Abstract
The goal of tissue engineering is the creation of a living device that can restore, maintain or improve tissue function. Behind this goal is a new idea that has emerged from twentieth century medicine, science and engineering. It is preceded by centuries of human repair and replacement with non-living materials adapted to restore function and cosmetic appearance to patients whose tissues have been destroyed by disease, trauma or congenital abnormality. The nineteenth century advanced replacement and repair strategies based on moving living structures from a site of normal tissue into a site of defects created by the same processes. Donor skin into burn wounds, tendon transfers, intestinal replacements into the urinary tract, toes to replace fingers are all examples. The most radical application is that of vital organ transplantation in which a vital part such as heart, lung or liver is removed from one donor, preserved for transfer and implanted into a patient dying of end-stage organ failure. Tissue engineering and regenerative medicine have advanced a general strategy combining the cellular elements of living tissue with sophisticated biomaterials to produce living structures of sufficient size and function to improve patients' lives. Multiple strategies have evolved and the application of nanotechnology can only improve the field. In our era, by necessity, any medical advance must be successfully commercialized to allow widespread application to help the greatest number of patients. It follows that business models and regulatory agencies must adapt and change to enable these new technologies to emerge. This brief review will discuss the science of nanotechnology and how it has been applied to this evolving field. We will then briefly summarize the history of commercialization of tissue engineering and suggest that nanotechnology may be of use in breeching the barriers to commercialization although its primary mission is to improve the technology by solving some remaining and vexing problems in its science and engineering aspects.
Keywords: tissue engineering, extracellular matrix, nanotechnology
1. Introduction
The goal of tissue engineering is the creation of a living device that can restore, maintain or improve tissue function (Langer & Vacanti 1993). Behind this goal is a new idea that has emerged from twentieth century medicine, science and engineering. It is preceded by centuries of human repair and replacement with non-living materials adapted to restore function and cosmetic appearance to patients whose tissues have been destroyed by disease, trauma or congenital abnormality. The nineteenth century advanced replacement and repair strategies based on moving living structures from a site of normal tissue into a site of defects created by the same processes. Donor skin into burn wounds, tendon transfers, intestinal replacements into the urinary tract, toes to replace fingers are all examples. The most radical application is that of vital organ transplantation in which a vital part such as heart, lung or liver is removed from one donor, preserved for transfer and implanted into a patient dying of end-stage organ failure. Tissue engineering and regenerative medicine have advanced a general strategy combining the cellular elements of living tissue with sophisticated biomaterials to produce living structures of sufficient size and function to improve patients' lives. Multiple strategies have evolved and the application of nanotechnology can only improve the field. In our era, by necessity, any medical advance must be successfully commercialized to allow widespread application to help the greatest number of patients. It follows that business models and regulatory agencies must adapt and change to enable these new technologies to emerge. This brief review will discuss the science of nanotechnology and how it has been applied to this evolving field. We will then briefly summarize the history of commercialization of tissue engineering and suggest that nanotechnology may be of use in breeching the barriers to commercialization although its primary mission is to improve the technology by solving some remaining and vexing problems in its science and engineering aspects.
2. Nanotechnology and extracellular matrix
Although for decades we have been able to grow cells as simple monolayers, the necessary cell patterning and complex multi-dimensional structures required for engineered tissues to function in vivo have only recently gained the foreground. An ever-increasing understanding of the interactions between cells and between cells and their environments has facilitated this transition. Tissue engineering and regenerative medicine have recently focused on the role of the extracellular matrix (ECM) in cell patterning, migration, proliferation and differentiation. The desire to engineer an ECM substitute capable of influencing cellular behaviour is the catalyst for interdisciplinary cooperation between cell biologists, biochemists, and physical and chemical engineers. Creation of ECM-mimicking scaffolds that facilitate the generation of high levels of cellular complexity is the purview of nanotechnology.
The ECM functions in vivo as the scaffold upon which cells proliferate and differentiate. The matrix is not, however, solely a static scaffolding. Signalling to cells from the ECM occurs by direct cell receptor–ECM ligand interactions, the sequestration of growth factors (GFs) by the ECM, spatial cues and mechanical force transduction (Prestwich 2007). Challenges to engineering such complex and multi-functional supports have been met with nanofabricating techniques that create porous, nanometre-sized fibre scaffolds with surface qualities that influence cell fate determination, allow regulation of specific protein-expression patterns and encourage cell-specific scaffold remodelling (Heydarkhan-Hagvall et al. 2007). These techniques can regulate surface topography down to the nanometre range and include nanoscale surface pattern fabrication, electrospinning and self-assembly fabrication (Ayres et al. 2010). Incorporating biological signals in the form of GFs, mechanical stresses, cell surface receptors and spatial cues can also influence cellular proliferation, differentiation, migration and three-dimensional organization. Such refinements enhance the complexity and biological mimicry necessary to develop biologically viable constructs and have been integrated into scaffold engineering and the applications of gene-activated matrices.
Nanofabrication techniques have been used to understand cell behaviour in response to spatial environment and applied to engineering multiple tissue types. This review will highlight nanotechnologies that have encouraged the transition from culturing simple cell monolayers to engineering highly organized cultures of differentiated cells within specialized structures using specific examples of recent accomplishments. Further, we will emphasize techniques that have addressed two long-standing challenges in tissue engineering: designing a matrix that can be remodelled by resident cells and mass transfer—nutrient and oxygen delivery and waste removal. Contributions of these techniques to complex organ generation will also be illustrated.
3. Nanoscale surface patterning
Cells respond to spatial cues from the extracellular environment with alterations in shape, adhesion, cytoskeletal arrangement and activation of intracellular signalling pathways. These types of cellular responses are investigated and manipulated using nanoscale surface-patterning techniques. Surface-patterning techniques encompass a wide range of fabrication methods that include electron-beam lithography, nanoimprint lithography, photolithography including micro electrical mechanical systems (MEMS), nanocontact printing, micromachining and three-dimensional printing. Lithography, when referenced in the microfabrication context, refers to the creation of a template pattern in a substrate using an engraving mechanism. The template pattern is used to recreate the original pattern in the manner of a stamp. Engraving mechanisms include ultraviolet or visible spectrum light, electron or ion beams and chemical treatments. Biological molecules can be ‘stamped’ onto various surfaces using the templates created by lithography. Alternatively, the biological substrate or tissue itself can be patterned using similar techniques (Lee et al. 2004; Truskett & Watts 2006; Torres et al. 2008). In some instances, the fabricated pattern is formed in the negative so that when used as a mould, multiple identical patterned surfaces can be cast from the template.
Surface patterning is enhancing our understanding of cell responses on the most basic levels. McBeath and colleagues established that cell shape and cytoskeletal arrangement influenced cell fate determination. Human mesenchymal stem cells grown on microcontact-printed fibronectin islands differentiated into adipocytes or osteoblasts depending on the size of the fibronectin island and the amount of cell spreading. They went on to show that the differentiation pattern was dependent upon activation of the RhoA signalling pathway that was modulated by the cell shape (McBeath et al. 2004). Gray and co-workers used micropatterned wells made from a pre-fabricated mould to investigate the effects of cell–cell contact on proliferation. Fibronectin-coated wells were patterned so that cells had contact with one to three other cells. The cells were precisely positioned within the wells using 3 µm electrodes that trapped cells in the well by dielectrophoresis. The cells contacting only one neighbour proliferated while cells with two or more neighbours were inhibited from proliferating. RhoA signalling was also found to play an important role in this contact-dependent proliferation (Gray et al. 2008). A growing understanding of the influence of mechanical forces on cell fate has been facilitated by patterning technology. Fu and colleagues fabricated polydimethylsiloxane (PDMS) microposts from silicone moulds to evaluate the role of substrate rigidity on cell fate. The micropost rigidity varied by post lengths. They showed that cell fate determinations were strongly influenced by increased substrate rigidity leading to enhanced cell spreading with actin fibre and focal adhesion formation, where decreased rigidity leads to rounded cells with microvillus formation (Fu et al. 2010).
More complex surface patterns have been used to spatially restrict cells within co-cultures or to present cells with differentially patterned molecules. The resulting patterns are useful in investigating cell–cell and cell–ECM ligand interactions. Takahashi and co-workers cultured hepatocytes on hyaluronic acid patterned by force lithography. Fibroblasts were then seeded onto collagen-coated free areas surrounding the hepatocyte islands. The resulting co-cultures produced albumin and induced hepatocyte cell–cell junction formation, neither of which occurred in monolayer cultures of hepatocytes (figure 1; Takahashi et al. 2009). Several investigators have explored the effect of adhesion ligand patterns on cell behaviours. By varying the density and spatial distribution of the integrin ligand YGRGD on the nanometre scale, Maheshwari and colleagues showed that cell migration and migration speed are dependent upon the presentation of clustered YGRGD integrin ligands. Non-clustered presentation patterns promoted cell adhesion but not migration (Maheshwari et al. 2000). Cavalcanti-Adam et al. used gold dots functionalized with arginine–glycine–aspartate (RGD) peptides deposited onto glass surfaces by diblock copolymer nanolithography with inter-dot distances between 28 and 110 nm to show that cell spreading and focal adhesion formation are strongly influenced by RGD ligand density (Cavalcanti-Adam et al. 2007). The density of RGD ligand also affects focal adhesion strength with inter-ligand distances greater than 90 nm inhibiting focal adhesion formation, while distances of 50 and 28 nm promote increasing adhesion strength with decreasing ligand spacing (Selhuber-Unkel et al. 2010). Nanoscale surface patterning has elucidated that many other cell behaviours are influenced by the surface patterns to which they are exposed. Orientation, neurite axonal extension, bone deposition and gene-expression patterns are all influenced by the specific surface patterns upon which cells are cultured (Johansson et al. 2006; Heydarkhan-Hagvall et al. 2007; Dalby et al. 2008; Kantawong et al. 2009; Lamers et al. 2010).
Figure 1.
Hyaluronic acid (HA) micropatterns and co-cultures. (a–c) Well-defined HA patterns were formed, as observed with a phase-contrast microscope. (d) Hepatocytes selectively adhered to the fibronectin-coated regions on the HA-patterned surface during the 6 h incubation period. (e) The HA surface, including the hepatocytes, was treated with collagen and seeded with fibroblasts. (f) The co-culture was visualized by fluorescence staining with anti-albumin antibody (black, hepatocytes) and DAPI (grey, fibroblasts) on day 3 of culture. (g,h) Fibroblasts and (i) PA6 cells selectively adhered to form different HA patterns. Hepatocytes observed in islands with diameters of (j) 100 µm, (k) 200 µm and (l) 400 µm and the surrounding fibroblasts after pre-labelling with fluorescent markers on day 3 of culture. Scale bars, (a–f, h–l) 500 µm; (g) 1 mm. Reprinted with permission from Elsevier.
Surface-patterning techniques are also proving valuable in engineering three-dimensional scaffolds. The growth of cells in three dimensions is limited to the nutrient supply and waste removal available by diffusion, known as mass transfer. Using surface patterning technology, biocompatible polymers can be patterned to create vascular capillary-like channels that keep all the cells in a three-dimensional structure close to the source of oxygen, nutrients and waste removal. Nalayanda and co-workers used photolithography to create a 70 µm thick PDMS microfluidics scaffold that sustained the culture of primary pulmonary cells at an air–fluid interface. With continuous media flow through the capillary-type fluid channels, they were able to show maintenance of cellular architecture for up to two weeks. The system also allowed for testing of cellular responses to mechanical changes in sheer stress by altering the flow rate through the channels. This revealed that cells exposed to high flow rates and thus higher sheer stress levels had decreased proliferation when compared with cells exposed to low flow (Nalayanda et al. 2010). Carraro and colleagues used MEMS photolithographic and etching protocols to create a patterned template that can be used to mould silicone wafers with a vascular-type branching pattern. The characteristics of the vascular-like network mimic the fluid dynamics found in the branching pattern of the vessels in the liver (figure 2a). By stacking two different patterned silicone wafers with a thin membrane intervening, a three-dimensional scaffold was created (figure 2b,c). The scaffold was seeded with hepatocytes that were separated by a permeable membrane from media flowing through the vascular-like channels. Hepatocytes seeded into the scaffolds demonstrated polarization and maintained protein production and oxidation capacity comparable to that seen in normal liver cells (Carraro et al. 2008). A similar patterning method was employed to engineer a vascular-like arrangement of channels simulating a lung capillary network. Thin collagen wafers patterned from the prefabricated templates emulate the gas exchange surface in the lung. The collagen wafers supported cultured pneumocytes and endothelial cells on opposite sides of the membrane. The flow of gas and blood by the cells allowed the exchange of carbon dioxide and oxygen mimicking the alveolar capillary membrane of the lung (Hoganson et al. 2008).
Figure 2.
(a) The microfluidics device was tested by flowing trypan blue through the channels to verify patency. (b) Cross-sectional view of the bilayer-assembled device showing the layers containing microfluidics channels, C, and posts supporting the parenchymal chamber, P, connected by the intervening nanoporous polycarbonate membrane, M. (c) Scanning electron micrograph of the finished device. Adapted from Cortiella et al. (2006). Reprinted with permission from Springer.
Machining technologies have given us tools to create nanoscale surface patterns that enhance our knowledge of how cells respond to their environment and each other. When it was recognized that certain structures could not be created without an intrinsic supply of nutrients and oxygen, micro- and nanofabrication technologies were adapted to create microfluidic channels for flow of oxygenated media and blood to cells throughout the device. These technologies facilitate engineering of multi-dimensional structures capable of carrying out complex physiological processes like detoxification and gas exchange. Currently, the available technology restricts many of the three-dimensional architectural supports to non-biological polymers that may limit compatibility with living organisms. Significant up-scaling will also be required before these systems can support the cellular mass required to function in an organ replacement or supplementation capacity.
4. Electrospinning
Electrospinning is a process by which nanoscale fibres are fabricated by the application of a magnetic field to a highly charged polymer solution that is forced through a small diameter nozzle (Sill & von Recum 2008). Aligned fibre and non-woven mesh fibre scaffolds can be created with fibre diameters down to the 100 nm range with a wide range of pore sizes. Electrospun fibre mats have large surface areas available to interact with the cell surfaces and varying levels of porosity that enable differing amounts of cellular infiltration (Sill & von Recum 2008). Porosity and high surface area to volume ratio of the mats also facilitate diffusion into the three-dimensional structures aiding in mass transfer. The material used to create electrospun fibres is carefully chosen for effect on strength, fibre diameter and cellular response. Alignment of the fibres can also affect cell behaviours. Naturally occurring matrix protein fibres including collagen, elastin and fibrinogen, synthetic fibres, such as poly(ɛ-caprolactone) (PCL) and poly(lactide-co-glycolide) (PLGA), and hybrid fibres can be created with elctrospinning (McManus et al. 2007b; Ayres et al. 2010). Hybrid fibres combine the superior mechanical properties of synthetic fibres with the enhanced cell binding and signalling qualities of biological fibres (Heydarkhan-Hagvall et al. 2008).
Electrospinning can produce randomly oriented fibres that create porous-mesh or aligned fibre mats. Aligned fibres have been found to mimic natural tissue architecture and encourage differentiation in multiple cell types. Aligned hybrid scaffolds of collagen and PCL fibres have been shown to promote directional alignment of skeletal muscle cells and myotubule formation mimicking normal skeletal muscle architecture (figure 3c,d; Choi et al. 2008). Aligned PCL nanofibrous meshes produced by electrospinning induce pluripotent mesenchymal stem cells to differentiate into fibrochondrocytes and produce an ECM similar to natural joint meniscus tissue (Baker & Mauck 2007). Groups interested in vascular graft engineering have found that aligned electrospun fibres can influence vascular cell phenotypes as well. Zhu et al. report that fibrin-coated aligned PCL fibres enhanced vascular endothelial cell adhesion and von Willebrand factor production over cells grown on polystyrene plates (figure 3a,b; Zhu et al. 2010). In a similar illustration of the effects of aligned electrospun fibres, Huijun and colleagues demonstrated that super-aligned poly-l-lactic acid fibres could promote peripheral blood endothelial cell proliferation and specify cell orientation. Cells grown on the aligned fibres also maintained markers of an endothelial phenotype and angiogenic potential (Lu et al. 2009).
Figure 3.
Scanning electron microscope images of electrospun poly(ɛ-caprolactone) (PCL) fibres. (ai), (aii) Aligned fibres with different magnification; (bi,bii) non-aligned fibres. Laser confocal microscopy images of F-actin staining in human skeletal muscle cells seeded on aligned electrospun PCL/collagen nanofibre meshes (c) and randomly oriented electrospun meshes (d) (600× magnification). Adapted from Choi et al. (2008) (ai,aii, bi,bii) and Zhu et al. (2010) (c,d). Reprinted with permission from Wiley (ai,aii,bi,bii) and Elsevier. Scale bars, (ai,bi), 200 µm; (aii,bii), 10 µm; (c,d) 100 µm.
Integration of a synthetic component with a biological component in electrospun fibres has also proven to be effective in inducing stem cell differentiation into cell-specific lineages. By including hydroxyapatite with poly-l-lactic acid in electrospun fibres, Spadaccio and co-workers were able to induce mesenchymal stem cells to differentiate along a chondrocyte pathway and to lay down a cartilage-type proteoglycan matrix (Spadaccio et al. 2009). Chondrocyte differentiation from mesenchymal stem cells has also been induced on poly-l-lactic acid/hyaluronic acid composite electrospun nanofibre meshes. These composites were used to encase a gelatinous core of hyaluronic acid to engineering intervertebral discs. The cells also produced their own collagen and proteoglycan-based ECM (Nesti et al. 2008). In addition to incorporating biological components into the fibres themselves, some groups have coated electrospun fibres with biologically active peptide sequences, such as RGD that activates the integrin receptor. Coating fibres with RGD can increase fibroblast adhesion, spreading and proliferation over cells grown on non-coated fibres (Kim & Park 2006).
One of the most sought-after characteristics in scaffold design is the capability of the implanted cells to remodel the scaffold into a more biologically compatible environment. Electrospun fibres composed of biological proteins can be used to facilitate remodelling as demonstrated by McManus and colleagues who seeded human bladder myocytes on to non-woven electrospun fibronectin scaffolds. The cells migrated into the scaffold and over time degraded the fibronectin and laid down a collagen matrix of their own (McManus et al. 2007a). Similarly, in fibrinogen mats seeded with rat cardiac myocytes, the cells completely degraded the electrospun fibres and laid down a new collagen matrix over 14 days (McManus et al. 2007b).
Electrospun mesh scaffolds can be manipulated into three-dimensional structures to re-create native architecture. With the goal of engineering a blood vessel replacement that mimics the mechanical stress–strain characteristics of a native vessel while maintaining biological signalling capabilities, Sell and colleagues created electrospun polydioxanone and elastin composites and fashioned the mats into tubes. They were able to demonstrate equivalent stress–strain characteristics and stitch retention strength as native femoral artery. The electrospun fibrous mats also promoted the in-growth of cells throughout the length of the tube (Sell et al. 2006). Inoguchi et al. created tubes from electrospun poly(l-lactide-co-ɛ-caprolactone) fibre mats. Testing under pulsatile flow showed that these tubes had elastic compliance properties similar to small-diameter blood vessels (Inoguchi et al. 2006). They went on to seed umbilical vein endothelial cells onto the luminal side of the tubes. Culture undergraded pulsatile flow led to the alignment of the cells and formation of oriented stress fibres within the cytoplasm as seen in in vivo vessels (Inoguchi et al. 2007). Electrospun mesh scaffolds rolled into tubes have also been used in an in vivo nerve injury model. The tubes were sutured into a surgically created defect in the sciatic nerve in rats. The PLGA/PCL fibre tubes facilitated neurite extension and myelination with functional re-enervation of target muscles, where control animals showed no demonstrable nerve healing (figure 4; Panseri et al. 2008).
Figure 4.
(a) Scanning electron microscope image of the electrospun PLGA/PCL nerve guide conduit. Scale bar, 500 µm. (b–d) Longitudinal sections of nerve regenerated within the implanted guide channel. In the conduit, the regenerated nerve bridged the 10 mm gap, reconnecting the two sciatic nerve stumps. (a) Four months after surgery, haematoxylin–eosin staining shows the presence of regenerated tissue filling the conduit lumen; decreased lumen diameter is observable at middle length of the guidance channel. Regenerated tissue positive to Bielschowsky staining (b) and to anti-β-tubulin antibody (c) shows nervous projections oriented along the major axis of the prosthesis bridging the 10 mm gap between the severed sciatic nerve stumps (image sequence collected at 4× magnification). Adapted from Panseri et al. (2008). Reprinted with permission from BioMed Central.
The variable composition and architecture of electrospun fibre scaffolds allows for a wide range of tissue-engineering applications. Alterations of the alignment of the fibres alone can affect cell phenotype and behaviour. Addition of biological components into the fibres allows researchers to direct pluripotent cell differentiation down specific pathways and enhances the remodelling of the man-made scaffold into a native ECM. Formation of three-dimensional structures from the electrospun mats has also shown potential for blood vessel replacement and in nerve regeneration. Patents for human applications of electrospun products have been reported. However, the use of organic solvents in the production process and the need for sterility of human implants has slowed the progress to clinical utility (Kumbar et al. 2008). Future applications of the technology are likely to incorporate non-organic solvents in the production process, novel fibre compositions and innovative three-dimensional structures.
5. Self-assembly
The spontaneous association of particles in solution to form fibrous structures is the basis of the nanotechnology known as self-assembly. The most common particles used in self-assembly for medical purposes are amphiphilic particles that interact in solution driven by shielding of hydrophobic regions, hydrogen bonding and electrostatic repulsing forces (Cui et al. 2010). The fibres obtained by self-assembly are not random, but show specific order based upon the type of particles in the assembly. Peptides with alternating positively and negatively charged amino acids form secondary structures similar to β-pleated sheets seen in native protein folding. Lipid–peptide conjugates can form α-helical strands and lipid micelles, which can incorporate into cell and organelle membranes (Cavalli et al. 2010). The incorporation of biologically active peptide sequences into self-assembling particles has been applied to induce specific cellular behaviour.
Cell surface receptors such as integrins and laminins interact with the ECM and transduce signals to the cell by activating signalling cascades that influence cell survival, proliferation and differentiation. Integration of the peptides that activate these receptors into self-assembling particles has been successfully used to influence cell fate and behaviour. In one example, Fmoc-FF (fluorenylmethoxycarbonyl-diphenylalanine) and Fmoc-RGD self-assemble into β-pleated sheets creating a three-dimensional hydrogel with nanofibrils containing exposed RGD sites that mimic integrin ligand presentation. The exposed RGD sites promote cell adhesion, spreading and proliferation in the hydrogels. The cells also mechanically deformed the hydrogel in a manner similar to the contraction seen in the later stages of wound healing (Zhou et al. 2009). Incorporation of the laminin-derived peptide, IKVAV, into two- and three-dimensional self-assembled nanofibre networks induced neural progenitor cell differentiation into neurites. Cells grown on surfaces coated with IKVAV alone showed minimal differentiation into neurites indicating that enhanced presentation of the signalling motif by the peptide amphiphile resulted in greater biological activity (Silva et al. 2004). Willcox et al. designed peptides that self-assemble into dimers with a leucine zipper coiled-coil domain conformation. One monomer contained functional peptide motifs while the other contained a surface grafting site. The surface grafting site attached the dimers to a hydrogel upon which cells were cultured. Dynamic influence on cell behaviour was achieved by adding monomers with different functional peptides into the cell culture media. Disassembly and reassembly of the peptides from the solution with the surface-grafted peptide led to changes in the functional peptide that was presented to cells. By switching the peptides in solution, the authors were able to induce a specifically timed switch in cell behaviour (Willcox et al. 2005).
Similar to electrospun fibres, one of the significant attributes of self-assembled nanoparticle scaffolds is the propensity for cellular remodelling of the scaffold itself. Although in many instances the self-assembling particles are biological in nature and therefore degradable, incorporation of specific cleavage sites into the particles can enhance degradation. In an example of such, polyethylene glycol hydrogels containing integrin-binding motifs as well as matrix metalloproteinase (MMP)-sensitive peptides promoted pluripotent cardiac progenitors to commit to a myocyte pathway. The incorporation of the MMP sites allowed remodelling of the existing matrix and increased expression of markers of myocyte terminal differentiation (Kraehenbuehl et al. 2008).
Self-assembly nanotechnology has also been applied to the challenge of mass transfer. The availability of oxygen and nutrients is dependent upon diffusion in cultured tissues and this limits the size of engineered tissues to the millimetre range. In electrospun mats, enhanced diffusion through the porous scaffold can partially overcome this limitation. The ultimate solution, however, is engineering a vascularized tissue. Pro-angiogenic nanomaterials have recently been created that encourage blood vessel growth into self-assembled, implantable structures. The particles contain heparin sulphate proteoglycan moieties that bind and stabilize pro-angiogenic proteins, such as vascular endothelial growth factor (VEGF) and the fibroblast growth factors (FGFs). These self-assembling particles create nanofibre structures (heparin-binding peptide amphiphile (HBPA) hydrogels) that promote angiogenesis. HBPA hydrogels with pro-angiogenic factors implanted into rat corneas significantly enhanced angiogenesis over heparin plus GFs or hydrogel alone (Rajangam et al. 2006). In a continuation of this technology, Stendahl and colleagues used HBPA hydrogels containing VEGF and FGF-2 to support engraftment of transplanted β-islet cells. The GF-containing hydrogels promoted angiogenesis into the tissue surrounding the transplanted islet cells and enhanced cell survival (figure 5; Rajangam et al. 2006; Stendahl et al. 2008). The systemic physiological repercussions were impressive with significantly increased number of recipients receiving the islet cells with the HBPAs plus GFs showing resolution of hyperglycaemia (Stendahl et al. 2008).
Figure 5.
Vascular endothelial growth factor and fibroblast growth factor-2 delivered via heparin-binding peptide amphiphile (HBPA) nanostructures significantly increase vascular density at the omental transplant site. CD31 staining of (a) HBPA-control (CNTRL) and (b) HBPA+ growth factor (GF) scaffolds retrieved from omenta on post-transplant day 14. CD31-positive cells are stained brown by DAB chromogen and cell nuclei are stained blue by haematoxylin. Arrows denote sections of PLLA filaments among the infiltrating cellular tissue. (c) Haematoxylin–eosin staining of an HBPA-GF scaffold retrieved on day 14. Erythrocytes in the lumens (see arrows) suggest that neovessels have functional characteristics. (d) Density of CD31-positive neovessels in HBPA scaffold specimens retrieved between days 11 and 14. Neovessel densities in HBPA-GF specimens were nearly eight times greater than those in HBPA-CNTRL specimens (error bars represent 95% CI). *p = 2.86 × 10−4 by Student's t-test. Reprinted with permission from Lippincott Williams & Wilkins. Scale bars, (a,b) 25 µm and (c) 30 µm.
Although out of the scope of this review, self-assembling particles are also being explored as local drug-delivery systems. As a recent example, self-assembling nanoparticles were used to deliver cytotoxic protein sequences to cancer cells leading to membrane disruption and cell death. The nanoparticles protect the protein from proteolysis and gave a magnitude of selectivity for tumour cells over non-transformed cell lines (Standley et al. 2010). In possible future applications, self-assembled particles may be applied to the treatment of drug-resistant bacteria or chemotherapy-resistant tumours by allowing the use of systemically toxic drugs that would be delivered directly to the target of interest.
The utility of self-assembled particles to influence cell behaviour combined with the ability of surrounding cells to degrade the assembled structure has recently renewed interest in this nanotechnology for regenerative medicine applications. Introduction of these particles as vehicles for GFs and drug-delivery systems has broadened the potential applications for the technology. Translation to the clinical arena may be expedited, as many similar hydrogel products already have applications in wound healing gels and have a proven safety profile having been through prior clinical trials.
6. Gene-activated matrices
Gene-activated matrices (GAMs) combine a tissue-engineering scaffold with plasmid DNA. Cellular degradation of the matrix releases the DNA that can be taken up by cells in the local environment leading to transient transfection of the cells and production of a protein of interest (Bonadio 2000). In in vivo studies of wound healing using GAMs, the efficiency of transfection has been high, up to 50 per cent, with plasmid-encoded gene expression documented for six weeks (Bonadio et al. 1999). Bone regeneration has been induced in animal models using GAMs that contain bone morphogenic protein (BMP) DNA with impressive results. Using collagen matrix supplemented with calcium phosphate to enhance BMP2 plasmid DNA uptake, Endo and colleagues were able to promote complete osseous bridging in a rat model of tibial bone defect creation over six weeks. No bridging was seen in control animals (figure 6; Endo et al. 2006). GAMs have also been used to promote wound healing in the central nervous system. Collagen-based matrices containing neural growth factors promote survival of rat optic nerves and dorsal root ganglia after injury. Transcription of plasmid DNA was also seen in the cerebral cortex indicating retrograde neuronal transport of the plasmid DNA (Berry et al. 2001). In addition, the presence of the GAM's collagen matrix scaffold at the site of injury was found to decrease scar formation signifying a dual benefit from the GAM implantation (Gonzalez et al. 2006).
Figure 6.
Soft X-ray photographs at (a) four and (b) six weeks after the operation. A critical size bone defect (5 mm in length) was prepared in the rat tibia and the defect treated with different implants. G1, bmp2-CaP-collagen; G2, bmp2-collagen; G3, collagen; G4, vacant plasmid vector-CaP-collagen; G5, untreated. Reprinted with permission from Mary Ann Liebert, Inc.
GAMs have shown utility in addressing the specific challenges of matrix remodelling and mass transfer. A chitosan–gelatin matrix complexed with plasmid DNA encoding transforming growth factor-β1 (TGF-β1) can support the growth of chondrocytes and the regeneration of cartilage. Upregulated cellular TGF-β1 expression enhanced maintenance of chondrocyte cell phenotype and modification of the chitosan–gelatin matrix to include type II collagen and proteoglycans. These findings demonstrate maturation of the GAM towards native cartilage (Guo et al. 2006). A similar strategy has been successful in supporting regrowth of periodontal ligament cells using chitosan–collagen matrix containing platelet-derived growth factor plasmid DNA. Cells maintained in the GAM produced periodontal ligament-like connective tissue after two weeks in culture (Peng et al. 2009). Christman and colleagues used pleiotrophin-encoding plasmid DNA in a fibrin glue matrix to enhance neovascularization in a rat model of myocardial infarction. Not only were the numbers of blood vessels in the infarcted area increased, flow through the vessels could also be confirmed (Christman et al. 2005).
Like the previously discussed technologies, GAMs have married nanotechnology with biological pathways to overcome barriers to matrix remodelling and mass transfer. GAM invocation of protein production over a sustainable time period coupled with a biocompatible vehicle delivers promise for further applications in tissue injury and repair. The consequences of possible recombination events between plasmid DNA and nuclear DNA require careful examination prior to introduction to human applications.
7. Engineering complex organs
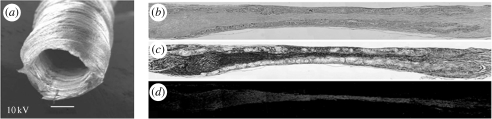
The greatest feat in tissue engineering is recreating organs that are made up of multiple cell types. Issues of cell differentiation, orientation, matrix remodelling and mass transfer must all be overcome for successful organ generation. The use of pluripotent cells that can differentiate into many cell lineages is currently one approach applied to address these issues. Cortiella et al. seeded pluriplotent sheep adult lung progenitor cells into engineered scaffolds of polyglycolic acid or Pleuronic-F 127. The constructs were implanted into nude mice and over the course of eight weeks, the cells differentiated into multiple lung-specific cell types and reordered the matrix to create alveolar-like structures. The cell–scaffold constructs could also be implanted into wedge resection sites in adult sheep lung and the structures incorporated into the healing area and maintained the alveolar-type architecture (figure 7; Cortiella et al. 2006). Gelfoam sponge scaffolds impregnated with rat stem cells have also been used to engineer new lung tissue. Andrade and colleagues introduced rat stem cells into gelfoam scaffolds and injected these constructs into adult rat lungs. Over time, the sponges were degraded and the foetal cells differentiated into lung-specific cell types in a conformation resembling the native alveoli. A patent vascular network infiltrating into the constructs was also demonstrated by perfusion studies suggesting in-growth angiogenesis leading to a vascularized tissue (Andrade et al. 2007).
Figure 7.
In vitro tissue-engineered lung. (a–f) Scanning electron micrographs of in vitro tissue-engineered ovine somatic lung progenitor cells seeded onto polyglycolic acid scaffolds. Directly after seeding (a) and after one week (b), two weeks (c), six weeks (d) and eight weeks (e) of culture in vitro. By six weeks, cultures developed morphological features of lung tissue. By eight weeks, tissue-engineered lung (e,f) contained structures similar to alveoli (a) with septa (arrows), compared with septa (arrows) in native lung (f) (scale bars, 10 µm). By eight weeks, engineered lung tissue grown in vitro was macroscopically visible and could be handled easily (g) (scale bar, 5 mm). Reproduced with permission from Mary Ann Liebert, Inc.
An alternative approach to engineering a biomimetic ECM is using an endogenous matrix that has been rendered immunologically inert and is then re-seeded with cells that populate that matrix and reconstruct the tissue. Ott and colleagues demonstrated the feasibility of this technique by decellularizing rat hearts by perfusing the coronary arteries with detergents until no demonstrable cellular components remained. The decellularized scaffold was then seeded with cardiac muscle cells and maintained in a bioreactor through which coronary flow was maintained with oxygenated culture media. After 8 days, the re-seeded heart matrix showed rhythmic contractions and could generate left ventricular pump function (figure 8; Ott et al. 2008). Similar methods have been used to repopulate decellularized lung ECM. Two groups have shown that these lungs can participate in gas exchange in rodent models for up to 6 h (Ott et al. 2010; Petersen et al. 2010).
Figure 8.
(a) Photographs of cadaveric perfusion decellularization of whole rat hearts perfused with sodium dodecyl sulphate (SDS) over 12 h. The heart becomes more translucent as cellular material is washed out from the right ventricle, then the atria and finally the left ventricle. (b) Haematoxylin–eosin staining of thin sections of SDS-treated heart showing no intact cells or nuclei. Maintenance of large vasculature conduits (asterisks). Scale bar, 200 µm. (c) Formation of a working perfused bioartificial heart-like construct by recellularization of decellularized cardiac ECM. Recellularized whole rat heart at day 4 of perfusion culture in a working heart bioreactor. (d) Representative functional assessment tracing of decellularized whole heart construct paced in a working heart bioreactor. Tracings of electrocardiogram (ECG), aortic pressure (afterload) and left ventricular pressure (LVP) of the paced construct on day 8 after recellularization, and on day 8 after stimulation with physiological (B50–100 mM) doses of phenylephrine. Adapted from Ott et al. (2008). Reprinted with permission from Nature Medicine.
The ability to apply our understanding of the complex cellular environment to engineering technologies that can re-create that specialized environment has paved the way for complex organ generation. The few illustrative examples above, chosen from many, highlight the strides that tissue engineering has taken in the last decade away from simple cell culture monolayers. Large-scale introduction of decellularized tissues into a clinical use may be hindered by the same organ shortage that limits cadaveric donor transplantation currently. Investment of resources into alternative sources of tissue may be necessary for the technology to impact the donor organ shortfall. Animal organs may represent a possible source of organs if a lack of immunogenicity can be assured.
8. Commercialization and nanotechnology
It was recognized early in the evolution of tissue engineering that successful commercialization was critical to the application of scientific innovation. Academic investigators created platforms of intellectual property that served as the basis for the creation of new companies or new divisions within existing medical device or pharmaceutical firms. In parallel, conversations were begun with regulatory agencies to ensure safety and efficiency as products developed. The most careful studies of the commercial development of the field have been done by Lysaght et al. (2008). Although his last report was before the most recent economic downturn, it suggests remarkable resiliency and growth commercially since he documents survival and growth after the downturn of 2000–2002. Many lessons were learnt that resulted in adaptations in the business model. Importantly, low hanging fruit using acellular devices that trigger a regenerative response have produced commercially successful products that can then fund more complex devices. Certainly, the application of nanotechnology as we have described can improve the regenerative response by more accurately guiding cells and their differentiation into the device. As well, the more involved replacement structures including large, complex tissues and organs needing augmentation of the blood supply have benefited from nanofabrication strategies. In many cases, this will allow commercialization because the product could fill an unmet need, where no good alternative exists and where in many cases the outcome is either fatal or extremely disabling. The future of the field appears brighter than ever not only because of the progress in science and commercialization, but also because of the infusion of new, young investigators committed to its goals.
Footnotes
One contribution to a Theme Supplement ‘Translation and commercialization of regenerative medicines’.
References
- Andrade C. F., Wong A. P., Waddell T. K., Keshavjee S., Liu M. 2007. Cell-based tissue engineering for lung regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L510–L518. ( 10.1152/ajplung.00175.2006) [DOI] [PubMed] [Google Scholar]
- Ayres C. E., Jha B. S., Sell S. A., Bowlin G. L., Simpson D. G. 2010. Nanotechnology in the design of soft tissue scaffolds: innovations in structure and function. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2, 20–34. ( 10.1002/wnan.55) [DOI] [PubMed] [Google Scholar]
- Baker B. M., Mauck R. L. 2007. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 28, 1967–1977. ( 10.1016/j.biomaterials.2007.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., et al. 2001. Sustained effects of gene-activated matrices after CNS injury. Mol. Cell. Neurosci. 17, 706–716. ( 10.1006/mcne.2001.0975) [DOI] [PubMed] [Google Scholar]
- Bonadio J. 2000. Tissue engineering via local gene delivery: update and future prospects for enhancing the technology. Adv. Drug Deliv. Rev. 44, 185–194. ( 10.1016/S0169-409X(00)00094-6) [DOI] [PubMed] [Google Scholar]
- Bonadio J., Smiley E., Patil P., Goldstein S. 1999. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 5, 753–759. ( 10.1038/10473) [DOI] [PubMed] [Google Scholar]
- Carraro A., et al. 2008. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed. Microdevices 10, 795–805. ( 10.1007/s10544-008-9194-3) [DOI] [PubMed] [Google Scholar]
- Cavalcanti-Adam E. A., Volberg T., Micoulet A., Kessler H., Geiger B., Spatz J. P. 2007. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 92, 2964–2974. ( 10.1529/biophysj.106.089730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli S., Albericio F., Kros A. 2010. Amphiphilic peptides and their cross-disciplinary role as building blocks for nanoscience. Chem. Soc. Rev. 39, 241–263. ( 10.1039/b906701a) [DOI] [PubMed] [Google Scholar]
- Choi J. S., Lee S. J., Christ G. J., Atala A., Yoo J. J. 2008. The influence of electrospun aligned poly(ɛ-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 29, 2899–2906. ( 10.1016/j.biomaterials.2008.03.031) [DOI] [PubMed] [Google Scholar]
- Christman K. L., Fang Q., Yee M. S., Johnson K. R., Sievers R. E., Lee R. J. 2005. Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials 26, 1139–1144. ( 10.1016/j.biomaterials.2004.04.025) [DOI] [PubMed] [Google Scholar]
- Cortiella J., Nichols J. E., Kojima K., Bonassar L. J., Dargon P., Roy A. K., Vacant M. P., Niles J. A., Vacanti C. A. 2006. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 12, 1213–1225. ( 10.1089/ten.2006.12.1213) [DOI] [PubMed] [Google Scholar]
- Cui H., Webber M. J., Stupp S. I. 2010. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 94, 1–18. ( 10.1002/bip.21328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby M. J., Gadegaard N., Wilkinson C. D. 2008. The response of fibroblasts to hexagonal nanotopography fabricated by electron beam lithography. J. Biomed. Mater. Res. A 84, 973–979. ( 10.1002/jbm.a.31409) [DOI] [PubMed] [Google Scholar]
- Endo M., Kuroda S., Kondo H., Maruoka Y., Ohya K., Kasugai S. 2006. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 12, 489–497. ( 10.1089/ten.2006.12.489) [DOI] [PubMed] [Google Scholar]
- Fu J., Wang Y. K., Yang M. T., Desai R. A., Yu X., Liu Z., Chen C. S. 2010. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7, 733–736. ( 10.1038/nmeth.1487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. M., Berry M., Greenlees L., Logan A., Baird A. 2006. Matrix-mediated gene transfer to brain cortex and dorsal root ganglion neurones by retrograde axonal transport after dorsal column lesion. J. Gene Med. 8, 901–909. ( 10.1002/jgm.919) [DOI] [PubMed] [Google Scholar]
- Gray D. S., Liu W. F., Shen C. J., Bhadriraju K., Nelson C. M., Chen C. S. 2008. Engineering amount of cell–cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp. Cell Res. 314, 2846–2854. ( 10.1016/j.yexcr.2008.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Zhao J., Chang J., Ding Z., Hong H., Chen J., Zhang J. 2006. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-β1 for chondrocytes proliferation. Biomaterials 27, 1095–1103. ( 10.1016/j.biomaterials.2005.08.015) [DOI] [PubMed] [Google Scholar]
- Heydarkhan-Hagvall S., Choi C. H., Dunn J., Heydarkhan S., Schenke-Layland K., Robb-MacLellan W., Beygui R. E. 2007. Influence of systematically varied nano-scale topography on cell morphology and adhesion. Cell Commun. Adhes. 14, 181–194. ( 10.1080/15419060701755594) [DOI] [PubMed] [Google Scholar]
- Heydarkhan-Hagvall S., Schenke-Layland K., Dhanasopon A. P., Rofail F., Smith H., Wu B. M., Shemin R., Beygui R. E., MacLellan W. R. 2008. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29, 2907–2914. ( 10.1016/j.biomaterials.2008.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoganson D. M., Pryor H. I., II, Vacanti J. P. 2008. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr. Res. 63, 520–526. [DOI] [PubMed] [Google Scholar]
- Inoguchi H., Kwon I. K., Inoue E., Takamizawa K., Maehara Y., Matsuda T. 2006. Mechanical responses of a compliant electrospun poly(L-lactide-co-ɛ-caprolactone) small-diameter vascular graft. Biomaterials 27, 1470–1478. ( 10.1016/j.biomaterials.2005.08.029) [DOI] [PubMed] [Google Scholar]
- Inoguchi H., Tanaka T., Maehara Y., Matsuda T. 2007. The effect of gradually graded shear stress on the morphological integrity of a HUVEC-seeded compliant small-diameter vascular graft. Biomaterials 28, 486–495. ( 10.1016/j.biomaterials.2006.09.020) [DOI] [PubMed] [Google Scholar]
- Johansson F., Carlberg P., Danielsen N., Montelius L., Kanje M. 2006. Axonal outgrowth on nano-imprinted patterns. Biomaterials 27, 1251–1258. ( 10.1016/j.biomaterials.2005.07.047) [DOI] [PubMed] [Google Scholar]
- Kantawong F., Burgess K. E., Jayawardena K., Hart A., Burchmore R. J., Gadegaard N., Oreffo R. O. C., Dalby M. J. 2009. Whole proteome analysis of osteoprogenitor differentiation induced by disordered nanotopography and mediated by ERK signalling. Biomaterials 30, 4723–4731. ( 10.1016/j.biomaterials.2009.05.040) [DOI] [PubMed] [Google Scholar]
- Kim T. G., Park T. G. 2006. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(d,l-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 12, 221–233. ( 10.1089/ten.2006.12.221) [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl T. P., Zammaretti P., Van der Vlies A. J., Schoenmarkers R. G., Lutolf M. P., Jaconi M. E., Hubbell J. A. 2008. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 29, 2757–2766. ( 10.1016/j.biomaterials.2008.03.016) [DOI] [PubMed] [Google Scholar]
- Kumbar S. G., Nukavarapu S. P., James R., Hogan M. V., Laurencin C. T. 2008. Recent patents on electrospun biomedical nanostructures: an overview. Recent Patents Biomed. Eng. 1, 68–78. [Google Scholar]
- Lamers E., et al. 2010. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 31, 3307–3316. ( 10.1016/j.biomaterials.2010.01.034) [DOI] [PubMed] [Google Scholar]
- Langer R., Vacanti J. P. 1993. Tissue engineering. Science 260, 920–926. ( 10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- Lee C. J., Blumenkranz M. S., Fishman H. A., Bent S. F. 2004. Controlling cell adhesion on human tissue by soft lithography. Langmuir 20, 4155–4161. ( 10.1021/la035467c) [DOI] [PubMed] [Google Scholar]
- Lu H., Feng Z., Gu Z., Liu C. 2009. Growth of outgrowth endothelial cells on aligned PLLA nanofibrous scaffolds. J. Mater. Sci. Mater. Med. 20, 1937–1944. ( 10.1007/s10856-009-3744-y) [DOI] [PubMed] [Google Scholar]
- Lysaght M. J., Jaklenec A., Deweerd E. 2008. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng. A 14, 305–315. ( 10.1089/tea.2007.0267) [DOI] [PubMed] [Google Scholar]
- Maheshwari G., Brown G., Lauffenburger D. A., Wells A., Griffith L. G. 2000. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113, 1677–1686. [DOI] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495. ( 10.1016/S1534-5807(04)00075-9) [DOI] [PubMed] [Google Scholar]
- McManus M., Boland E., Sell S., Bowen W., Koo H., Simpson D., Bowlin G. 2007a. Electrospun nanofibre fibrinogen for urinary tract tissue reconstruction. Biomed. Mater. 2, 257–262. ( 10.1088/1748-6041/2/4/008) [DOI] [PubMed] [Google Scholar]
- McManus M. C., Boland E. D., Simpson D. G., Barnes C. P., Bowlin G. L. 2007b. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. J. Biomed. Mater. Res. A 81, 299–309. ( 10.1002/jbm.a.30989) [DOI] [PubMed] [Google Scholar]
- Nalayanda D. D., Wang Q., Fulton W. B., Wang T. H., Abdullah F. 2010. Engineering an artificial alveolar-capillary membrane: a novel continuously perfused model within microchannels. J. Pediatr. Surg. 45, 45–51. ( 10.1016/j.jpedsurg.2009.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesti L. J., Li W. J., Shanti R. M., Jiang Y. J., Jackson W., Freedman B. A., Kuklo T. R., Giuliani J. R., Tuan R. S. 2008. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng. A 14, 1527–1537. ( 10.1089/ten.tea.2008.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott H. C., Matthiesen T. S., Goh S. K., Black L. D., Kren S. M., Netoff T. I., Taylor D. A. 2008. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221. ( 10.1038/nm1684) [DOI] [PubMed] [Google Scholar]
- Ott H. C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., Vacanti J. P. 2010. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933. ( 10.1038/nm.2193) [DOI] [PubMed] [Google Scholar]
- Panseri S., Cunha C., Lowery J., Del Carro U., Taraballi F., Amadio S., Vescovi A., Gelain F. 2008. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transactions. BMC Biotechnol. 8, 39 ( 10.1186/1472-6750-8-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Cheng X., Zhuo R., Lan J., Wang Y., Shi B., Li S. 2009. Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J. Biomed. Mater. Res. A 90, 564–576. ( 10.1002/jbm.a.32117) [DOI] [PubMed] [Google Scholar]
- Petersen T. H., et al. 2010. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541. ( 10.1126/science.1189345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich G. D. 2007. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J. Cell. Biochem. 101, 1370–1383. ( 10.1002/jcb.21386) [DOI] [PubMed] [Google Scholar]
- Rajangam K., Behanna H. A., Hui M. J., Han X., Hulvat J. F., Lomasney J. W., Stupp S. I. 2006. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 6, 2086–2090. ( 10.1021/nl0613555) [DOI] [PubMed] [Google Scholar]
- Selhuber-Unkel C., Erdmann T., Lopez-Garcia M., Kessler H., Schwarz U. S., Spatz J. P. 2010. Cell adhesion strength is controlled by intermolecular spacing of adhesion receptors. Biophys. J. 98, 543–551. ( 10.1016/j.bpj.2009.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. A., McClure M. J., Barnes C. P., Knapp D. C., Walpoth B. H., Simpson D. G., Bowlin G. L. 2006. Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomed. Mater. 1, 72–80. ( 10.1088/1748-6041/1/2/004) [DOI] [PubMed] [Google Scholar]
- Sill T. J., von Recum H. A. 2008. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006. ( 10.1016/j.biomaterials.2008.01.011) [DOI] [PubMed] [Google Scholar]
- Silva G. A., Czeisler C., Niece K. L., Beniash E., Harrington D. A., Kessler J. A., Stupp S. I. 2004. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355. ( 10.1126/science.1093783) [DOI] [PubMed] [Google Scholar]
- Spadaccio C., Rainer A., Trombetta M., Vadala G., Chello M., Covino E., Denaro V., Toyoda Y., Genovese J. A. 2009. Poly-l-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann. Biomed. Eng. 37, 1376–1389. ( 10.1007/s10439-009-9704-3) [DOI] [PubMed] [Google Scholar]
- Standley S. M., et al. 2010. Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 70, 3020–3026. ( 10.1158/0008-5472.CAN-09-3267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl J. C., Wang L. J., Chow L. W., Kaufman D. B., Stupp S. I. 2008. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation 86, 478–481. ( 10.1097/TP.0b013e3181806d9d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Yamazoe H., Sassa F., Suzuki H., Fukuda J. 2009. Preparation of coculture system with three extracellular matrices using capillary force lithography and layer-by-layer deposition. J. Biosci. Bioeng. 108, 544–550. ( 10.1016/j.jbiosc.2009.06.013) [DOI] [PubMed] [Google Scholar]
- Torres A. J., Wu M., Holowka D., Baird B. 2008. Nanobiotechnology and cell biology: micro- and nanofabricated surfaces to investigate receptor-mediated signaling. Annu. Rev. Biophys. 37, 265–288. ( 10.1146/annurev.biophys.36.040306.132651) [DOI] [PubMed] [Google Scholar]
- Truskett V. N., Watts M. P. 2006. Trends in imprint lithography for biological applications. Trends Biotechnol. 24, 312–317. ( 10.1016/j.tibtech.2006.05.005) [DOI] [PubMed] [Google Scholar]
- Willcox P. J., Reinhart-King C. A., Lahr S. J., DeGrado W. F., Hammer D. A. 2005. Dynamic heterodimer-functionalized surfaces for endothelial cell adhesion. Biomaterials 26, 4757–4766. ( 10.1016/j.biomaterials.2004.11.060) [DOI] [PubMed] [Google Scholar]
- Zhou M., Smith A. M., Das A. K., Hodson N. W., Collins R. F., Ulijn R. V., Gough J. E. 2009. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 30, 2523–2530. ( 10.1016/j.biomaterials.2009.01.010) [DOI] [PubMed] [Google Scholar]
- Zhu Y., Cao Y., Pan J., Liu Y. 2010. Macro-alignment of electrospun fibers for vascular tissue engineering. J. Biomed. Mater. Res. B 92, 508–516. ( 10.1002/jbm.b.31544) [DOI] [PubMed] [Google Scholar]