Abstract
Individual differences in circadian phase preference (“chronotype”) are linked to sleep schedule variability, psychosocial functioning, and specific properties of the circadian clock. While much is known about the development, distribution, and variability of chronotype in adolescents and adults, assessment in prepubertal children has been hindered by a lack of appropriate, reliable, and valid measures. This study presents a detailed description of the assessment of children’s chronotype by the Children’s ChronoType Questionnaire (CCTQ). The CCTQ is a parent-report, 27-item mixed-format questionnaire resulting in multiple measures of chronotype in 4- to 11-yr-old children: the midsleep point on free days (MSF), a morningness/eveningness scale (M/E) score, and a five-point chronotype (CT) score. The study provides validity data using actigraphy as well as test-retest reliability data for all three chronotype measures and sleep/wake parameters. Overall, the findings indicate moderate to strong agreement between the three measures, adequate associations between chronotype measures and sleep/wake parameters assessed by actigraphy, and excellent temporal stability (reliability). (Author correspondence: oskar.jenni@kispi.uzh.ch)
Keywords: Chronotype, Midsleep point, Morningness/eveningness, Validity, Children
INTRODUCTION
Chronotype is an individual difference characteristic reflecting the time of day at which individuals are “at their best” (Guthrie, 1995; Kerkhof, 1985). While some people prefer to wake up early in the morning and are most alert in the first part of the day, others prefer to wake up later, as their peak time of the day is in the evening, and they prefer to go to bed late at night (Cofer et al., 1999; Tankova et al., 1994). Studies on adults and adolescents show morning types (also called “larks”) have an earlier sleep schedule (e.g., Carskadon et al., 1993; Horne & Östberg, 1976; Kerkhof & Lancel, 1991; Mecacci & Zani, 1983), earlier circadian temperature phase (e.g., Bailey & Heitkemper, 2001; Duffy et al., 1999; Kerkhof, 1991; Kerkhof & Van Dongen, 1996; Mongrain et al., 2004), and earlier melatonin secretion pattern (Laberge et al., 2000), and report fewer difficulties with sleepiness and attention (Giannotti et al., 2002) than evening types (“owls”). Thus, individual differences in chronotype are linked to sleep schedule variability, psychosocial functioning, and specific properties of the circadian clock. Moreover, chronotype, at least in young adults, has recently been linked with specific personality traits (e.g., Digdon & Howell, 2008; Jankowski & Ciarkowska, 2008; Soehner et al., 2007; Tonetti et al., 2009).
Chronotype is also referred to as ‘morningness/eveningness’ (M/E) preference, which reflects an individual’s standing on a continuum between two extremes (Natale & Cicogna, 2002). Chronotype is assessed through self-report questionnaires. In adults, Horne and Östberg’s Morningness-Eveningness Questionnaire (MEQ; Horne & Östberg, 1976) estimates M/E preference by asking respondents about their preferred timing of sleep and daily activities. The MEQ has been validated across a variety of samples (e.g., Chelminski et al., 1997; Posey & Ford, 1981; Taillard et al., 2004), translated into several languages (e.g., Mecacci & Zani, 1983), and revised into other versions, such as Smith’s Composite Scale of Morningness (CSM; Smith et al., 1989) and Adan and Almirall’s rMEQ (Adan & Almirall, 1991). To evaluate M/E preference in adolescents, Carskadon and colleagues (1993) modified adult measures of chronotype (Horne & Östberg, 1976; Smith et al., 1989) into an adolescent-friendly self-report of daily preference. In contrast to these multi-item measures, Roenneberg and colleagues (2003) developed the Munich ChronoType Questionnaire (MCTQ), which estimates an individual’s circadian preference by a single phase-reference point, the mid-sleep point on free days (MSF). The self-report MCTQ has been used in adults, adolescents, and children as young as 10 yrs of age (Roenneberg, n. d.). The MCTQ’s validity in adults and adolescents is evidenced by strong concordance with MEQ scores (MSF: r = −.73; Zavada et al., 2005) and CSM (MSF: r = −.62; Randler, 2008b). Reliability and validity data for the MSF in children, however, have not been reported. Furthermore, a parent-reported version for the assessment of chronotype in prepubertal children is not currently available.
The assessment of individual chronotype is important not only for the diagnosis and treatment of circadian sleep disorders (Baehr et al., 2000) and for predicting the ability to adapt to specific work schedules (Costa et al., 1989, 2006; Pisarski et al., 2006), but also for improving daytime performance of individuals by matching sleep schedules to circadian biology (Silva, 2008). In particular, extreme evening types are at higher risk than morning types of not obtaining sufficient sleep and of performing poorly due to discordance between their individual circadian rhythm and social demands, such as work and school schedules (Takeuchi et al., 2001; Wittman et al., 2006). Evidence is also accumulating that subjects have more difficulties in maintaining sleep when sleep is scheduled at adverse circadian phases (Silva, 2008).
Overall, little is known about the development, distribution, and variability of chronotype in prepubertal children. Childhood sleep problems, such as bedtime resistance, sleep onset delay, prolonged nighttime wakings, and difficulties waking in the morning, are common parental complaints, affecting approximately 25% of children during the first 10 yrs of life (Jenni et al., 2005b; Owens, 2007). Some have argued that behavioral sleep problems during childhood may occur because individual sleep and circadian characteristics are not matched with parental expectations or family and school schedules (Jenni & O’Connor, 2005; Takeuchi et al., 2001). Although individual differences in chronotype may contribute to the development and maintenance of sleep problems in prepubertal children, assessment of this construct has been hindered by lack of appropriate, reliable, and valid measures.
Based upon the previous work of Roenneberg and colleagues (Roenneberg et al., 2003, 2004; Zavada et al., 2005) and Carskadon and colleagues (Carskadon et al., 1993), we developed the Children’s Chronotype Questionnaire (CCTQ). The CCTQ is a 27-item, mixed-format parent-report scale that provides three individual measures of chronotype in 4- to 11-yr-old children: MSF, a multi-item morningness/ eveningness scale (M/E), and a five-point chronotype item (CT). The purpose of this study was as follows:
to describe the chronotypes of prepubertal children as assessed by these three individual measures;
to examine the concordance (validity) between children’s chronotype measures and sleep/wake parameters (parental reports and actigraphic estimates);
to assess associations between the three children’s chronotype measures; and
to examine test-retest reliability of chronotype measures and sleep/wake parameters in children.
METHODS
Subjects
Children were recruited as part of three individual studies in the greater Zurich area of Switzerland. In the first two studies, researchers recruited 135 children from 34 of 270 Zurich kindergartens (children between 4 and 7 yrs of age attend kindergarten for 2 yrs for about 4 h/ day, with school start times between 08:15 and 08:30 h.). Of these children, 117 were enrolled in the study and included in the data analysis. The first study was conducted in 2006/2007 (see Werner et al., 2008), and the second study was completed in 2008. In the third study, 46 children were recruited from primary schools in the greater Zurich area (children attend primary school five days/week for about 6 h/day, starting between 07:45 and 08:15 h) and from a special school program for gifted children; 35 of these children were included in this analysis (n = 19 recruited from primary schools, n = 16 recruited from school program for gifted children). In total, parents of 179 children agreed to participate after initial contact, and 152 children were selected for the analysis (75 girls and 77 boys, mean age 6.70 ± 1.5 [SD] yrs, range = 4–11 yrs). At the time of assessment, 80 children (53%) were the eldest sibling or an only child, and 72 children (47%) had an older sibling. None of the children took regular naps.
Overall, 29 children were excluded for any of the following reasons:
parents had insufficient language skills or the questionnaire was not filled out completely (n = 12);
several families reported data for two or more children, but only one child was included in the data analysis based upon random selection (n = 16); and
children had a self-reported pubertal development score ≥3 (n = 1; Carskadon et al., 1993).
The actigraphic validity analysis included data from a sub-sample of 85 children (50 kindergarten and 35 primary school children). Their parents filled out the questionnaire prior to actigraphy monitoring, and devices were returned by postal mail. The test-retest reliability analysis was performed on a sub-sample of 43 children whose parents received and returned questionnaires on two occasions by postal mail.
All families agreeing to participate received a letter including a description of the investigation and a study enrolment form. The study procedure was explained by the researchers, and written informed consent was obtained from all parents. Families participating in either of the first two studies (see above) were rewarded with a gift certificate from a book shop. All studies were approved by the local research ethics committee, were performed according to the Declaration of Helsinki, and met the ethical standards of this journal (Portaluppi et al., 2008).
Measures
The Children’s ChronoType Questionnaire (CCTQ)
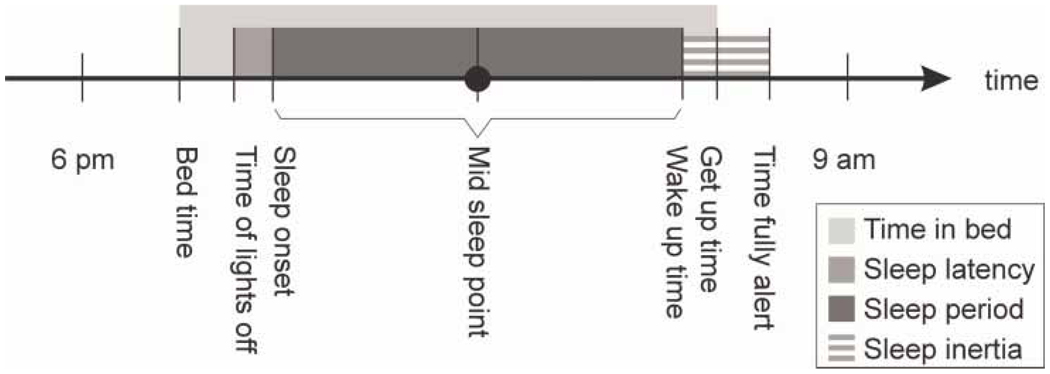
The CCTQ (see Appendix) is an adaptation of the Munich Chrono-Type Questionnaire (MCTQ; Roenneberg, 2004) and Morningness/ Eveningness Scale for Children (MESC; Carskadon et al., 1993). The CCTQ includes a short demographics section about age, sex, birth order, family size, and education level. Parents respond to a number of open-ended questions about sleep/wake parameters for both scheduled and free days (bedtime, time of lights-off, sleep latency in min, wake-up time, get-up time, time fully alert). Scheduled days (SC) are defined as those when the children’s sleep/wake patterns are directly influenced by individual or family activities (e.g., school or athletics). Free days (FR) are defined as those when the children’s sleep/wake patterns are “free” from any influence of individual or family activities. Computed variables included (see Figure 1):
time in bed, defined as the difference between bedtime and get-up time;
sleep onset, defined as sleep latency added to time of lights-off;
sleep period, defined as the difference between sleep onset in the evening and wake-up time in the morning;
sleep inertia, defined as the difference between wake-up time and time being fully alert; and
midsleep point, defined as sleep onset + sleep period/2.
APPENDIX.
Children’s ChronoType Questionnaire (CCTQ)
| Demographics: Please answer the following questions or choose the best answer. | |
|---|---|
| Individual completing the questionnaire: □ Mother □ Father □ Other_________ | |
| Today’s Date: ____/____/____ (day/month/year) | Child’s Sex: □ Male □ Female |
| Child’s Birth Date: ____/___/___ (day/month/year) Child’s Age: _______years |
Child’s Birth Order: _________ Is he/she an Only Child? □ Yes □ No |
| How many children are included in your nuclear family? ________ Do all children in your family have the same biological parents? □ Yes □ No | |
| Child’s current level of education: □ Preschool □ Kindergarten □ Grade ________ If he/she attends school, how many days/week? _________ |
□ Not attending school How many hours/day? _________ |
| Does he/she go to Day Care or After-School Care? If yes, how many days/week? __________ |
□ Yes □ No How many hours/day? __________ |
| Directions: The following questions ask about sleep/wake patterns during “Scheduled Days” in contrast to “Free Days”. Think about your child’s behavior during recent weeks when answering these questions. For questions with changing conditions (e.g., child goes to day care at 7:00am 1 day/week and 9:00am 3 days/week), fill in or select the most frequent or common answer. | |
|
Scheduled Days Child’s sleep-wake pattern is directly influenced by individual or family activities (e.g., by school, day care, work, athletics etc.) | |
| On Scheduled Days, my child … | |
| 1. …wakes up at _____:_____ am | |
| 2. …regularly wakes up: □ by him/herself □ with help from a family member □ with an alarm clock | |
| 3. …gets up at ______:_____ am | |
| 4. …is fully awake by_____:_____ am | |
| 5. …takes regular naps: □ Yes □ No If yes, he/she naps ______ days/week. If no, why does he/she not nap? If yes, he/she sleeps for ____ minutes/nap. _____________________________ On nights before Scheduled Days… | |
| 6. …my child goes to bed (body in bed) at _____:_____pm | |
| 7. …my child is ready to fall asleep (lights turned out) at ______:_____ pm | |
| 8. …it takes him/her _____ minutes to fall asleep (after lights turned out). | |
|
Free Days Child’s sleep/wake pattern is “free” from the influence of individual or family activities (e.g., by school, day care, work, athletics etc.) | |
| On Free Days, my child … | |
| 9. …normally wakes up at ____:____ am | |
| 10. …wakes at his/her normal time on scheduled days, but then goes back to sleep after waking: □ Yes □ No if yes, my child goes back to sleep for ___ minutes after waking. | |
| 11. …gets up by ____:____ am | |
| 12. …is fully awake by____:____ am | |
| 13. …takes regular naps: □ Yes □ No If yes, he/she naps ______ days per week. If no, why does he/she not nap? If yes, he/she sleeps for _____ minutes per nap. _________________________ | |
| On nights before Free Days… | |
| 14. …my child goes to bed (body in bed) at ____:____ pm | |
| 15. …my child is ready to fall asleep (lights turned out) at _____:_____ pm | |
| 16. …it takes him/her ______ minutes to fall asleep (after lights turned out). | |
| Directions: For each of the following questions, please select the answer that best describes your child. Make your judgments based on how the behavior of your child was in recent weeks. There are no “right” or “wrong” answers. | |
| 17. *If your child has to be awakened, how difficult do you find it to wake your child up in the morning? a. very difficult b. fairly difficult c. moderate difficult d. slightly difficult e. not at all difficult/my child has never to be awakened | |
| 18. *How alert is your child during the first half hour after having awakened in the morning? a. not at all alert b. slightly alert c. moderate alert d. fairly alert e. very alert | |
| 19. Considering your child’s “feeling best” rhythm, at what time would your child get up if he/she could decide by him/herself and if he/she were entirely free to plan the day (e.g., vacation)? a. prior to 6:30 am b. 06:30–7:14 am c. 7:15 – 9:29 am d. 9:30 – 10:14 am e. after 10:15 am | |
| 20. Considering your child’s “feeling best” rhythm, at what time would your child go to bed if he/she could decide by hirn/herself and if he/she were entirely free to plan the next day (e.g., weekend)? a. prior to 6:59 pm b. 7:00 – 7:59 pm c. 8:00–9:59 pm d. 10:00 – 10:59 pm e. after 11:00 pm | |
| 21. Let’s assume that your child has to be at peak performance for a test that will be mentally exhausting for 2 hours. Considering your child’s “feeling best” rhythm and that you are entirely free to plan your child’s day, which ONE of the three time intervals would you choose for the test? a. 7:00 – 11:00 am b. 11:00 am - 3:00 pm c. 3:00 – 8:00 pm | |
| 22. Let’s assume that you have decided to enroll your child in an athletic activity (e.g., swimming). The only class available meets twice a week at 7 to 8 am. How do you think he/she will perform? a. would be in very good form b. would be in good form c. would be in reasonable form d. would find it difficult e. would find it very difficult | |
| 23. At what time in the evening does your child seem tired and in need of sleep? a. prior to 6:30 pm b. 6:30 – 7:14 pm c. 7:15 – 9:29 pm d. 9:30–10:14pm e. after 10:15 pm | |
| 24. *If your child had to get up every day at 6 am, what do you think it would that be like for him/her? a. very difficult b. rather difficult c. moderate difficult d. a little difficult, but not a great problem e. not at all difficult | |
| 25. *If your child always had to go to bed at ________, what do you think it would be like for him/her? (for 2 years old: 06:00 pm; for 2 to 4 years old: 06:30 pm; for 4 to 8 years old: 07:00 pm; for 8 to 11 years old: 07:30 pm) a. very difficult b. rather difficult c. moderate difficult d. a little difficult, but not a great problem e. not at all difficult | |
| 26. When your child wakes up in the morning, how long does it take to be fully awake? a. 0 minutes (i.e., immediately) b. 1 to 4 minutes c. 5 to 10 minutes d. 11 to 20 minutes e. ≥ 21 minutes | |
| Directions: After answering the above questions, you may have a feeling which “Chronotype” or “Time-of-Day type” your child is. For example, if your child would like to sleep quite a bit longer on “Free Days” compared to “Scheduled Days” or if it is difficult for your child to get out of bed on Monday mornings, then he/she is more likely to be an Evening Type person (a “Night Owl”). If your child, however, regularly wakes up and feels perky once he/she gets out of bed, and your child prefers to go to bed rather early than late, then he/she is more likely a Morning Type person (a “Morning Lark”). Please categorize your child using one of the following choices. Please choose only one category! | |
| 27. My child is… □ Definitely a Morning Type □ Rather a Morning Type than an Evening Type □ Neither a Morning nor an Evening Type □ Rather an Evening Type than a Morning Type □ Definitely an Evening Type □ I do not know | |
The M/E score is derived by adding points from answers 17–26 (a=1, b=2, c=3, d=4, d=5), except as indicated by *, where point values has to be reversed.
FIGURE 1.
Parent-reported sleep/wake parameters computed from items on the Children’s Chrono-Type Questionnaire (CCTQ).
The CCTQ includes three different parent-report measures of children’s chronotype. The midsleep point on free days (MSF) is computed as the midpoint of the sleep period only on free days. As many individuals compensate for a sleep deficit accumulated during scheduled days by sleeping in on free days (sleep deficit acting as a confounder for sleep period on free days), Roenneberg corrected MSF for the confounding sleep deficit based on the individual weekly average sleep need (MSFsc). The average sleep need is defined as
(for correction algorithm for MSF, see supplement to Roenneberg et al., 2004). The Morningness/Eveningness (M/E) scale score is derived from responses to 10 questions (see Appendix items 17–26) about preferred timing of going to bed, getting up in the morning, taking a cognitive test, and completing physical activities, as well as the child’s most prevalent behavior in recent weeks (e.g., sleepiness after awakened in the morning and in the evening). M/E scale-scores range from 10 (extreme morningness) to 49 (extreme eveningness). Morning types are classified by a M/E scale score of ≤23, intermediate types by a score of 24–32, and evening types by a score ≥33. Cronbach’s alpha for the 10 items (.81) was similar to that for the adolescent version of Carskadon and colleagues (1993); corrected item-total correlations were on average .49 and ranged from .31 to .71. Chronotype (CT) is a single-item measure. Parents read a short description of different chronotypes and selected one of five categories that best represents their child’s circadian phase preference (i.e., definitely a morning type, rather a morning type than an evening type, neither/nor type, rather an evening type than a morning type, or definitely an evening type). CT scores range from 1 (definitely a morning type) to 5 (definitely an evening type). This measure has been widely used in sleep and circadian research, such as Horne and Östberg (1976) and Roenneberg et al. (2003), with response set varying from 3 to 7 categories.
Pubertal Development
All children were assessed by the self-rating scale for pubertal development (Carskadon et al., 1993). The scale is an adaptation of the interview-based puberty rating scale by Peterson (1984), including five items for rating physical development, an overall maturation measure, and a categorical maturation score designed to be similar to Tanner (1962) staging categories. The puberty scores are categorized separately for girls and boys as pre-pubertal, early pubertal, mid-pubertal, late pubertal, and post-pubertal. Children with a pubertal score ≥3 were excluded from this data analysis, as sleep regulatory mechanisms change during the course of puberty (Carskadon et al., 1993).
Actigraphy
A total of 85 children were monitored continuously at home with an actigraph (AW4, Actiwatch Plus®, Cambridge Neurotechnology, Cambridge, UK) for 6 to 14 consecutive nights and days (median = 8). Data were analyzed in 1 min epochs and translated into sleep measures by the software Actiware 5® using the scoring procedures described by Acebo et al. (2005). The scoring interval was defined as 30 min before the reported bedtime to 30 min after the reported rising time. Data were evaluated at a medium-sensitivity threshold. Actigraphic sleep measures for the analysis included the following:
bedtime, as indicated in the diary;
sleep start time, defined as the first min of at least three consecutive min of scored sleep after bedtime;
sleep end time, defined as the last min of at least five consecutive min of scored sleep just prior to the reported rise time;
assumed sleep (“nocturnal sleep period”), defined as the difference between sleep start time and sleep end time;
sleep latency, defined as the difference between bedtime and sleep start time; and
midsleep point, defined as sleep start time + assumed sleep/2.
Actigraphs were attached to the non-dominant wrist of the children and removed only during times when it could get wet. Children were monitored during the academic year, including one or two weekends, but not during school vacation. Data for each actigraph measure were aggregated (averaged) separately for weekday (scheduled, SC) and weekend (free, FR) nights, which were used as the units of analysis. All public vacation days were counted as free days. The total number of monitored nights was 917 (SC range = 4–14; FR range = 1–6). Individual actigraphy nights were discarded if the child was sick (4/917 nights), if the actigraph was off for all or parts of the night (3/917), if parents had forgotten to fill out the diary (9/917), or if the diary indicated unusual external motion that would mask sleep, such as sleeping in the car (2/917).
Diary
Parents completed a sleep diary on each study day that sleep was assessed with actigraphy. Diary reports were recorded in 15 min intervals. Bedtime was indicated by a greater-than sign (>); estimated sleep start and sleep end were noted by starting and ending a continuous line. Parents also noted any type of activity that may have influenced the scoring of actigraphic data, such as illness, intervals the actigraph was off of the child, or car rides (see Acebo et al., 2005). This diary has been used clinically at our center for several years (Werner et al., 2008).
Statistical Analysis
Descriptive results are presented as means and standard deviations (SD). Because parents commonly reported their children’s sleep/wake times to the nearest full, half, or quarter hour, rather than to the nearest min (e.g., 8:15 p.m. bedtime rather than 8:07 p.m. bedtime), many variables from the CCTQ showed significant skewness and/or kurtosis. As a consequence, we used nonparametric tests for all parameters to test equality of means (Wilcoxon-Test) and Spearman correlations to measure associations. Simple and quadratic regression (quadratic term was never significant) and analysis of variance were used to describe the relationship between sleep/wake parameters and demographic variables (age, sex, birth order, and type of day). Effect size in SD units (Cohen’s d) was computed for actigraphy and questionnaire mean (M) comparisons and for scheduled and free day mean (M) comparisons (d=Msample1 – Msample2/ SDpooled) (Cohen, 1962). Test-retest reliability coefficients were determined with Pearson correlations, except for the chronotype measure CT, which was assessed with Spearman correlations. All analyses were performed with two-tailed tests, and p < 0.05 was considered significant. SPSS (14.0J for Windows; SPSS Inc., Chicago, Illinois, USA) was used for all statistical analyses.
RESULTS
Parental Reports of Children’s Sleep/Wake Parameters on Scheduled (SC) and Free Days (FR)
Descriptive statistics for the sleep/wake parameters are shown separately for scheduled and free days in Table 1 (parameters are illustrated in Figure 1). Mean differences between scheduled and free days were significant for all sleep/wake parameters. On free days, children went to bed later and got up at later times, slept about 20 min longer, and had shorter sleep latencies and sleep inertia estimates than on scheduled days.
TABLE 1.
Descriptive statistics for parent-reported sleep/wake parameters on scheduled and free days from the Children’s ChronoType Questionnaire (CCTQ) and linear regression coefficients by age (n = 152)
| Scheduled days* |
Free days* |
Statistics | Scheduled days† |
Free days† |
|
|---|---|---|---|---|---|
| Bedtime | 20:17 (0:31) | 20:47 (0:46) | p < 0.001, d = 0.77 | 0:09 (0:01)‡ | 0:16 (0:02)‡ |
| Time of lights-off | 20:35 (0:36) | 21:02 (0:48) | p < 0.001, d = 0.66 | 0:11 (0:02)‡ | 0:18 (0:02)‡ |
| Sleep latency | 0:12 (0:09) | 0:11 (0:10) | p ≤ 0.001, d = 0.07 | 0:01 (0:00:29) | |
| Sleep onset | 20:47 (0:38) | 21:13 (0:50) | p < 0.001, d = 0.59 | 0:13 (0:02)‡ | 0:19 (0:02)‡ |
| Wake-up time | 7:07 (0:25) | 7:51 (0:46) | p < 0.001, d = 1.23 | − 0:01 (0:01) | 0:09 (0:02)‡ |
| Get-up time | 7:16 (0:25) | 8:00 (0:48) | p < 0.001, d = 1.20 | −0:00:04(0:01) | 0:11 (0:02)‡ |
| Time fully alert | 7:29 (0:36) | 8:05 (0:53) | p < 0.001, d = 0.81 | − 0:01 (0:02) | 0:10 (0:03)‡ |
| Sleep period | 10:20 (0:40) | 10:38 (0:45) | p < 0.001, d = 0.43 | − 0:11 (0:02)‡ | |
| Time in bed | 10:59 (0:34) | 11:14 (0:47) | p < 0.001, d = 0.37 | − 0:10 (0:02)‡ | −0:05 (0:02)‡ |
| Sleep inertia | 0:22 (0:23) | 0:14 (0:20) | p < 0.001, d = 0.33 | 0:01 (0:01) | |
| Midsleep point | 1:58 (0:26) | 2:32 (0:43) | p < 0.001, d = 1.00 | 0:06 (0:01)‡ | 0:14 (0:02)‡ |
| MSFsc | 2:26 (0:40) | 0:13 (0:02)‡ | |||
Reported as mean (standard deviation), in hours:minutes
Reported as slope coefficient (standard error). When no interaction between age and type of day (scheduled versus free) existed, the common slope was reported (analysis of covariance); otherwise, separate slopes are reported.
Significant effect of age (p ≤ .05)
Because age is a predictor of many sleep/wake parameters, age effects were examined by simple regression. Table 1 reports coefficients of age with standard error (SE). Older children went to bed later, had later sleep onsets and shorter sleep periods, and spent less time in bed than younger children. Sleep latency and sleep inertia on both (SC, FR) types of days were not influenced by age. Wake-up time, get-up time, and time fully alert were later for older children only on FR days. After controlling for age, girls had longer sleep latencies and woke up later than boys (p < .05). Sleep/wake parameters were not associated with birth order.
Children’s Chronotype Measures
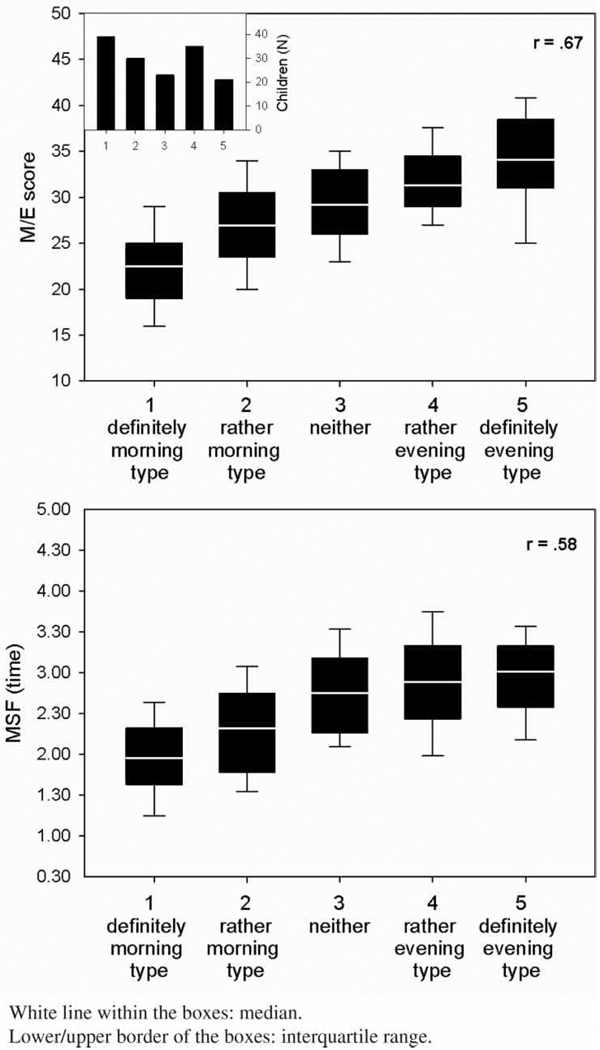
MSF and MSFsc did not show any significant deviation from a normal Gaussian distribution. Although statistically significant (p < .001), MSF and MSFsc means differed by only 6 min [.10 h; MSF = 2.53 (.71) versus MSFsc = 2.43 (.67)], with a small effect size (d = .15). MSF and MSFsc were both significantly related to children’s age (older children had later MSFs) and sex (girls had later MSFs than boys), but not to birth order. The distribution of the M/E score did not show any significant deviation from normality, with a mean of 28.2 (SD = 6.0; range = 15 to 43). The M/E score was not associated with age, sex, or birth order. The distribution of the CT is presented in the inset of Figure 2. Thirty-nine parents (26%) classified their children as definitely morning type, 30 (20%) as rather morning type than evening type, 23 (15%) as neither nor type, 35 (23%) as rather evening type than a morning type, and 21 (14%) as definitely evening type. Age, sex, and birth order were not related to CT.
FIGURE 2.
Distribution of ChronoType (CT) scores (upper left insert) and associations with morningness/eveningness (M/E) scores and midsleep point on free days (MSF).
Concordance between Parental Report of Sleep/Wake Parameters and Chronotype Measures
Validity of the three measures of children’s chronotype was first examined by determining concordance with sleep/wake parameters (see Table 2). All three measures of children’s chronotype were significantly related to time going to bed, time of lights-off, sleep latency, sleep onset, wake-up time, get-up time, and time fully alert. Across chronotype measures, the highest correlations were with MSFsc, such as for sleep onset (r = .93) and time of lights-off (r = .92) on free days. Later chronotypes had later sleep start times, later get-up times, and later times to be fully alert. While chronotype as measured by M/E or CT was not related to sleep period on SC or FR days, chronotype as measured by MSF was related to sleep period on SC days (r = −.37), and MSFsc was related to sleep period on both type of days (SC: r = −.32; FR: r = −.24).
TABLE 2.
Spearman correlations between parent-reported sleep/wake parameters and midsleep point on free days (MSF), corrected midsleep point on free days (MSFsc), morningness/eveningness (M/E) scores, and chronotype (CT) scores (n = 152)
| MSF | MSFsc | M/E score | CT | ||
|---|---|---|---|---|---|
| Bedtime | SC | 0.59* | 0.57* | 0.33* | 0.23* |
| FR | 0.76* | 0.82* | 0.31* | 0.31* | |
| Time of lights-off | SC | 0.68* | 0.64* | 0.43* | 0.36* |
| FR | 0.86* | 0.92* | 0.40* | 0.40* | |
| Sleep latency | SC | 0.31* | 0.31* | 0.23† | 0.29* |
| FR | 0.18† | 0.19† | 0.25† | 0.29* | |
| Sleep onset | SC | 0.70* | 0.66* | 0.46* | 0.41* |
| FR | 0.87* | 0.93* | 0.42* | 0.45* | |
| Wake-up time | SC | 0.46* | 0.45* | 0.52* | 0.40* |
| FR | 0.89* | 0.75* | 0.63* | 0.59* | |
| Get-up time | SC | 0.51* | 0.47† | 0.55* | 0.41† |
| FR | 0.87* | 0.75* | 0.63* | 0.57* | |
| Time fully alert | SC | 0.53* | 0.48† | 0.68* | 0.50* |
| FR | 0.82* | 0.69* | 0.66* | 0.59* | |
| Sleep period | SC | − 0.37* | − 0.32* | −0.12 | −0.18 |
| FR | −0.05* | − 0.24† | 0.16 | 0.13 | |
| Time in bed | SC | −0.16 | −0.16 | 0.11 | 0.06 |
| FR | 0.08 | −0.09 | 0.29† | 0.22† | |
| Sleep inertia | SC | 0.28† | 0.21† | 0.45* | 0.27* |
| FR | −0.00 | −0.03 | 0.28* | 0.10 | |
| M/E-score | 0.584* | 0.516* | 0.672* | ||
| CT | 0.581* | 0.524* | 0.672* |
Note. Correlation coefficients are reported for scheduled (SC) and free (FR) days.
p ≤ .001.
p ≤ .05.
As shown in Table 1, children significantly delayed their sleep/wake patterns from scheduled to free days (e.g., bedtime for 30 min; get-up time for 44 min, and slept on average 18 min longer on free than scheduled days). We found a positive correlation between the difference in sleep period on SC and FR days with children’s chronotype. Earlier chronotypes extended their sleep period less on FR days than later chronotypes (MSF: r = .33, p < .001; M/E-score: r = .32, p < .001; CT: r = .29, p < .001). The difference between sleep period on SC and FR days was not related to MSFsc (r = .04, p ≥ .05). Furthermore, later chronotypes (all three chronotype measures) had longer sleep inertia on SC days, and later chronotypes (M/E and CT measures) reported a longer time in bed on FR than earlier chronotypes (see Table 2).
Relations between Chronotype Measures
The three different measures of the children’s chronotype were significantly correlated (r = .52 to r = .67; see Table 2). Figure 2 illustrates the monotonic relationships between CT and the two other chronotype measures (MSF and M/E). While the association between M/E and CT appears to be linear, the association between MSF and CT suggests a levelling off in the two evening classes (moderate and definitely).
Parent-Reported and Actigraphically Estimated Sleep/Wake Parameter Comparisons
On a sub-sample of 85 children, parent-reported sleep/wake parameters were compared to measures derived from actigraphy (see Table 3). On average, parents reported significantly earlier sleep onsets, later wake-up times, and longer sleep periods than estimated by actigraphy. Discrepancies between the two measures, such as earlier parental report of sleep onset time and later parental report of wake time as computed by actigraphy, were on average approximately the same. Thus, the finding of no significant differences in midsleep point on SC and FR days was not surprising. Parental reports of sleep latency were significantly shorter than corresponding actigraphic estimates. In contrast, MSFsc computed from actigraphic measures was 12 min later than from the CCTQ (p = .006, d = .27).
TABLE 3.
Comparison of actigraphic estimates of sleep/wake parameters and corresponding parent reports from the Children’s ChronoType Questionnaire (CCTQ; n = 85)
| Actigraphy |
CCTQ |
Statistics |
||||
|---|---|---|---|---|---|---|
| Scheduled days* | Free days* | Scheduled days* | Free days* | Scheduled days† | Free days‡ | |
| Bedtime/time of lights-off | 20:49 (0:43) | 21:31 (0:57) | 20:41 (0:40) | 21:13 (0:55) | p < 0.01, d = 0.19 | p < 0.001, d =0.32 |
| Sleep start/sleep onset | 21:08 (0:42) | 21:51 (0:56) | 20:55 (0:43) | 21:25 (0:58) | p < 0.001, d = 0.32 | p < 0.001, d =0.46 |
| Sleep end/wake-up time | 07:00 (0:26) | 07:42 (0:44) | 07:08 (0:28) | 07:57 (0:50) | p < 0.01, d = 0.29 | p < 0.001, d =0.32 |
| Assumed sleep/sleep period | 09:49 (0:38) | 09:55 (0:40) | 10:13 (0:43) | 10:32 (0:50) | p < 0.001, d = 0.59 | p < 0.001, d =0.83 |
| Sleep latency | 0:20 (0:11) | 0:20 (0:14) | 0:14 (0:10) | 0:12 (0:11) | p < 0.001, d = 0.56 | p < 0.001, d =0.68 |
| Midsleep point | 02:03 (0:31) | 02:48 (0:47) | 02:01 (0:29) | 02:40 (0:48) | NS | NS |
| MSFsc | 02:46 (0:47) | 02:34 (0:46) | p < 0.01, d = 0.27 | |||
Reported as mean (standard deviation), in hours:minutes.
Wilcoxon Signed-Rank Test between actigraphy and questionnaire data for scheduled days.
Wilcoxon Signed-Rank Test between actigraphy and questionnaire data for free days.
Abbreviation: NS = not significant.
Concordance between Actigraphic Estimates of Sleep/Wake Parameters and Chronotype Measures
Validity of chronotype measures was also assessed by examining concordance between actigraphically estimated sleep/wake parameters (SC and FR) and the three chronotype measures. Spearman correlations are presented in Table 4. Independent of type of day (SC, FR), later chronotypes had later bedtimes, sleep start times, and sleep end times. Sleep latency, as assessed by actigraphy, was not significantly related to any parent-report measure of children’s chronotype. Assumed sleep assessed by actigraphy was negatively related to the MSF and MSFsc, but not to M/E or CT. Concordance between parent-reported and actigraphically estimated MSF was high (r = .78; for MSFsc: r = .70).
TABLE 4.
Spearman correlations between actigraphic estimates ofsleep/wake parameters and parent reports of mid-sleep point on free days (MSF/MSFsc), morningness/eveningness scale (M/E) scores, and chronotype (CT) scores (n = 85)
| Actigraphy | Children’s ChronoType Questionnaire (CCTQ) |
||||
|---|---|---|---|---|---|
| MSF | MSFsc | M/E | CT | ||
| Bedtime | SC | 0.70* | 0.72* | 0.39* | 0.30† |
| FR | 0.74* | 0.74* | 0.44* | 0.40* | |
| Sleep latency | SC | 0.05 | −0.03 | 0.003 | 0.01 |
| FR | −0.04 | −0.05 | 0.09 | 0.15 | |
| Sleep start | SC | 0.70* | 0.70* | 0.41* | 0.32† |
| FR | 0.75* | 0.74* | 0.45* | 0.43* | |
| Sleep end | SC | 0.46* | 0.45* | 0.45* | 0.34† |
| FR | 0.64* | 0.56* | 0.65* | 0.51* | |
| Assumed sleep | SC | − 0.49* | − 0.51† | −0.10 | −0.10 |
| FR | − 0.31† | − 0.38* | 0.05 | 0.00 | |
| Midsleep point | SC | 0.67* | 0.66* | 0.50* | 0.37* |
| FR | 0.78* | 0.73* | 0.57* | 0.50* | |
| MSFsc | 0.73* | 0.70* | 0.52* | 0.47* | |
Note. SC = scheduled days; FR = free days
p ≤ .001
p ≤ .05
Test-Retest Reliability
The CCTQ was administered twice within 2–4 weeks (range between the two administrations: 14–37 days, mean = 20 days) to parents of 46 children (23 girls, 50%) who were on average 7.7 yrs old (range: 4.4– 11.0 yrs). Standard deviations of the sleep/wake parameters of the first and second administration were approximately the same, and mean differences between the two administrations were not significant for any parameter (p>.05). The reliability was moderate-to-high for most sleep/ wake parameters (r = .58 to r = .94; see Table 5) and high for the three chronotype measures [r = .91 (p < .001) for MSF; r = .79 (p < .001) for MSFsc; r = .94 (p < .001) for M/E; and r = .84 (p < .001) for CT]. The time between the two administrations and whether the questionnaires had been filled out on the same type of day (i.e., SC or FR) did not significantly influence the differences between the two administrations.
TABLE 5.
Test-retest reliability (Pearson correlations) within 2–4 weeks for parent-reported sleep/wake parameters, midsleep point on scheduled and free days, and corrected midsleep point on free days (MSFsc; n = 46)
| Scheduled days | Free days | ||
|---|---|---|---|
| Bedtime | 0.90 | 0.88 | |
| Time of lights-off | 0.90 | 0.85 | |
| Sleep latency | 0.74 | 0.58 | |
| Sleep onset | 0.92 | 0.85 | |
| Wake-up time | 0.89 | 0.91 | |
| Get-up time | 0.91 | 0.91 | |
| Time fully alert | 0.94 | 0.89 | |
| Sleep period | 0.94 | 0.79 | |
| Time in bed | 0.92 | 0.82 | |
| Sleep inertia | 0.78 | 0.70 | |
| Midsleep point | 0.87 | 0.91 | |
| MSFsc | 0.79 |
All correlations are p ≤ .001.
Note. Reliability coefficients for M/E and CT are presented in the text.
DISCUSSION
This study describes the assessment of chronotype in children between 4 and 11 yrs of age using three different measures: the midsleep point on FR days (MSF), the Morningness/Eveningness scale (M/E) score, and a five-point chronotype (CT) score. To our knowledge, no parent-report questionnaire with adequate reliability and validity is available for the assessment of children’s chronotype in prepubertal children. We adapted measures of morningness/eveningness used in adolescents and adults from Horne and Östberg (1976), Smith et al. (1989), and Carskadon et al. (1993), and combined them with other measures used in the literature, such as the MSF and CT, into a single questionnaire (CCTQ). This study provides validity data for the CCTQ using actigraphy, as well as 2–4 week test-retest reliability data. Overall, the findings indicate moderate to strong agreement between the three chronotype measures, adequate associations between sleep/wake parameters (parent-report and actigraphy) and chronotype measures, and excellent temporal stability for all three chronotype measures (reliability).
Comparisons between the three chronotype measures and parental reports of sleep/wake parameters suggest stronger relationships between sleep/wake parameters and MSF/MSFsc than between sleep/wake parameters and M/E or CT. Higher correlations with MSF/MSFsc may be explained by the fact these measures are computed derivations of the reported sleep onset and sleep period, while assessment of M/E and CT require methodologically distinct responses from parents. The M/E score is a sum score of multiple items measuring children’s “best” time to sleep, take a cognitive test, and do physical activities, as well as children’s level of sleepiness at different times of the day. Likewise, the CT is an overall parental impression of the child’s chronotype using five response choices. Our results indicate that later chronotypes as measured by MSF/MSFsc, M/E, and CT are more likely to have later bedtimes, lights-off times, and sleep onset times; longer sleep latencies; and later wake-up and get-up times; and take longer to be fully alert in the morning than earlier chronotypes, independent of the type of day (i.e., SC or FR). These findings are consistent with previous reports on circadian preference with adolescents and adults (e.g., Carskadon et al., 1993; Roenneberg et al., 2003), suggesting that the CCTQ adequately measures chronotype in prepubertal children.
The validity of parent-reported sleep/wake parameters and chrono-type measures was examined by objective data (actigraphy). The relationship between bedtime, sleep onset, wake-up time, and child’s chronotype was verified with estimates from actigraphy. The finding that sleep latency was significantly related to child’s chronotype was not verified with estimates from actigraphy. This may due to parents’ difficulty in providing accurate estimates of sleep latency, especially for later chronotypes (i.e., children’s sleep onset is later than their parents or for children who require little to no assistance in falling asleep at bedtime). While many sleep/wake parameters significantly differ between actigraphy and questionnaire data, midsleep point on scheduled and free days did not. This finding indicates objective validity for the chronotype measure MSF. The significant discrepancies between actigraphy and questionnaire data in sleep/wake parameters are well documented in the literature (Acebo et al., 2005; Sadeh et al., 1991, 1994; Werner et al., 2008) and may be explained by methodological differences; for example, actigraphy estimates sleep/wake patterns based on movements during specified time intervals, while subjective reports may be influenced by recall, experiences, and expectations and are not primarily based on a particular time window.
Relations between the three different chronotype measures (MSF/ MSFsc, M/E, and CT) were moderate to high. The strongest correlation was between M/E and CT, which may be explained by the sequence of filling out the CT after the two other measures. That is, parents may have become more in tune with the chronotype construct after completing questions resulting in MSF and M/E. High correlations between M/E scores and CT have been also reported in adult populations by Roenneberg [r = –.2.80; chronotype self-assessment on a seven-point scale; see Roenneberg et al. (2007)].
Although the correlations between the three different chronotype measures in our study were moderate to high, some incorrect classifications of morning types as evening types and vice-versa may have occurred. Comparing, for example, the M/E- and CT-scores classified into three groups (morning types, intermediate types, and evening types), our data may suggest that extreme misclassifications were rare. Because an honest false classification rate cannot be provided by our analysis, further studies should compare parent-reported chronotype measures with physiological circadian parameters (e.g., dim light melatonin onset, which may provide additional validity data for classifying children’s chronotype).
The test-retest analysis of sleep/wake parameters and the three chrono-type measures suggests excellent temporal stability. A test-retest period of 2–4 weeks was chosen according to Knapp and Brown (1995), who showed that a time period of 2–4 weeks is not too short (the shorter the interval, the more the answers of the first administration may be recalled, thereby producing an artificially high estimate of the instrument) or too long (the longer the interval, the more likely the true scores may have changed). The reliability coefficient for sleep latency and sleep inertia was influenced by two individual subjects for whom the difference between the two administrations was about 0.5 h (range of remaining values: 20.25 to 0.33 h). When the data of these subjects were removed from analysis, the correlation was higher. We cannot distinguish whether the moderate test-retest correlation of sleep latency and sleep inertia is due to more variability of a child’s behavior (e.g., difficulty falling asleep due to stressful events) or to less reliability of the parent report (e.g., if a child does not need parents assistance to fall asleep).
Data from many reports on adolescents and adults show that individuals delay their sleep on average by 1–3 h from SC to FR days and sleep longer on FR days, which has been interpreted as an accumulated sleep deficit (e.g., Carskadon et al., 1993; Roenneberg et al., 2004). These findings prompted Roenneberg and colleagues to correct the MSF for the accumulated sleep deficit during the work week (see the appendix in Roenneberg et al., 2004). Our data indicate the delaying pattern is already evident in prepubertal children, although to a lesser degree than in older children and adults. We found prepubertal children delay on average their sleep onset for 26 min and wake-up time for 44 min and, therefore, sleep 18 min longer on FR than SC days. Compared to Wolfson and Carskadon (1998), 15-yr-old adolescents go to bed 106 min later and get up 220 min later on weekends, oversleeping 114 min. The age effect on sleep/wake patterns (e.g., Carskadon et al., 1998; Iglowstein et al, 2003; Randler, 2008a) is likely influenced by environmental factors, such as increasing nighttime activity and setting own bedtime, and biological factors, such as maturation of the circadian system and the sleep/wake homeostatic regulatory processes (Carskadon et al., 1993; Jenni & LeBourgeois, 2006; Jenni et al., 2005a). As a consequence, we corrected the MSF for the accumulated sleep deficit as suggested by Roenneberg et al. (2004).
We note that study participation was voluntary, and the study population represents a small community sample, with an imbalance between the number of children aged 4–7 and those aged 7–11 yrs of age. Furthermore, we did not collect concurrent self-reported data from school children, and parents were not asked to report sleep/wake parameters to specified precision (e.g., 5 or 10 min), which may have resulted in significant deviation from normality for many sleep/wake parameters. Although this study presents findings in need of replication, including in different cultural groups (e.g., Caci et al., 2005), we still believe the CCTQ is a convenient, brief, and easy-to-administer questionnaire providing three different chronotype measures. Which of these measures may be recommended for clinical or research use depends on particular questions and aims.
Our results indicate that 4- to 11-yr-old children already delay their sleep/wake patterns and “oversleep” about 15 min between SC and FR days. As a result, prepubertal children, especially those with later chrono-type classifications, may have difficulty obtaining sufficient sleep. Because eveningness is associated with increased daytime sleepiness; greater emotional, attentional, and behavioral problems; and poorer school achievement; knowing the individual’s circadian phase preference may help the clinician dealing with these difficulties. We propose the CCTQ be used in future studies, including those with clinical populations (e.g., sleep disorders, learning disorder, behavioral problems). We conclude that all three measures included in the CCTQ (MSF, M/E, and CT) are equally valid and reliable measures for the assessment of chronotype in prepubertal children between 4 and 11 yrs of age.
ACKNOWLEDGMENT
We thank all children and their families for participating and Dr. Luciano Molinari for statistical advice.
This work was supported by research grants from the Claus Cramer Stiftung, the Theodor und Ida Herzog-Egli Stiftung, and the Zurich Center for Integrative Human Physiology (ZIHP) of the University of Zurich.
Footnotes
Copyright of Chronobiology International: The Journal of Biological & Medical Rhythm Research is the property of Taylor & Francis Ltd and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Adan A, Almirall H. Horne and Östberg morningness-eveningness questionnaire: A reduced scale. Pers. Ind. Differ. 1991;12:241–253. [Google Scholar]
- Baehr EK, Revelle W, Eastmann CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:119–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Bailey S, Heitkemper M. Circadian rhythmicity of cortisol and body temperature: Morning-ness-eveningness effects. Chronobiol. Int. 2001;18:249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- Caci H, Adan A, Bohle P, Natale V, Pornpitakpan C, Tilley A. Transcultural properties of the composite scale of morningness: The relevance of the “morning affect” factor. Chronobiol. Int. 2005;22:523–540. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Wolfson A, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness as a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Chelminski F, Ferraro R, Petros T, Plaud JJ. Horne and Ostberg questionnaire: A score distribution in a large sample of young adults. Pers. Ind. Differ. 1997;23:647–652. [Google Scholar]
- Cofer LF, Grice JW, Sethre-Hofstad L, Radi CJ, Zimmermann LK, Palmer-Seal D, Santa-Maria G. Developmental perspectives on morningness-eveningness and social interactions. Human Develop. 1999;41:163–198. [Google Scholar]
- Cohen J. The statistical power of abnormal-social psychological research: A review. J. Abnorm. Soc. Psychol. 1962;65:145–153. doi: 10.1037/h0045186. [DOI] [PubMed] [Google Scholar]
- Costa G, Lievore F, Casaletti G, Gaffuri E, Folkard S. Circadian characteristics influencing inter-individual differences in tolerance and adjustment to shiftwork. Ergonomics. 1989;32:373–385. doi: 10.1080/00140138908966104. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Akerstedt T. Influence of flexibility and variability of working hours on health and well-being. Chronobiol. Int. 2006;23:1125–1137. doi: 10.1080/07420520601087491. [DOI] [PubMed] [Google Scholar]
- Digdon NL, Howell AJ. College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiol. Int. 2008;26:1029–1046. doi: 10.1080/07420520802553671. [DOI] [PubMed] [Google Scholar]
- Duffy J, Dijk D, Hall E, Czeisler C. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J. Investig. Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Salvatore O. Circadian preference, sleep and daytime beahaviour in adolescence. J. Sleep. Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Guthrie JP. Additional validity evidence for a measure of morningness. J. Appl. Psychol. 1995;80:186–190. [Google Scholar]
- Horne JA, Ostberg O. A self-assessement questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jankowski KS, Ciarkowska W. Diurnal variation in energetic arousal, tense arousal and hedonic tone in extreme morning and evening types. Chronobiol. Int. 2008;25:577–595. doi: 10.1080/07420520802261770. [DOI] [PubMed] [Google Scholar]
- Jenni OG, LeBourgeois MK. Understanding sleep-wake regulation and sleep disorders during childhood: The value of a model Curr. Opin. Psychiatry. 2006;19:282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni OG, O’Connor BB. Children’s sleep: An interplay between culture and biology. Pediatrics. 2005;115:204–216. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon M. Homeostatic sleep regulation in adolescents. Sleep. 2005a;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Fuhrer HZ, Iglowstein I, Molinari L, Largo RH. A longitudinal study of bed sharing and sleep problems among Swiss children in the first 10 years of life. Pediatrics. 2005b;115:233–240. doi: 10.1542/peds.2004-0815E. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA. Inter-individual differences in the human circadian system: A review. Biol. Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA. Differences between morning-types and evening-types in the dynamics of EEG slow wave activity during night sleep. Electroencephalogr. Clin. Neuro. physiol. 1991;78:197–202. doi: 10.1016/0013-4694(91)90033-z. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Lancel M. EEG slow wave activity, REM sleep, and rectal temperature during night and day sleep in morning-type and evening-type subjects. Psychophysiology. 1991;28:678–688. doi: 10.1111/j.1469-8986.1991.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HPA. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci. Lett. 1996;218:153–156. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- Knapp T, Brown J. Ten measurement commandments that often should be broken. Res. Nurs. Health. 1995;18:465–469. doi: 10.1002/nur.4770180511. [DOI] [PubMed] [Google Scholar]
- Laberge L, Carrier J, Lespérance P, Lambert C, Vitaro F, Tremblay R, Montplaisir J. Sleep and circadian phase characteristics of adolescent and young adult males in a naturalistic summertime condition. Chronobiolnt. 2000;17:489–501. doi: 10.1081/cbi-100101059. [DOI] [PubMed] [Google Scholar]
- Mecacci L, Zani A. Morningness-eveningness preferences and sleep-waking diary data of morning and evening types in student and worker samples. Ergonomics. 1983;26:1147–1153. doi: 10.1080/00140138308963450. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationship between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J. Biol. Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Natale V, Cicogna P. Morningness-eveningness dimension: Is it really a continuum? Pers. Ind. Diff. 2002;32:809–816. [Google Scholar]
- Owens J. Classification and epidemiology of childhood sleep disorders. Sleep Med. Clin. 2007;2:353–361. [Google Scholar]
- Peterson A. The Early Adolescence Study: An overview. J. Early. Adolesc. 1984;4:103–106. [Google Scholar]
- Pisarski A, Brook C, Bohle P, Gallois C, Watson B, Winch S. Extending a model of shift-work tolerance. Chronobiol. Int. 2006;23:1363–1377. doi: 10.1080/07420520601055316. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky M. Ethical and methodoligical standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Posey T, Ford J. The morningness-eveningness preference of college students as measured by the Horne and Ostberg Questionnaire. Chronobiol. Int. 1981;7:141–144. [Google Scholar]
- Randler C. Differences in sleep and circadian preference between Eastern and Western German adolescents. Chronobiol. Int. 2008a;25:565–575. doi: 10.1080/07420520802257794. [DOI] [PubMed] [Google Scholar]
- Randler C. Morningness-eveningness comparison in adolescents from different countries around the world. Chronobiol. Int. 2008b;25:1017–1028. doi: 10.1080/07420520802551519. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. Munich chronotype questionnaire. Munchen: Ludwig-Maximilians-University Munchen, Institut für Medizinische Psychologie,; [accessed December 11, 2008]. (n. d.). Available at: http://www.imp.med.uni-muenchen.de/index.html. [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr. Biol. 2004;14:1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Akllebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep. Med. Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–499. [PubMed] [Google Scholar]
- Sadeh A, Lavie P, Scher A. Sleep and temperament. Maternal perceptions of temperament of sleep-disturbed toddlers. Early Educ. Develop. 1994;5:311–322. [Google Scholar]
- Silva EJ. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neuro-sci. 2008;122:928–935. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Soehner AM, Kenndy KA, Monk TH. Personality correlates with sleep-wake variables. Chronobiol. Int. 2007;24:875–888. doi: 10.1080/07420520701648317. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Chastang J-F, Bioulac B. Validation of Horne and Ostberg Morningness-Eveningness Questionnaire in a middle-aged population of French workers. J. Biol. Rhythms. 2004;19:76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Inoue M, Watanabe N, Yamashita Y, Hamada M, Kadota G, Harada T. Parental enforcement of bedtime during childhood modulates preference of Japanese junior high school students for eveningness chronotype. Chronobiol. Int. 2001;18:823–829. doi: 10.1081/cbi-100107517. [DOI] [PubMed] [Google Scholar]
- Tankova I, Adan A, Buela-Casal G. Circadian typopology and individual differences. A review. Pers. Ind. Differ. 1994;16:671–684. [Google Scholar]
- Tanner J. Growth at adolescence. 2nd ed. Blackwell: Oxford; 1962. p. 325. [Google Scholar]
- Tonetti L, Fabbri M, Natale V. Relationship between circadian typology and big five personality domains. Chronobiol. Int. 2009;26:337–347. doi: 10.1080/07420520902750995. [DOI] [PubMed] [Google Scholar]
- Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary and questionnaire for children’s sleep patterns. Arch. Pediatr. Adolesc. Med. 2008;162:350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- Wittman M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wolfson A, Carskadon M. Sleep schedules and daytime functioning in adolescents. Child Develop. 1998;69:875–887. [PubMed] [Google Scholar]
- Zavada A, Gordijn M, Beersma D, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Östberg’s morningness-eveningness score. Chronobiol. Int. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]