Abstract
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have the ability (i) to duplicate indefinitely while maintaining pluripotency and (ii) to differentiate into cell types of all three embryonic germ layers. These two properties of ESCs and iPSCs make them potentially suitable for tissue engineering and cell replacement therapy for many different diseases, including Parkinson's disease, diabetes and heart disease. However, one critical obstacle in the clinical application of ESCs or iPSCs is the risk of teratoma formation. The emerging field of molecular imaging is allowing researchers to track transplanted ESCs or iPSCs in vivo, enabling early detection of teratomas.
Keywords: stem cells, tumorigenicity, molecular imaging, teratoma, differentiation, regenerative medicine
1. Introduction
In 1981, mouse embryonic stem cells (ESCs) were first isolated from the mouse blastocyst (Evans & Kaufman 1981; Martin 1981). A decade later, human ESCs (hESCs) were isolated from the human blastocyst (Thomson et al. 1998). ESC lines are created from the in vitro culture and expansion of the inner cell mass of the blastocyst stage embryo (Evans & Kaufman 1981; Martin 1981; Thomson et al. 1998). Subsequent studies found that it was possible to confer totipotency or pluripotency to somatic cells by transferring the nuclear contents of somatic cells into oocytes (Wilmut et al. 1997), or by fusion with hESCs (Tada et al. 2001; Cowan et al. 2005). This led Takahashi and Yamanaka to believe that certain factors, important in the maintenance of ESCs, could induce pluripotency in somatic cells and create mouse induced pluripotent stem cells (iPSCs), a process termed cellular reprogramming (Takahashi & Yamanaka 2006). It was first described using the exogenous expression of four genes, including two pluripotency transcription factors (i.e. Oct4 and Sox2) and two proto-oncogenes (i.e. c-Myc and Klf4; Takahashi & Yamanaka 2006). The first mouse iPSC line was created in 2006 (Takahashi & Yamanaka 2006), and a year later human iPSC lines were created independently by James Thomson's and Shinya Yamanaka's groups (Takahashi et al. 2007; Yu et al. 2007).
The pluripotent state of hESCs and iPSCs is indicated by several cellular characteristics, including the following: (i) expression of transcription factors (e.g. Nanog, Oct4 and Sox2), and cell surface molecules (e.g. SSEA3, SESA4, Tra-1-60 and Tra-1-81), (ii) high activity of the enzymes alkaline phosphatase and telomerase, and (iii) typical cell morphology (e.g. small cells with a high nucleus to cytoplasm ratio and prominent nucleoli growing in flat colonies; Thomson et al. 1998; Amit et al. 2000; Takahashi et al. 2007). The gold standard for pluripotency is to demonstrate a cell line's ability to form tissue of all three germ layers by creating chimeras (Okita et al. 2007) or tetraploid complementation (Zhao et al. 2009) with mouse iPSCs, or by forming teratomas with human iPSCs. Teratomas are defined as benign germ cell tumours (GCTs), which consist of mature, well-differentiated tissues (mature teratomas), or embryonic, less-differentiated tissues (immature teratomas) with a normal karyotype (Pierce et al. 1960; Oosterhuis & Looijenga 2005). The ability of the first hESC line to form tissue composed of all three germ layers was demonstrated by teratoma assay in immunodeficient SCID mice. Histology showed that every mouse had developed tumours composed of gut epithelium (endoderm); cartilage, bone, smooth muscle and striated muscle (mesoderm); and neural epithelium, embryonic ganglia, and stratified squamous epithelium (ectoderm; Thomson et al. 1998). This assay was later used to test pluripotency in vivo for human iPSCs (Takahashi et al. 2007; Yu et al. 2007).
Owing to the pluripotency of ESCs and iPSCs, they are considered to possess great potential for regenerative medicine, including possible treatment of Parkinson's disease (Yang et al. 2008), diabetes (D'Amour et al. 2006) and heart disease (Cao et al. 2008). Pre-clinical studies in animal models, however, have reported teratoma formation occurring upon injection of mouse ESC (mESC)-derived beating embryoid bodies (EBs; Cao et al. 2006; Xie et al. 2007) and ESC-derived neuroprogenitors (Chaudhry et al. 2009), highlighting a major hurdle that we have to overcome before clinical translation of pluripotent cells can be realized. The issue being addressed now is how teratoma formation occurs within differentiated ESC- or iPSC-derived cell lines. Although not fully understood, it is believed to be the result of remnant, undifferentiated cells present within the transplanted cell population. This hypothesis is further reinforced by findings that transplantation of a fairly pure hESC-derived cardiomyocyte population (82.6 ± 6.6%, range 71–95%) into immunosupressed rats did not result in teratoma formation after four weeks (Laflamme et al. 2007), whereas intracardiac injection of undifferentiated mESCs into immunodeficient rats resulted in teratoma formation in all of them after only three to four weeks (Cao et al. 2006; Nussbaum et al. 2007). Taking pluripotent cells from bench to bedside will therefore partly depend on the ability to purify the cell population and to detect teratoma formation early. The latter can be achieved with the non-invasive tracking of pluripotent cells in vivo with molecular imaging as discussed later.
2. The link between pluripotency and tumorigenicity
The link between pluripotency and tumorigenicity was discovered as early as the 1960s. At that time, researchers studied teratocarcinomas, the malignant form of teratomas (Pierce et al. 1960). The malignancy of teratocarcinomas lies in the presence of embryonic carcinoma (EC) cells, which are capable of self-renewal as well as differentiation into a variety of tissues (Andrews et al. 2005). Transplantation of a single EC cell resulted in the formation of a teratocarcinoma, which is composed of many differentiated tissues (Kleinsmith & Pierce 1964; Andrews et al. 2005). Subsequent studies showed that teratomas and teratocarcinomas could also be formed by the transplantation of normal mouse embryos or embryonic genital ridges to the adult host, demonstrating that tumour-initiating cells were also present within normal embryos (Stevens & Hummel 1957; Stevens 1964, 1967, 1968). When the first hESCs were isolated in 1998, similarities were observed between the newly isolated ESCs and EC cells, including the expression of the pluripotency markers (e.g. SSEA3, SSEA4, TRA 1–60, TRA 1–81, telomerase and alkaline phosphatase) and the ability to form teratomas in immunodeficient mice (Thomson et al. 1998). Furthermore, Andrews and co-workers hypothesized that EC cells might have a stronger selection for mutations that would increase self-renewal capacities and limit differentiation. Similarly, mutations have been observed when growing hESCs in culture over longer passages (Andrews et al. 2005). For example, unbalanced chromosomal translocation involving the long arm of chromosomes 1 and 9, resulting in karyotypic change 46,XX,der(9)t(1;9)(q31;q22), has been reported in H9 cells at passage 120 compared with passage 48 (Xie et al. in press). Passaging of three subcultures of hESC line H7 (H7.S0, H7.S6, H7.S9) and the hESC line H14 over a period of several months resulted in a gain of the complete long arm of chromosome 17 (17q; Draper et al. 2004), which is also very commonly seen in EC cells derived from teratocarcinomas (Andrews et al. 2005). In addition to a gain of chromosome 17, a gain of chromosome 12 has also been observed in a few subpopulations of these cells. The pluripotency marker Nanog is sited on this chromosome (Draper et al. 2004). The gain of one or more isochromosomes on the short arm of chromosome 12 is associated with testicular GCTs in humans (Mostert et al. 1998). Further evidence for the role in chromosome 12 in the tumorgenicity of pluripotent cells was provided by Sperger and co-workers, who compared the gene expression of five hESC lines, 36 GCT cell lines, 14 samples of normal testis and 17 somatic cell lines. Significance analysis of microarrays in this study identified 1760 cDNAs in ESCs, 1299 cDNAs in EC cells and 1518 cDNAs in seminomas that were significantly more highly expressed than the control group of differentiated cells. They found 330 genes that were shared by ESCs, EC cells and seminomas, and among these genes was POU5F1 (Oct4). Furthermore, genes sited on chromosome 12 were highly represented (p < 0.00001) among the genes highly overexpressed in EC cells when compared directly with ESCs (Sperger et al. 2003).
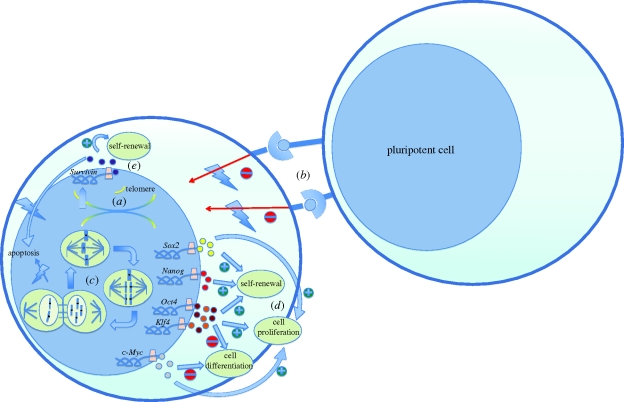
The molecular basis of the tumorigenicity of pluripotent cells lies in their cancer-resembling properties, namely their ability to self-renew and proliferate, which are promoted by several molecular processes and have been described in detail by Blum & Benvenisty (2008, 2009). These properties include self-renewal, rapid proliferation, lack of contact inhibition and telomerase activity (figure 1). On closer examination of the karyotype of ESCs, polyploidy has been observed in mESCs despite an intact spindle assembly checkpoint (SAC; Malashicheva et al. 2002). Mantel and co-workers demonstrated that mESCs and hESCs have an intact SAC, but that mitotic failure-induced polyploidy does not lead to apoptosis of these cells (Mantel et al. 2007). Mitotic errors often occur in rapidly proliferating cells and the SAC, typically leading to apoptosis of these cells and the necessity for genome maintenance. Uncoupling of this mechanism will lead to karyotypic abnormalities in ESC culture (figure 1). In addition, when inducing double-strand breaks in the DNA of mouse ESCs and mouse EBs by the DNA-damaging agent etoposide, ESCs were resistant to apoptosis whereas the EBs underwent caspase-3-dependent apoptosis. This suggests that polyploidy tolerance in ESCs will give way to apoptosis upon lineage-specific differentiation. This hypothesis has been confirmed for mouse as well as human mononuclear polyploidy/aneupoloid cells, indicating that checkpoint–apoptosis uncoupling is an intrinsic behaviour of mESCs and hESCs (Mantel et al. 2007).
Figure 1.
The molecular basis of the tumorigenicity of pluripotent cells. (a) Increased telomerase activity results in extended lifespan of the pluripotent cells by adding telomere ends to the chromosomes. (b) Lack of contact inhibition allows for proliferation and cell growth, even if pluripotent cells are in contact with neighbouring pluripotent cells. (c) Spindle assembly checkpoint (SAC) uncoupling results in failure to induce apoptosis of ESCs with karyotypic abnormalities such as mitotic failure-induced polyploidy. (d,e) Overexpression of the pluripotency markers leads to a highly proliferative, undifferentiated and self-renewal state. Survivin (BIRC5), in addition to the self-renewal properties, functions as an anti-apoptotic gene.
The examination of gene expression in several human tumours has shown that the pluripotency markers used in reprogramming somatic cells to iPSCs are directly oncogenic (e.g. c-Myc and Klf4) or are involved in tumorigenesis (e.g. Sox2, Nanog, Knoepfler (2009); and Oct3/4, Palma et al. (2008)) Targets of Nanog, Sox2 and Oct4 that encode for transcription regulators, are overexpressed and prove especially active in high-grade breast tumours (Ben-Porath et al. 2008). Upregulation of Nanog and Oct4 is also associated with poor outcomes in patients with oral cancer (Chiou et al. 2008). The pluripotent genes Oct3/4, Sox2, Nanog, c-Myc and Klf4 were also present in prostate tumour cell lines, as well as in primary prostate tumour tissue. Furthermore, injection of these tumour cells containing the pluripotent genes created strong tumorigenicity in immunodeficient mice (Bae et al. 2010).
Using a mouse model in which Oct4 could be activated by doxycycline administration, Hochedlinger and co-workers evaluated the effects of Oct4 overexpression in somatic differentiation and tumorigenesis. Oct4-induced dysplasia of the epithelial tissues could be seen as early as 5 days after initiating doxycycline. Dysplasia was only seen in the epithelial progenitor and/or stem cells, and Oct4 overexpression had no effect on the cellular phenotype of differentiated cells. Withdrawal of doxycycline caused a complete reversal of the tumour phenotype, again showing the association of Oct4 with tumorigenesis (Hochedlinger et al. 2005).
The biological link between pluripotency and tumorigenicity can perhaps best be described by c-Myc. Although not required for the induction of pluripotency in iPSCs, c-Myc augments the reprogramming ability of Oct4, Sox2 and Klf4 (Takahashi et al. 2007), inhibits differentiation and promotes proliferation (figure 1). Myc proteins have been shown by Eilers & Eisenman (2008) to influence several thousand genes. The majority of these are upregulated and involved in cell growth; the few genes that are downregulated are involved in cell cycle arrest, cell adhesion and cell–cell communication. The influence of Myc on these nuclear processes is oncogenic as shown by the many tumours described in this review (Eilers & Eisenman 2008). However, not all members of the Myc family possess the same properties. L-Myc for instance has a decreased transformation activity and only few human cancers are associated with an aberrant expression of L-Myc. A recent study reported the creation of iPSCs with L-Myc instead of c-Myc and chimeric mice derived from these L-Myc iPSCs showed reduced tumorigenicity (Nakagawa et al. 2010).
Recently, 21 genes were found to be highly expressed in hESCs as well as in teratomas (Blum et al. 2009). These genes were then credited as known oncogenes, if they were involved in cell-cycle progression, inhibition of apoptosis, signal transduction, transcription or translation. Of these 21 genes, Survivin (BIRC5) was found to be the strongest candidate gene. Survivin is an anti-apoptotic gene that is also involved in mitotic regulation and is expressed in the majority of cancers (Ambrosini et al. 1997; Li et al. 1998; figure 1). It was highly expressed in hESCs and teratomas and downregulated in mature EBs (Blum et al. 2009). From these insights into the gene expression of hESCs and iPSCs, it can be concluded that tumour formation is not dependent on the presence of EC-like cells, but is more an intrinsic property of pluripotent cells. Furthermore, this tumorigenicity of pluripotent cells is reduced upon differentiation. The dilemma now facing investigators is that in reducing the tumorigenicity of stem cells, the very essence of stem cells that makes them useful also has to be reduced, namely self-renewal and pluripotency.
3. Molecular imaging for tracking cell fate
In the last few decades, the field of medical imaging has advanced enormously, giving rise to different in vivo imaging technologies such as magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasound and the charge-coupled device (CCD) camera (Blasberg & Tjuvajev 2003). These modalities have been used in the field of nuclear medicine, in which radiolabelled imaging probes are injected into living subjects to provide information about structure and functionality of different organ systems. Although the field of nuclear medicine gave physicians non-invasive insights into the anatomy of organs, a better understanding of changes in biological processes at the cellular and molecular levels in disease was needed. This need gave rise to the field of molecular imaging, in which the interaction of an imaging probe with a molecular target (DNA, RNA or protein) can be detected using the appropriate imaging system.
In addition to cardiovascular, neuronal and oncological applications, molecular imaging is also used in tracking the survival and proliferation of stem cells in vivo. For the latter purpose, two approaches can be used to label the stem cells before transplantation. The first approach is directly labelling the cells by incubation with a contrast agent such as nanoparticles, magnetic particles or radionuclides. The second approach is by a reporter-gene construct transferred into the cells by a vector-delivery system. Transcription and translation of the reporter gene will result in a reporter protein that can either be an intracellular enzyme, a cell surface receptor, a transmembrane protein or an intracellular storage protein.
3.1. Nanoparticle imaging
Semiconductor quantum dots (QDs) can be manipulated to emit different wavelengths of light and are very photostable, making them interesting options for in vivo tracking of stem cells. In addition, QDs provide brighter and more stable signals for molecular and cellular imaging (Dubertret et al. 2002; Jaiswal et al. 2003; Lin et al. 2007). However, QDs tend to aggregate in the cytosol, are difficult to deliver to the cells and have non-specific binding to multiple molecules.
3.2. Iron particle imaging
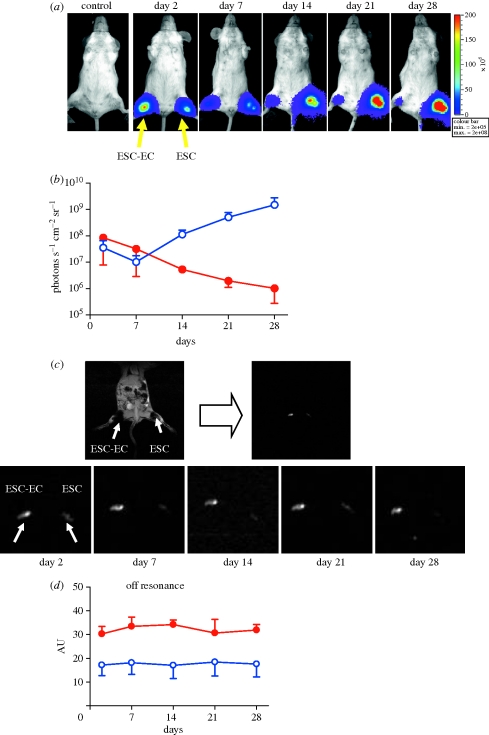
The monitoring of stem cells with MRI is mostly based on endocytosis of a contrast agent such as superparamagnetic iron oxide particles (SPIOs) or ultrasmall superparamagnetic iron oxide particles (USPIOs) that can elicit changes in T2 relaxivity, which allows their detection in vivo. Usage of MRI for the tracking of mesenchymal stem cells labelled by furomoxides has been described by several groups (Kraitchman et al. 2003; Amado et al. 2005; Arai et al. 2006). MRI signals in these studies could be detected from three weeks to two months. However, one concern is that the MRI signals can still be present even when the transplanted cells are no longer alive. This is because the iron particles can be engulfed by nearby cells, especially macrophages (figure 2; Li et al. 2008).
Figure 2.
Comparison of physical labelling versus reporter gene labelling for tracking cell fate. Human ESCs were stably transduced with Fluc-eGFP double-fusion reporter gene as well as incubated with superparamagnetic iron oxide (SPIO). Immunodeficient SCID mice were injected with either undifferentiated ESCs or ESCs differentiated into endothelial cells (ESC–EC). (a) Representative animal injected with 1 × 106 ESC–ECs (right hind limb) shows significant bioluminescence activity at day 2, which decreases progressively over the next four weeks owing to acute donor cell death. In contrast, undifferentiated ESCs (left hind limb) proliferated dramatically over four weeks owing to teratoma formation. (b) Detailed quantitative analysis of bioluminescence signals from all animals transplanted with hESCs (blue open circles) versus hESC–ECs (red filled circles). Signal activity is expressed as photons s−1 cm−2 sr−1. Note that the y-axis is shown as a logarithmic scale. (c) Representative in vivo gradient-recalled echo (GRE) imaging of the animals as shown in (a) and (b). MR signals showed no significant difference between undifferentiated ESCs and differentiated human ESC–ECs from day 2 to day 28. MR image by GRE at day 28 shows bulking expansion of the left hind limb injected with undifferentiated ESCs owing to teratoma formation (arrow head). (d) Detailed quantitative analysis of GRE signals from all animals transplanted with hESCs (blue open circles) and hESC–ECs (red filled circles) (signal activity is expressed as authority unit (AU)). The persistent MRI signal is owing to macrophages engulfing SPIO particles from transplanted cells that have died. Overall, unlike reporter gene imaging, MRI was unable to distinguish cell viability from cell proliferation as its signals remained relatively constant over the four-week time span. Reprinted with permission from Li et al (2008).
3.3. Radionuclide imaging
Stem cell imaging by directly labelling cells with radionuclides is the only direct labelling approach that has been used in human studies, using either bone marrow-derived stem cells (Hofmann et al. 2005) or circulating progenitor cells (Kang et al. 2006; Schachinger et al. 2008). Tracking these cells using SPECT, PET and gamma camera imaging is realized by a labelling agent that is introduced into the cells of interest before transplantation. The major advantage of radionuclide imaging over MRI is its higher imaging sensitivity. However, these cells can only be tracked for several hours to days, depending on their individual decay half-lives (e.g. 99mTc: 6 h; 111In: 2.8 days; 18F: 109 min). Another problem is that radionuclides can leak into other non-target cells and hence give rise to false-positive signals.
3.4. Reporter gene imaging
Reporter gene imaging is based on a reporter gene linked to a promotor/enhancer. These DNA sequences are transcribed into mRNAs and will lead to the production of reporter proteins that will interact with exogenously administered reporter probes. This reaction will lead to a detectable signal, which can be visualized by a CCD camera, SPECT, PET or MRI. As mentioned earlier, this can be (i) an intracellular enzyme that interacts with an exogenous reporter probe to give rise to a detectable signal (Cao et al. 2006), (ii) a cell surface receptor that binds to an exogenously delivered probe (e.g. dopamine 2-like receptor; MacLaren 1999), (iii) a transmembrane protein that mediates in the intracellular uptake of radioisotopes (e.g. sodium/iodide symporter; Miyagawa et al. 2005), or (iv) an intracellular storage protein that actively concentrates endogenous contrast elements (e.g. intracellular iron in ferritin-bound form; Liu et al. 2009a).
For small animal imaging, several reporter gene constructs have been developed. A double-fusion construct containing the firefly luciferase (Fluc) and enhanced green fluorescence protein (eGFP) reporter gene has been used in several studies (van der Bogt et al. 2006; Cao et al. 2007; Lee et al. 2009). In these studies, the Fluc enzyme interacts with the reporter probe d-luciferin to generate low-energy photons (2–3 eV) that can be detected by an ultrasensitive CCD camera. In addition, the eGFP allows confirmation of in vivo imaging signals with traditional ex vivo post-mortem histology. A triple-fusion construct for monitoring the in vivo survival, proliferation and migration of mESCs after intramyocardial transplantation was reported by Cao et al. (2006). This triple-fusion construct used Fluc for high-throughput bioluminescence imaging (BLI), monomeric red fluorescent protein (mRFP) for fluorescence-activated cell sorting and fluorescence microscopy, and herpes simplex virus truncated thymidine kinase (HSVttk) for deep tissue PET imaging (Cao et al. 2006). For PET reporter gene imaging, the HSVttk phosphorylates its radioactive substrate, 9-(4-[18F]-fluoro-3-[hydroxymethyl]butyl)guanine ([18F]-FHBG), intracellularly. The [18F] generates two 511 keV photons that can be detected by PET. Compared with BLI, PET imaging provides higher anatomical details and allows imaging in large animals and humans (Yaghoubi et al. 2009).
Finally, it should be mentioned that stable integration of the various reporter genes (Fluc, eGFP, mRFP and HSVttk) into cellular chromosomes allows the mother cell to pass on the reporter genes to daughter cells. Hence, this genetic approach can truly evaluate survival and proliferation, unlike physical approaches such as iron particle, QD or radionuclide labelling. However, the main disadvantage here is the risk of altering cellular behaviours from gene insertion. Although previous genomic (Wu et al. 2006a) and proteomic (Wu et al. 2006b) studies have not shown any significant adverse effects of mouse ESCs expressing the triple-fusion reporter genes, further optimization using safer site-specific integration approaches is probably needed in the future (Keravala et al. 2009).
3.5. Imaging for tumorigenicity
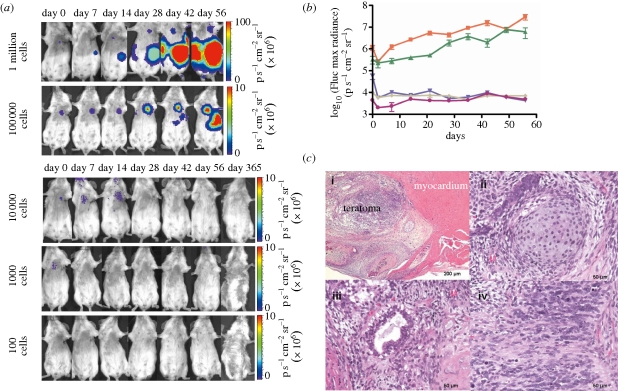
Several imaging modalities have been described here to track the survival and proliferation of pluripotent cells. Owing to the apparent risk of teratoma formation after transplantation of pluripotent cells, it is imperative that molecular imaging be used to detect these tumours at an early stage. In this pre-clinical setting of transplanting pluripotent cells, several groups have looked at the different imaging modalities for the detection of tumour formation in small animals. BLI of mice and rats using Fluc is most commonly used for tumour detection, because it is easy to use and very sensitive (figure 3). Teratoma formation can be detected by a strong BLI signal, even before a palpable tumour is formed (van der Bogt et al. 2006; Lee et al. 2009). Using BLI, Lee et al. have found that they could inject a maximum of 1 × 103 undifferentiated ESCs in the skeletal muscle and 1 × 104 undifferentiated hESCs in the cardiac muscle of immunodeficient mice without teratoma formation (Lee et al. 2009). These findings provided important pre-clinical insights into the effects of hESC numbers and local niches on teratoma development as well as the kinetics of teratoma formation. In addition, BLI together with PET imaging have provided insights into teratoma ablation using a reporter–suicide gene construct (figure 4; Cao et al. 2007). However, Fluc is not suited for clinical application because of the limited penetration of the signal through tissue in larger animals. Furthermore, BLI only gives a two-dimensional image and reveals little spatial information. In contrast, MRI and PET provide a higher spatial resolution and can be used in clinical imaging (Kraitchman et al. 2003; Hinds et al. 2003; Yaghoubi et al. 2009), but with significantly lower detection threshold. Taking into account that every imaging modality has its advantages and drawbacks, combining two or several imaging modalities may provide a better solution. This multimodality approach together with the continuing technological advances will help scientists take tissue engineering and cell-replacement therapies into the clinical phase in the near future.
Figure 3.
Potential for varying numbers of hESCs to form teratomas following intramyocardial injection. (a) Different numbers of undifferentiated Fluc-eGFP-positive hESCs were injected intramyocardially into SCID mice. BLI reveals tumour development in animals transplanted with 1 × 105 or 1 × 106 cells but not in animals receiving 1 × 104 cells or less. (b) Quantitative analysis of the kinetics of teratoma development using BLI. Orange squares, 1 million; green triangles, 100 000; violet inverted triangles, 10 000; grey diamonds, 1000; pink circles, 100. (c) Representative hematoxylin and eosin staining of intramyocardial teratoma: (i) low-power view of intramyocardial teratoma, (ii) cartilage (mesoderm), (iii) mucinous glandular epithelium (endoderm), and (iv) neural tissue (ectoderm) confirm the presence of cellular derivatives from all three germ layers. Reprinted with permission from Lee et al. (2009).
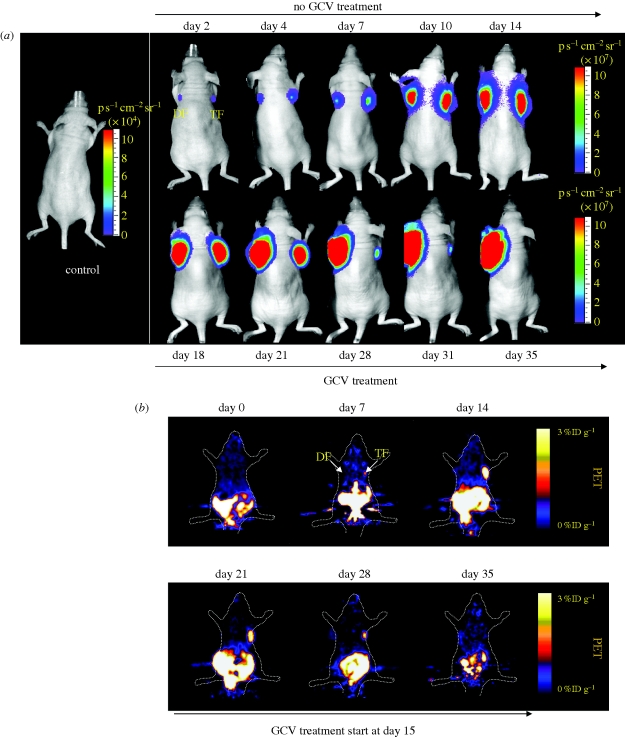
Figure 4.
Ablation of teratoma formation using HSVttk reporter and suicide gene. mESCs were stably transduced with a triple-fusion (ESC-TF) reporter gene that consisted of monomeric red fluorescence protein (mRFP), Fluc and HSVttk or a double-fusion (ESC-DF) reporter gene that consisted of enhanced GFP and Fluc. Stably transduced ESC-TF and ESC-DF cells (1 × 106 each group) were transplanted into right and left shoulders of adult nude mice, respectively. (a) BLI and (b) PET imaging were performed at various time points using d-luciferin and [18F]-FHBG reporter probes, respectively. The marked difference in PET activities is consistent with the expression of HSVttk reporter gene in ESC-TF cells but not ESC-DF cells. Ganciclovir (GCV) treatment was started at week 2 and continued until week 5. After three weeks, ESC-TF cells (right shoulder) were completely ablated by GCV treatment, whereas ESC-DF cells were not affected as they lack HSVttk. Reprinted with permission from Cao et al. (2007).
4. Discussion
The field of regenerative medicine using pluripotent stem cells holds great promise if clinical hurdles can be overcome. One significant problem is the tumorigenic property of pluripotent cells. This is highlighted by a recent case report involving a child that received foetal neural stem cells as a treatment for the neurodegenerative disease ataxia telangiectasia but later developed multifocal glioneural tumour from these transplanted neural stem cells (Amariglio et al. 2009). As gene expression of pluripotent cells becomes widely investigated, more and more correlations in gene expression between pluripotent cells and cancer cells are being found. Not only are some of the pluripotent markers used in the creation of iPSCs directly oncogenic, other pluripotent genes are found to be highly associated with tumour differentiation as well.
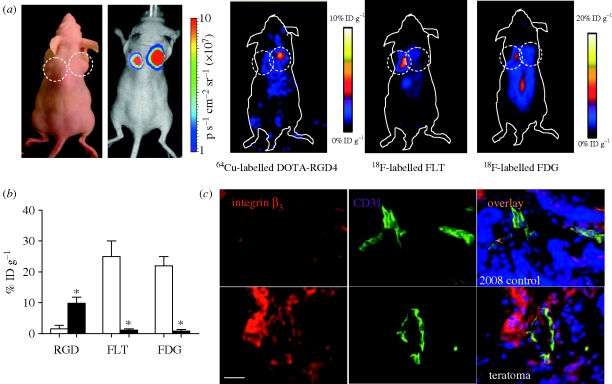
Because of the tumorigenicity of pluripotent cells, tracking them in vivo is of high importance for future clinical application, and several imaging modalities can be used for this purpose. To make regenerative medicine therapies safe, one option is to use a reporter–suicide gene mechanism that would allow for the in vivo tracking of the transplanted pluripotent cells and would target these cells for elimination in the case of tumour formation (figure 4; Cao et al. 2007). Although this study showed ablation of teratomas formed from ESCs, the construct is still based on genetically modifying the pluripotent cells by viral transduction, which has tumorigenic potential as well. Future approaches therefore should focus on site-specific genomic integration approaches such as zinc finger nuclease (Hockemeyer et al. 2009) or phiC31 integrase (Liu et al. 2009b) to minimize potential adverse effects to the cells. Alternatively, one could bypass the reporter gene technique by designing molecular probes that target cell surface receptors of teratoma, as was recently demonstrated using 64Cu-labelled RGD tetramer that targets αvβ3 integrin receptors on hESC-derived teratomas (figure 5; Cao et al. 2009).
Figure 5.
Non-inasive de novo imaging of hESC-derived teratoma formation. MicroPET imaging of 2-deoxy-2-[18F]fluoro-d-glucose ([18F]-FDG), 3′-deoxy-3′-[18F]-fluorothymidine ([18F]-FLT) and 64Cu-DOTA-RGD4 on ESC-derived teratoma. (a) Static photograph and bioluminescence imaging show teratoma formation by H9 hESC-DF cells at the right shoulder and tumour formation by the control 2008 cell line at the left shoulder, which were confirmed by bioluminescence imaging (7.3 ± 2.5 × 107 versus 4.5 ± 1.6 × 107 photons s−1 cm−2 sr−1, respectively, p = n.s.). Specific and prominent uptake of 64Cu-DOTA-RGD4 was found in vascularized teratoma, but not in control human ovarian carcinoma cell line (2008) tumour with low integrin expression (p < 0.01). By contrast, hESC-derived teratomas had low uptake of [18F]-FDG and [18F]-FLT, whereas the 2008 ovarian cancer xenografts had both high [18F]-FDG and [18F]-FLT uptake. (b) Quantification of different microPET imaging patterns between ESC-derived teratoma (filled bars) and control human ovarian carcinoma (unfilled bars). *p < 0.01. (c) Higher expression of β3 integrin (red) was observed in ESC-derived teratoma (bottom) than that in control 2008 tumour (upper), which can be partly co-localized with CD31 staining (green; blue: DAPI; scale bar = 50 µm). Reprinted with permission from Cao et al. (2009).
Ultimately, clear insights into the gene expression of pluripotent cells will allow researchers to select a pluripotent cell population with a reduced tumorigenic signature. In addition, the advances in the field of molecular imaging will allow for (i) labelling of these cells without adverse effects to cellular function, (ii) tracking them in vivo with high sensitivity, spatial resolution, and over long periods of time without loss of signal (Thomson et al. 1998), and (iii) targeting these cells upon tumour formation. These exciting novel developments will pave the way for safe regenerative medicine.
Acknowledgements
This work was in part supported by NIH HL099776, HL091453 and EB009589.
Footnotes
One contribution to a Theme Supplement ‘Translation and commercialization of regenerative medicines’.
References
- Amado L. C., et al. 2005. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl Acad. Sci. USA 102, 11 474–11 479. ( 10.1073/pnas.0504388102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N., et al. 2009. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, e1000029 ( 10.1371/journal.pmed.1000029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G., Adida C., Altieri D. C. 1997. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3, 917–921. ( 10.1038/nm0897-917) [DOI] [PubMed] [Google Scholar]
- Amit M., Carpenter M. K., Inokuma M. S., Chiu C.-P., Harris C. P., Waknitz M. A., Itskovitz-Eldor J., Thomson J. A. 2000. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278. ( 10.1006/dbio.2000.9912) [DOI] [PubMed] [Google Scholar]
- Andrews P. W., et al. 2005. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem. Soc. Trans. 33, 1526–1530. [DOI] [PubMed] [Google Scholar]
- Arai T., et al. 2006. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn. Reson. Med. 55, 203–209. ( 10.1002/mrm.20702) [DOI] [PubMed] [Google Scholar]
- Bae K. M., et al. 2010. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J. Urol. 183, 2045–2053. ( 10.1016/j.juro.2009.12.092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I., Thomson M. W., Carey V. J., Ge R., Bell G. W., Regev A., Weinberg R. A. 2008. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507. ( 10.1038/ng.127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasberg R. G., Tjuvajev J. G. 2003. Molecular-genetic imaging: current and future perspectives. J. Clin. Invest. 111, 1620–1629. ( 10.1172/JCI18855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Benvenisty N. 2008. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 100, 133–158. ( 10.1016/S0065-230X(08)00005-5) [DOI] [PubMed] [Google Scholar]
- Blum B., Benvenisty N. 2009. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle 8, 3822–3830. [DOI] [PubMed] [Google Scholar]
- Blum B., Bar-Nur O., Golan-Lev T., Benvenisty N. 2009. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat. Biotechnol. 27, 281–287. ( 10.1038/nbt.1527) [DOI] [PubMed] [Google Scholar]
- Cao F., et al. 2006. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 113, 1005–1014. ( 10.1161/CIRCULATIONAHA.105.588954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Drukker M., Lin S., Sheikh A. Y., Xie X., Li Z., Connolly A. J., Weissman I. L., Wu J. C. 2007. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells 9, 107–117. ( 10.1089/clo.2006.0E16) [DOI] [PubMed] [Google Scholar]
- Cao F., et al. 2008. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE 3, e3474 ( 10.1371/journal.pone.0003474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Li Z., Lee A., Liu Z., Chen K., Wang H., Cai W., Chen X., Wu J. C. 2009. Noninvasive de novo imaging of human embryonic stem cell-derived teratoma formation. Cancer Res. 69, 2709–2713. ( 10.1158/0008-5472.CAN-08-4122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Fecek C., Lai M. M., Wu W.-C., Chang M., Vasquez A., Pasierb M., Trese M. T. 2009. Fate of embryonic stem cell derivatives implanted into the vitreous of a slow retinal degenerative mouse model. Stem Cells Dev. 18, 247–258. ( 10.1089/scd.2008.0057) [DOI] [PubMed] [Google Scholar]
- Chiou S.-H., et al. 2008. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 14, 4085–4095. ( 10.1158/1078-0432.CCR-07-4404) [DOI] [PubMed] [Google Scholar]
- Cowan C. A., Atienza J., Melton D. A., Eggan K. 2005. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373. ( 10.1126/science.1116447) [DOI] [PubMed] [Google Scholar]
- D'Amour K. A., et al. 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401. ( 10.1038/nbt1259) [DOI] [PubMed] [Google Scholar]
- Draper J. S., et al. 2004. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22, 53–54. ( 10.1038/nbt922) [DOI] [PubMed] [Google Scholar]
- Dubertret B., Skourides P., Norris D. J., Noireaux V., Brivanlou A. H., Libchaber A. 2002. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762. ( 10.1126/science.1077194) [DOI] [PubMed] [Google Scholar]
- Eilers M., Eisenman R. N. 2008. Myc's broad reach. Genes Dev. 22, 2755–2766. ( 10.1101/gad.1712408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. ( 10.1038/292154a0) [DOI] [PubMed] [Google Scholar]
- Hinds K. A., et al. 2003. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102, 867–872. ( 10.1182/blood-2002-12-3669) [DOI] [PubMed] [Google Scholar]
- Hochedlinger K., Yamada Y., Beard C., Jaenisch R. 2005. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477. ( 10.1016/j.cell.2005.02.018) [DOI] [PubMed] [Google Scholar]
- Hockemeyer D., et al. 2009. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857. ( 10.1038/nbt.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wollert K. C., Meyer G. P., Menke A., Arseniev L., Hertenstein B., Ganser A., Knapp W. H., Drexler H. 2005. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 111, 2198–2202. ( 10.1161/01.CIR.0000163546.27639.AA) [DOI] [PubMed] [Google Scholar]
- Jaiswal J. K., Mattoussi H., Mauro J. M., Simon S. M. 2003. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21, 47–51. ( 10.1038/nbt767) [DOI] [PubMed] [Google Scholar]
- Kang W. J., Kang H.-J., Kim H.-S., Chung J.-K., Lee M. C., Lee D. S. 2006. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J. Nucl. Med. 47, 1295–1301. [PubMed] [Google Scholar]
- Keravala A., Lee S., Thyagarajan B., Olivares E. C., Gabrovsky V. E., Woodard L. E., Calos M. P. 2009. Mutational derivatives of PhiC31 integrase with increased efficiency and specificity. Mol. Ther. 17, 112–120. ( 10.1038/mt.2008.241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Pierce G. B., Jr 1964. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 24, 1544–1551. [PubMed] [Google Scholar]
- Knoepfler P. S. 2009. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27, 1050–1056. ( 10.1002/stem.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraitchman D. L., Heldman A. W., Atalar E., Amado L. C., Martin B. J., Pittenger M. F., Hare J. M., Bulte J. W. M. 2003. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation 107, 2290–2293. ( 10.1161/01.CIR.0000070931.62772.4E) [DOI] [PubMed] [Google Scholar]
- Laflamme M. A., et al. 2007. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024. ( 10.1038/nbt1327) [DOI] [PubMed] [Google Scholar]
- Lee A. S., Tang C., Cao F., Xie F., van der Bogt K., Hwang A., Connolly A. J., Robbins R. C., Wu J. C. 2009. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 8, 2608–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Ambrosini G., Chu E. Y., Plescia J., Tognin S., Marchisio P. C., Altieri D. C. 1998. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396, 580–584. ( 10.1038/25141) [DOI] [PubMed] [Google Scholar]
- Li Z., Suzuki Y., Huang M., Cao F., Xie X., Connolly A. J., Yang P. C., Wu J. C. 2008. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells 26, 864–873. ( 10.1634/stemcells.2007-0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., et al. 2007. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 7, 67 ( 10.1186/1472-6750-7-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., et al. 2009a. Noninvasive monitoring of embryonic stem cells in vivo with MRI transgene reporter. Tissue Eng. C 15, 739–747. ( 10.1089/ten.tec.2008.0678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., et al. 2009b. Generation of platform human embryonic stem cell lines that allow efficient targeting at a predetermined genomic location. Stem Cells Dev. 18, 1459–1472. ( 10.1089/scd.2009.0047) [DOI] [PubMed] [Google Scholar]
- MacLaren D. C., et al. 1999. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 6, 785–791. ( 10.1038/sj.gt.3300877) [DOI] [PubMed] [Google Scholar]
- Malashicheva A. B., Kisliakova T. V., Savatier P., Pospelov V. A. 2002. Embryonal stem cells do not undergo cell cycle arrest upon exposure to damaging factors. Tsitologiia 44, 643–648. [PubMed] [Google Scholar]
- Mantel C., et al. 2007. Checkpoint-apoptosis uncoupling in human and mouse embryonic stem cells: a source of karyotpic instability. Blood 109, 4518–4527. ( 10.1182/blood-2006-10-054247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638. ( 10.1073/pnas.78.12.7634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M., Beyer M., Wagner B., Anton M., Spitzweg C., Gansbacher B., Schwaiger M., Bengel F. M. 2005. Cardiac reporter gene imaging using the human sodium/iodide symporter gene. Cardiovasc. Res. 65, 195–202. ( 10.1016/j.cardiores.2004.10.001) [DOI] [PubMed] [Google Scholar]
- Mostert M. C., et al. 1998. Identification of the critical region of 12p over-representation in testicular germ cell tumors of adolescents and adults. Oncogene 16, 2617–2627. ( 10.1038/sj.onc.1201787) [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Takizawa N., Narita M., Ichisaka T., Yamanaka S. 2010. Promotion of direct reprogramming by transformation-deficient Myc. Proc. Natl Acad. Sci. USA 107, 14 152–14 157. ( 10.1073/pnas.1009374107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum J., et al. 2007. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 21, 1345–1357. ( 10.1096/fj.06-6769com) [DOI] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. 2007. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317. ( 10.1038/nature05934) [DOI] [PubMed] [Google Scholar]
- Oosterhuis J. W., Looijenga L. H. J. 2005. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222. ( 10.1038/nrc1568) [DOI] [PubMed] [Google Scholar]
- Palma I., Peñab R.-Y., Contrerasb A., Ceballos-Reyesc G., Coyoteb N., Erañab L., Kofman-Alfaroa S., Queipoa G. 2008. Participation of OCT3/4 and β-catenin during dysgenetic gonadal malignant transformation. Cancer Lett. 263, 204–211. ( 10.1016/j.canlet.2008.01.019) [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Jr, Dixon F. J., Jr, Verney E. L. 1960. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab. Invest. 9, 583–602. [PubMed] [Google Scholar]
- Schachinger V., et al. 2008. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation 118, 1425–1432. ( 10.1161/CIRCULATIONAHA.108.777102) [DOI] [PubMed] [Google Scholar]
- Sperger J. M., et al. 2003. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl Acad. Sci. USA 100, 13 350–13 355. ( 10.1073/pnas.2235735100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. C. 1964. Experimental production of testicular teratomas in mice. Proc. Natl Acad. Sci. USA 52, 654–661. ( 10.1073/pnas.52.3.654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. C. 1967. Origin of testicular teratomas from primordial germ cells in mice. J. Natl Cancer Inst. 38, 549–552. [PubMed] [Google Scholar]
- Stevens L. C. 1968. The development of teratomas from intratesticular grafts of tubal mouse eggs. J. Embryol. Exp. Morphol. 20, 329–341. [PubMed] [Google Scholar]
- Stevens L. C., Hummel K. P. 1957. A description of spontaneous congenital testicular teratomas in strain 129 mice. J. Natl Cancer Inst. 18, 719–747. [PubMed] [Google Scholar]
- Tada M., Takahama Y., Abe K., Nakatsuji N., Tada T. 2001. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 11, 1553–1558. ( 10.1016/S0960-9822(01)00459-6) [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. ( 10.1016/j.cell.2006.07.024) [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. ( 10.1016/j.cell.2007.11.019) [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. ( 10.1126/science.282.5391.1145) [DOI] [PubMed] [Google Scholar]
- van der Bogt K. E., Swijnenburg R. J., Cao F., Wu J. C. 2006. Molecular imaging of human embryonic stem cells: keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle 5, 2748–2752. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A. E., McWhir J., Kind A. J., Campbell K. H. 1997. Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813. ( 10.1038/385810a0) [DOI] [PubMed] [Google Scholar]
- Wu J. C., et al. 2006a. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol. Genomics 25, 29–38. ( 10.1152/physiolgenomics.00254.2005) [DOI] [PubMed] [Google Scholar]
- Wu J. C., et al. 2006b. Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics 6, 6234–6249. ( 10.1002/pmic.200600150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Cao F., Sheikh A. Y., Li Z., Connolly A. J., Pie X., Li R.-K., Robbins R. C., Wu J. C. 2007. Genetic modification of embryonic stem cells with VEGF enhances cell survival and improves cardiac function. Cloning Stem Cells 9, 549–563. ( 10.1089/clo.2007.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Hiona X., Lee A. S., Cao F., Huang M., Li Z., Cherry A., Pei X., Wu J. C. In press Effects of long term culture on human embryonic stem cell aging. Stem Cells Dev. ( 10.1089/scd.2009.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoubi S. S., Jensen M. C., Satyamurthy N., Budhiraja S., Paik D., Czernin J., Gambhir S. S. 2009. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 6, 53–58. ( 10.1038/ncponc1278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Zhang Z. J., Oldenburg M., Ayala M., Zhang S. C. 2008. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 26, 55–63. ( 10.1634/stemcells.2007-0494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. ( 10.1126/science.1151526) [DOI] [PubMed] [Google Scholar]
- Zhao X. Y., et al. 2009. iPS cells produce viable mice through tetraploid complementation. Nature 461, 86–90. ( 10.1038/nature08267) [DOI] [PubMed] [Google Scholar]