atrial side which is delivered percutaneously via a preloaded catheter (figure 1). The Watchman device is designed to be permanently implanted at, or slightly distal to, the opening of the LAA to trap potential emboli before they exit the LAA. It is implanted during an interventional procedure, performed either with conscious sedation or general anaesthesia, with fluoroscopic and transoesophageal echocardiography (TOE) guidance (figure 2). Patients require a 24–48 hour hospital stay. It is available in diameters ranging from 21 to 33 mm to accommodate the variability in LAA dimensions.
Figure 1.
Watchman (Atritech) LAA closure device. A self-expanding nitinol frame structure with fixation barbs and a permeable polyester fabric.
Figure 2.
(Acknowledgement—TOE images taken by Beth Unsworth, Imperial College, using Philips iE33 echocardiography system with live three-dimensional transoesophageal imaging) (a) short axis (‘en face’) view of the ostia (immediate opening) of the left atrial appendage. (b) Watchman device in position following deployment in the LAA.
The multicentre PROTECT-AF study compared the Watchman device with warfarin in over 700 patients with a CHADS2 risk score of 1 or more (Holmes et al. 2009). Patients remained on warfarin for the first six weeks to allow endothelialization of the device into the LAA. Following a satisfactory six week TOE patients were swapped to aspirin and clopidogrel for 4.5 months before remaining just on aspirin alone. Following confirmation of LAA closure on TOE, 86 and 92 per cent of patients stopped warfarin therapy at 45 days and 12 months, respectively. The primary efficacy of the device (occurrence of all types of stroke, cardiovascular or unexplained death, or systemic emboli up to 3 years) was non-inferior to warfarin with fewer haemorrhagic strokes in the device group versus the controls. The primary safety endpoint (combined major bleeding, serious pericardial effusion and device embolization) was significantly greater in the device group than in the control group and was observed peri-procedurally.
Current guidelines recommend the Watchman for patients on anticoagulation which requires continuation for a temporary period post-procedure; however, these recommendations may change following the outcome of the ASAP study (LAA closure using the Watchman device in patients with contraindications to warfarin; Reddy et al. 2010).
5. Conclusion
The economic and personal cost of a stroke is high and new therapies to reduce this burden must be explored further. Although undesirable, procedural complications are inevitable with any interventional therapy. In comparison with the Watchman device with an early safety hazard during implantation, the safety hazard of warfarin occurs late and persists throughout the duration of lifelong therapy. With broadening experience these immediate complications are likely to fall and although long-term safety data are required for the Watchman the impact of this therapeutic intervention could potentially change the future of thromboembolic risk management in AF patients. Once the device endothelializes into the LAA the originating source of thrombi is completely obliterated and therefore the impact not only on stroke prevention but on patient quality of life and long-term financial cost to the healthcare system are far reaching.
Although the current indication for the Watchman device is as an alternative for patients who are unable to take long-term warfarin therapy, some patients and clinicians would be willing to accept an immediate procedural risk to avoid long-term concerns about stroke or warfarin therapy. Our own experience as the first implanting center in the UK has already revealed a significant demand from patients who merely find lifelong drug therapy unacceptable.
Footnotes
One contribution to a Theme Supplement ‘Translation and commercialization of regenerative medicines’.
References
- Aberg H. 1969. Atrial Fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med. Scand. 185, 373–379. ( 10.1111/j.0954-6820.1969.tb07351.x) [DOI] [PubMed] [Google Scholar]
- Al-Saady N. M., Obel O. A., Camm A. J. 1999. Left atrial appendage: structure, function and role in thromboembolism. Heart 82, 547–554. ( 10.1136/hrt.82.5.547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birman-Deych E., Radford M., Nilasena D. S., Gage B. F. 2006. Use and effectiveness of warfarin in medicare beneficiaries with atrial fibrillation. Stroke 37, 1070–1074. ( 10.1161/01.STR.0000208294.46968.a4) [DOI] [PubMed] [Google Scholar]
- Blackshear J. L., Odel J. A. 1996. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61, 755–759. ( 10.1016/0003-4975(95)00887-X) [DOI] [PubMed] [Google Scholar]
- Connolly S., Ezekowitz M., Yusuf S., Eikelboom J. & RE-LY steering committee and investigators 2009. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151. [DOI] [PubMed] [Google Scholar]
- Falk R. H. 2001. Atrial fibrillation. N. Engl. J. Med. 344, 1067–1078. ( 10.1056/NEJM200104053441407) [DOI] [PubMed] [Google Scholar]
- Gage B. F., Waterman A. D., Shannon W., Boechler M., Rich M. W., Radford M. J. 2001. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J. Am. Med. Assoc. 285, 2864–2870. ( 10.1001/jama.285.22.2864) [DOI] [PubMed] [Google Scholar]
- Go A. S., Hylek E. M., Borowsky L. H., Phillips K. A., Selby J. V., Singer D. E. 1999. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann. Intern. Med. 131, 927–934. [DOI] [PubMed] [Google Scholar]
- Heerimga J., van der Kuip D. A., Hofman A., Kors J. A., van Herpen G., Stricker B. H. Ch., Stijnen T., Lip G. Y. H., Witteman J. C. M. 2006. Prevalence, incidence and lifetime risk of developing atrial fibrillation: the Rotterdam Study. Eur. Heart J. 27, 949–953. ( 10.1093/eurheartj/ehi825) [DOI] [PubMed] [Google Scholar]
- Heuschmann P. U., Kolominsky-Rabas P. L., Kremer R., Neundörfer B. 1998. Pflege- und Versorgungsbedarf nach schlaganfall am beispiel des populationsbasierten Erlanger schlaganfall projektes. Akt Neurologie 25(Suppl. 3), 168. [Google Scholar]
- Holmes D. R., Reddy V. Y., Turi Z. G., Doshi S. K., Sievert H., Buchbinder M., Mullin C. M., Sick P., Protect AF Investigators 2009. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374, 534–542. ( 10.1016/S0140-6736(09)61343-X) [DOI] [PubMed] [Google Scholar]
- Hylek E. M., Go A. S., Chang Y., Jensvold N. G., Henault L. E., Selby J. V., Singer D. E. 2003. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N. Engl. J. Med. 349, 1019–1026. ( 10.1056/NEJMoa022913) [DOI] [PubMed] [Google Scholar]
- Hylek E. M., D'Antonio J., Evans-Molina C., Shea C., Henault L. E., Regan S. 2006. Translating the results of randomized trials into clinical practice. The challenge of warfarin candidacy among hospitalised elderly patients with atrial fibrillation. Stroke 37, 1075–1080. ( 10.1161/01.STR.0000209239.71702.ce) [DOI] [PubMed] [Google Scholar]
- Lip G., Kakar P., Watson T. 2007. Atrial fibrillation—the growing epidemic. Heart 93, 542–543. ( 10.1136/hrt.2006.110791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D. M., et al. 2004. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation 110, 1042–1046. ( 10.1161/01.CIR.0000140263.20897.42) [DOI] [PubMed] [Google Scholar]
- Manning W. J., Weintraub R. M., Waksmonski C. A., Haering J. M., Rooney P. S., Maslow A. D., Johnson R. D., Douglas P. S. 1995. Accuracy of transoesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 123, 817–822. [DOI] [PubMed] [Google Scholar]
- McCormick D., Gurwitz J. H., Goldberg R. J., Becker R., Tate J. P., Elwell A., Radford M. J. 2001. Prevalence and quality of warfarin use for patients with atrial fibrillation in the long-term care setting. Arch. Intern. Med. 16, 2458–2463. [DOI] [PubMed] [Google Scholar]
- Muller I., Massberg S., Zierhut W., Binzc C., Schusterd A., Rüdiger-von Hochc S., Braunb S., Gawaza M. 2002. Effects of aspirin and clopidogrel versus oral anticoagulation on platelet function and on coagulation in patients with nonvalvular atrial fibrillation (CLAFIB). Pathophysiol. Haemost. Thromb. 32, 16–24. ( 10.1159/000057284) [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. 2006. The management of atrial fibrillation. Clinical guideline CG036. London, UK: NICE. [Google Scholar]
- Niewlaat R., et al. 2006. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur. Heart J. 27, 3018–3026. ( 10.1093/eurheartj/ehl015) [DOI] [PubMed] [Google Scholar]
- Ostermayer S. H., et al. 2005. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J. Am. Coll. Cardiol. 46, 9–14. ( 10.1016/j.jacc.2005.03.042) [DOI] [PubMed] [Google Scholar]
- Reddy V. Y., Neuzil P., Sick P., Sievert H. 2010. LAA closure using the Watchman device in patients with contraindications to warfarin: preliminary results from the ‘ASA Plavix Registry’ (ASAP). Poster p05-57 Presented at the Heart Rhythm Society 31st Annual Scientific Sessions, Denver, Colorado, May, 2010.
- Scottish Intercollegiate Guidelines Network. 2007. Cardiac arrhythmias in coronary heart disease. Clinical guideline 94. Edinburgh, UK: SIGN. [Google Scholar]
- Sievert H., et al. 2002. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 105, 1887–1889. ( 10.1161/01.CIR.0000015698.54752.6D) [DOI] [PubMed] [Google Scholar]
- Tsai L. M., Chen J. H., Lin L. J., Yang Y. J. 1990. Role of transoesophageal echocardiography in detecting left atrial thrombus and spontaneous echo contrast in patients with mitral valve disease or non-rheumatic atrial fibrillation. J. Formos. Med. Assoc. 89, 270–274. [PubMed] [Google Scholar]
- Waldo A. L., Becker R. C., Tapson V. F., Colgan K. J., NABOR Steering Committee 2005. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J. Am. Coll. Cardiol. 46, 1729–1736. ( 10.1016/j.jacc.2005.06.077) [DOI] [PubMed] [Google Scholar]
- Wolf P. A., Abbott R. D., Kannel W. B. 1991. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 982–988. [DOI] [PubMed] [Google Scholar]