Abstract
Accumulating evidence implicates nicotinic acid adenine dinucleotide phosphate (NAADP) in the control of Ca2+-dependent functions. Little, however, is known concerning its role in the vascular endothelium, a major regulator of blood pressure. Here, we show that NAADP acetoxymethyl ester (NAADP-AM), a cell-permeant NAADP analog, increases cytosolic Ca2+ concentration in aortic endothelial cells. We demonstrate that these signals and those evoked by acetylcholine are blocked by disrupting acidic organelles with bafilomycin A1. In contrast, Ca2+ signals in response to thrombin are only partially inhibited by bafilomycin A1 treatment, and those to ATP were insensitive, suggesting that recruitment of acidic stores is agonist-specific. We further show that NAADP-evoked Ca2+ signals hyperpolarize endothelial cells and generate NO. Additionally, we demonstrate that NAADP dilates aortic rings in an endothelium- and NO-dependent manner. Finally, we show that intravenous administration of NAADP-AM into anesthetized rats decreases mean arterial pressure. Our data extend the actions of NAADP to the endothelium both in vitro and in vivo, pointing to a previously unrecognized role for this messenger in controlling blood pressure.
Keywords: Calcium, Calcium Imaging, Endothelium, Lysosomes, Nitric Oxide
Introduction
Nicotinic acid adenine dinucleotide phosphate (NAADP)4 is a potent and widespread Ca2+-mobilizing messenger first described in sea urchin eggs (1, 2). Although its mechanism of action is subject to debate, much evidence indicates that NAADP mobilizes Ca2+ from acidic, lysosome-like organelles (3) through activation of novel Ca2+-permeable channels (1, 4). These channels have recently been identified as members of the two-pore channel family (5–9). Importantly, mobilization of so-called “acidic Ca2+ stores” (10) by NAADP is often linked to mobilization of the well established endoplasmic reticulum Ca2+ stores through the process of Ca2+-induced Ca2+ release (11). NAADP is thus thought to act as trigger during agonist-evoked Ca2+ signaling (2). The functional positioning of NAADP-sensitive Ca2+ stores upstream of those targeted by the messengers, inositol 1,4,5-trisphosphate (IP3) and cyclic ADP-ribose, raises the possibility that agonist-evoked Ca2+ signals previously ascribed to endoplasmic reticulum Ca2+ release might also involve NAADP and acidic organelles.
Using isolated cells or tissues, NAADP-induced Ca2+ signaling has been implicated in the regulation of several physiological functions such as egg fertilization (12, 13), T lymphocyte activation (14), muscle contraction (15), and neuronal differentiation (16). The role of NAADP in vivo, however is not known, although a recent study demonstrated that a newly described NAADP antagonist could prevent T cell motility in an animal model of multiple sclerosis (17).
In the cardiovascular system, a role for NAADP has been demonstrated in agonist-evoked Ca2+ signaling in pulmonary (15, 18) and coronary arteries (19, 20), cardiac myocytes (21), and the renal microcirculation (22). Direct measurements of NAADP have confirmed its messenger role in response to endothelin-1 (15) and β-adrenoreceptor agonists (21). What role NAADP plays in the vascular endothelium has yet to be explored. A variety of extracellular cues such as acetylcholine mediate changes in cytosolic Ca2+ concentration within endothelial cells (23). These signals drive production of vasodilators such as nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor. The endothelium is thus critical for regulating vascular tone and blood pressure.
Like many other cell types, changes in cytosolic Ca2+ levels within the endothelium are generally ascribed to mobilization of endoplasmic reticulum Ca2+ stores through activation of IP3 or ryanodine receptors (23). Given emerging evidence implicating NAADP in Ca2+ signaling pathways attributed to more traditional messengers (24, 25), we examined the role of NAADP in the endothelium. By combining Ca2+, voltage, and NO imaging of single cultured endothelial cells with measurement of contractility ex vivo and blood pressure in vivo, we show that NAADP-sensitive Ca2+ stores are present in the endothelium, that they are recruited in an agonist-specific manner, and that their mobilization is sufficient to regulate contractility and blood pressure.
EXPERIMENTAL PROCEDURES
HAEC Culture
Human aortic endothelial cells (HAECs) were grown in M199 medium and the serum concentration reduced from 20% to 0.5% prior to experimentation.
Measurement of Ca2+
Intracellular Ca2+ concentration ([Ca2+]i) was measured by the microfluorimetric technique, as described previously (6).
Measurement of Membrane Potential
Bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3), Invitrogen), a slow response voltage-sensitive dye, was used to assess relative changes in membrane potential of single cells, as described previously (26). Upon membrane hyperpolarization, the dye concentrates in the cell membrane, leading to a decrease of fluorescence intensity, whereas depolarization results in a sequestration of the dye into cytosol and is associated with an increase in the fluorescence intensity (27).
Measurement of NO
Intracellular NO was monitored with (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate (DAF-FM), a pH-insensitive fluorescent dye that emits increased fluorescence upon reaction with an active intermediate of NO formed during spontaneous oxidation of NO to NO2− (28, 29).
Measurement of Contractility
Mechanical activity of vascular rings from rat thoracic aorta was measured using an isometric force transducer (30).
Measurement of Blood Pressure
Arterial pressure of anesthetized rats was measured using a high sensitivity pressure transducer.
Statistics
Statistical significance between groups was tested using one-way analysis of variance followed by Bonferroni test.
Full details of all Methods are provided in the Supplemental Text.
RESULTS AND DISCUSSION
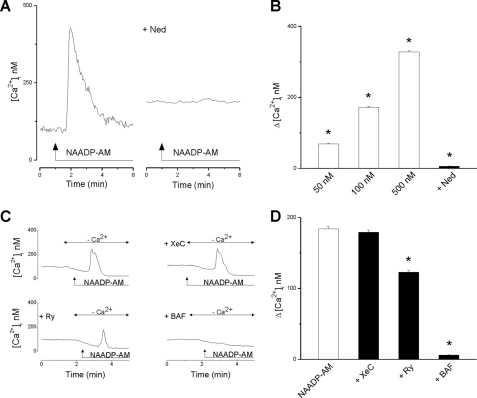
To probe the presence of functional NAADP-sensitive Ca2+ channels in the endothelium, we first examined the effect of NAADP-AM, a cell-permeant analog of NAADP (21, 31), on the [Ca2+]i in HAECs. Stimulation of cells with NAADP-AM induced a transient increase in [Ca2+]i (Fig. 1A), which was abolished by pretreatment with Ned-19, a blocker of NAADP signaling (32). The effect of NAADP-AM on [Ca2+]i was concentration-dependent (Fig. 1B). A delay of 30–90 s was noted between the moment of NAADP-AM administration and the increase in [Ca2+]i. Application of NAADP-AM also elevated [Ca2+]i in Ca2+-free saline (Fig. 1C), indicating that NAADP mobilizes intracellular Ca2+ stores. This response was blocked by pretreatment with bafilomycin A1 (Fig. 1C), a V-type ATPase inhibitor (33), confirming the involvement of an acidic store as reported in a variety of other cell types (3, 15, 16, 19, 20). Previous studies have shown that in intact cells, NAADP evokes Ca2+ signals from acidic Ca2+ stores that are then subsequently amplified by ryanodine receptors (18), IP3 receptors (34), or both (11). We therefore tested the effects of ryanodine and xestospongin C on NAADP-AM-evoked Ca2+ signals in endothelial cells. Pretreatment with ryanodine caused a partial (33%) reduction in the evoked response, whereas xestospongin C was without effect (Fig. 1C). These data, summarized in Fig. 1D, provide the first evidence for functional NAADP-sensitive Ca2+ channels in endothelial cells that, as in pancreatic beta cells, appear largely uncoupled from Ca2+ release from the endoplasmic reticulum (35).
FIGURE 1.
NAADP mobilizes Ca2+ from acidic calcium stores in endothelial cells. A, representative trace (left) showing the effect of NAADP-AM (500 nm) on [Ca2+]i of a single HAEC. In another HAEC (right trace), pretreatment with Ned-19 (Ned, 1 μm, 15 min) abolished the effect of NAADP-AM (500 nm). B, summary data quantifying the peak changes in [Ca2+]i (Δ[Ca2+]i) in response to the indicated concentration of NAADP-AM (n = 72) and the blocking effect of Ned-19 (n = 27). C, representative traces showing the effect of NAADP-AM (500 nm) on [Ca2+]i in Ca2+-free saline. Cells were untreated or preincubated with bafilomycin A1 (BAF, 1 μm, 1 h), ryanodine (Ry, 1 μm, 10 min), or xestospongin C (XeC, 10 μm, 15 min). D, summary data quantifying Δ[Ca2+]i in response to 500 nm NAADP-AM in the absence or presence of the indicated inhibitor (n = 74). *, = p < 0.05. Error bars indicate S.E.
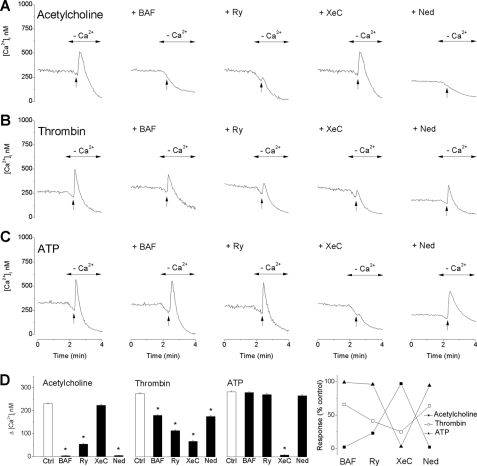
Having established the presence of acidic NAADP-sensitive Ca2+ stores in endothelial cells, we next probed their recruitment by Ca2+-mobilizing vasoactive agonists. To achieve this, we compared [Ca2+]i upon stimulation with acetylcholine, thrombin, and ATP in the absence and presence of bafilomycin A1 (Fig. 2). All three agonists evoked comparable cytosolic Ca2+ signals in Ca2+-free saline (Fig. 2, A–C). The responses to acetylcholine were abolished by bafilomycin A1, whereas those to thrombin were only partially blocked (Fig. 2, A–C). In stark contrast, the ATP responses were unaffected (Fig. 2, A–C). We also probed the contribution of endoplasmic reticulum Ca2+ stores to agonist-evoked Ca2+ signals by examining the effect of ryanodine and xestospongin C to block ryanodine and IP3 receptors, respectively. As shown in Fig. 2, A–C, the responses to acetylcholine were substantially reduced by ryanodine but unaffected by xestospongin C. In contrast, the responses to thrombin were substantially reduced by both ryanodine and xestospongin C, whereas the responses to ATP were unaffected by ryanodine but inhibited by xestospongin C. Moreover, pretreatment with Ned-19 (33) abolished acetylcholine-evoked Ca2+ signals, reduced the responses to thrombin, and did not significantly affect the responses to ATP (Fig. 2, A–C).
FIGURE 2.
Acidic Ca2+ stores in endothelial cells are recruited in an agonist-specific manner. A–C, representative traces showing the effect of 10 μm acetylcholine (A), thrombin (B), and ATP (C) on [Ca2+]i in Ca2+-free saline. Cells were untreated (first column) or preincubated with bafilomycin A1 (BAF, 1 μm, 1 h; second column), ryanodine (Ry, 1 μm, 10 min; third column), xestospongin C (XeC, 10 μm, 15 min; fourth column), or Ned-19 (Ned, 1 μm, 15 min, fifth column). D, summary data quantifying Δ[Ca2+]i in response to the agonists in the absence or presence of the indicated inhibitor (n = 6 for each condition). The far right panel expresses responses of all three agonists in the presence of the inhibitors as the percentage of the control (Ctrl) responses. *, = p < 0.05. Error bars indicate S.E.
A summary of the effects of the inhibitors on the evoked Ca2+ signals is shown in Fig. 2D. We conclude: (i) that acetylcholine-evoked Ca2+ signals involve recruitment of acidic Ca2+ stores and ryanodine receptors with little contribution of IP3 receptors; (ii) that thrombin-evoked Ca2+ signals involve recruitment of acidic Ca2+ stores, ryanodine receptors, and IP3 receptors; and (iii) that ATP-evoked Ca2+ signals appear independent of acidic Ca2+ stores and ryanodine receptors and are instead driven primarily by IP3 receptors. Agonist-evoked Ca2+ signals in endothelial cells thus involve differential recruitment of intracellular Ca2+ pools. Our results with thrombin and ATP are consistent with data in other cell types where thrombin was shown in part to recruit acidic stores in platelets (36), and ATP was shown to act largely independently of acidic stores in hippocampal neurons and glia (37). This is the first report implicating acidic Ca2+ stores in acetylcholine action. These findings contrast those in pancreatic beta (35) and myometrial (34) cells where acidic Ca2+ stores did not contribute to Ca2+ signals evoked by acetylcholine. Together, this raises the possibility that coupling of the specific receptors to acidic Ca2+ stores is malleable and likely cell type-specific.
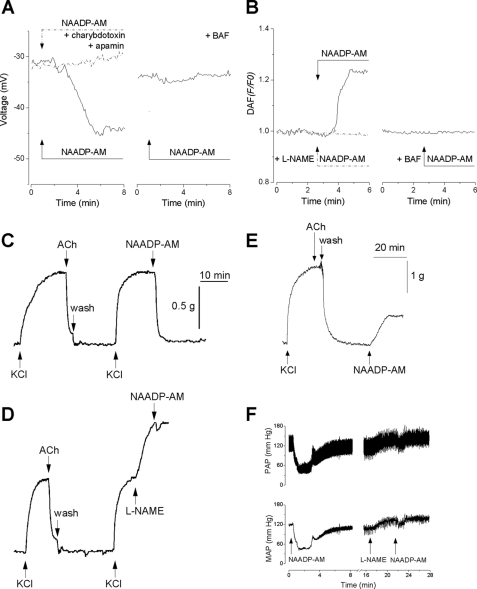
Agonist stimulation of endothelial cells results in hyperpolarization due to the activation of Ca2+-dependent K+ channels (38). Hyperpolarization increases the driving force for Ca2+ entry, which likely contributes to NO synthesis via activation of endothelial nitric oxide synthase. Hyperpolarization is also transmitted to smooth muscle cells, which contributes to vasodilatation through attenuating the opening of voltage-sensitive Ca2+ channels (38). To determine whether NAADP-mediated Ca2+ signals could mediate electrical responses in endothelial cells, we examined the effect of NAADP-AM on membrane potential using the slow response voltage-sensitive dye DiBAC4. The resting membrane potential of HAECs was −47 ± 6.2 mV (n = 57). Administration of NAADP-AM induced a hyperpolarization of 12 ± 2.6 mV (n = 19) (Fig. 3A). Endothelial cells express several types of Ca2+-activated K+ channels such as high and intermediate conductance Ca2+-activated K+ channels, which are blocked by charybdotoxin, and small conductance Ca2+-activated K+ channels, which are blocked by apamin. The effect of NAADP-AM on membrane potential was abolished by pretreatment with a combination of apamin and charybdotoxin (n = 12) consistent with the activation of Ca2+-activated K+ channels (Fig. 3A). Pretreatment with bafilomycin A1 also abolished the response (n = 15), indicating that the effects of NAADP were due to mobilization of an acidic store (Fig. 3A). Thus, NAADP can mediate hyperpolarization in addition to depolarization as reported recently in medulla neurons (39).
FIGURE 3.
NAADP is vasoactive. A, left, effect of NAADP-AM (500 nm) on membrane potential in a control cell (solid line) and a cell pretreated for 15 min with 1 μm apamin and 50 nm charybdotoxin (dashed trace). Right, effect of bafilomycin A1 pretreatment (BAF, 1 μm, 1 h). Data are representative of 12–19 cells. B, left, effect of NAADP-AM (500 nm) on NO production in a control cell (solid line) and a cell pretreated for 15 min with 10 μm l-NAME (dashed trace). Right, effect of bafilomycin A1 pretreatment (BAF, 1 μm, 1 h). Data are representative of 18–34 cells. C–E, the effect of KCl (40 mm), acetylcholine (ACh, 10 μm), and NAADP-AM (500 nm) on contractility of aortic rings with the endothelium intact (C and D) or following its removal (E). In D, l-NAME (100 μm) was added prior to NAADP-AM (500 nm). Washout of the various agents is marked. Data are representative of 5–6 preparations. F, effect of intravenous injection of NAADP-AM (300 μl, 1 μm) on pulsatile arterial pressure (PAP, top) and mean arterial pressure (MAP, bottom) in anesthetized rats in the absence (left traces) or presence of (right traces) of l-NAME (5 mg/kg). Data are representative of 5–6 animals.
To further probe the downstream effects of NAADP, we measured NO levels using DAF-FM. Administration of NAADP-AM increased DAF-FM fluorescence by 24 ± 0.4% (n = 31) (Fig. 3B) in the absence but not the presence of the nitric oxide synthase inhibitor l-NAME (n = 18) (Fig. 3B). As with the electrical responses, the effects of NAADP-AM were abolished by pretreatment with bafilomycin A1 (n = 34) (Fig. 3B). Taken together, the data in Fig. 3, A and B, show that NAADP mobilizes Ca2+ from acidic Ca2+ stores and that these signals are capable of regulating membrane potential and NO synthesis.
To examine the role of NAADP-mediated Ca2+ signals in vasomotor responses, we next characterized the effects of NAADP-AM on the contractility of aortic rings, with and without endothelium. As shown in Fig. 3C, administration of NAADP-AM to aortic rings with intact endothelium precontracted with KCl produced a rapid and marked relaxation (91.6 ± 3.8%, n = 6). This effect was similar to that evoked by acetylcholine (85.2 ± 2.7%, n = 11), which is known to activate endothelial nitric oxide synthase in endothelial cells. Administration of l-NAME to precontracted aortic rings produced a supplemental contraction (82.8 ± 5.9%, n = 5). Under these conditions, the relaxation to NAADP after l-NAME administration was reduced to 7.5 ± 0.6% (n = 5; Fig. 3D). These data are consistent with endothelial cells as targets for NAADP-AM. Accordingly, in denuded preparations, which were unresponsive to acetylcholine, NAADP-AM failed to evoke relaxation but instead evoked a significant contraction (37 ± 3%, n = 5), likely reflecting an action on smooth muscle cells (Fig. 3E).
The effects of NAADP have been extensively characterized in isolated cells and tissues, but corresponding in vivo data are currently lacking. Given the ability of NAADP to regulate arterial contractility ex vivo, the final set of experiments was untaken to examine the effects of NAADP-AM on blood pressure in anesthetized rats. The basal mean arterial pressure was 108 ± 3.7 mm Hg (n = 6). Intravenous administration of NAADP-AM induced a decrease in mean arterial pressure by 51.6 ± 5.9 mm Hg (n = 5). The effects of NAADP-AM were partially sensitive to l-NAME. Thus, in the presence of l-NAME, the mean arterial pressure before NAADP-AM administration was 132 ± 4.2 mm Hg (n = 5). Subsequent administration of NAADP-AM reduced mean arterial pressure by 13.6 + 5.3 mm Hg (Fig. 3F). These data suggest that NAADP-AM mediates vasodilatation in vivo by both NO-dependent and NO-independent means.
To conclude, we provide the first evidence that NAADP-sensitive Ca2+ stores are present in endothelial cells and that they are recruited by select physiologically relevant vasoactive agonists. We identify hyperpolarization and NO production as new downstream effectors for this messenger and show that NAADP is capable of regulating contractility ex vivo, and importantly, blood pressure in vivo. Although the role of NAADP in smooth muscle cells in mediating contraction is established (15, 18–20), our data showing that NAADP in neighboring endothelial cells is coupled to opposing, relaxation pathways highlight the versatility of this messenger in regulating vascular tone. When coupled with the demonstrated agonist-specific recruitment of acidic Ca2+ stores, this renders NAADP an attractive therapeutic target in combating endothelium dysfunction.
This work was supported, in whole or in part, by National Institutes of Health Grants HL90804 (to E. B.), HL51314 (to N. J. D.), and HL086699 (to M. M.). This work was also supported by the Biotechnology and Biological Sciences Research Council Grants BB/D012694/1 (to G. C. C.) and BB/G013721/1 (to S. P.).

The on-line version of this article (available at http://www.jbc.org) contains Supplemental Text.
- NAADP
- nicotinic acid-adenine dinucleotide phosphate
- NAADP-AM
- NAADP acetoxymethyl ester
- DAF-FM
- (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate
- DiBAC4(3)
- bis-(1,3-dibutylbarbituric acid) trimethine oxonol
- HAEC
- human aortic endothelial cell
- HBSS
- Hanks' balanced salt solution
- IP3
- inositol 1,4,5-trisphosphate
- l-NAME
- l-NG-nitroarginine methyl ester.
REFERENCES
- 1.Lee H. C., Aarhus R. (1995) J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 2.Guse A. H., Lee H. C. (2008) Sci. Signal. 1, re10. [DOI] [PubMed] [Google Scholar]
- 3.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 4.Berridge G., Dickinson G., Parrington J., Galione A., Patel S. (2002) J. Biol. Chem. 277, 43717–43723 [DOI] [PubMed] [Google Scholar]
- 5.Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A.M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) Pflugers. Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brailoiu E., Hooper R., Cai X., Brailoiu G. C., Keebler M. V., Dun N. J., Marchant J. S., Patel S. (2010) J. Biol. Chem. 285, 2897–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel S., Marchant J. S., Brailoiu E. (2010) Cell Calcium 47, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S., Docampo R. (2010) Trends Cell Biol. 20, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancela J. M., Churchill G. C., Galione A. (1999) Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 12.Churchill G. C., O'Neill J. S., Masgrau R., Patel S., Thomas J. M., Genazzani A. A., Galione A. (2003) Curr. Biol. 13, 125–128 [DOI] [PubMed] [Google Scholar]
- 13.Lim D., Kyozuka K., Gragnaniello G., Carafoli E., Santella L. (2001) FASEB J. 15, 2257–2267 [DOI] [PubMed] [Google Scholar]
- 14.Berg I., Potter B. V., Mayr G. W., Guse A. H. (2000) J. Cell Biol. 150, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. (2004) J. Biol. Chem. 279, 54319–54326 [DOI] [PubMed] [Google Scholar]
- 16.Brailoiu E., Churamani D., Pandey V., Brailoiu G. C., Tuluc F., Patel S., Dun N. J. (2006) J. Biol. Chem. 281, 15923–15928 [DOI] [PubMed] [Google Scholar]
- 17.Cordiglieri C., Odoardi F., Zhang B., Nebel M., Kawakami N., Klinkert W. E., Lodygin D., Lühder F., Breunig E., Schild D., Ulaganathan V. K., Dornmair K., Dammermann W., Potter B. V., Guse A. H., Flügel A. (2010) Brain. 133, 1930–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boittin F. X., Galione A., Evans A. M. (2002) Circ. Res. 91, 1168–1175 [DOI] [PubMed] [Google Scholar]
- 19.Zhang F., Zhang G., Zhang A. Y., Koeberl M. J., Wallander E., Li P. L. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H274–H282 [DOI] [PubMed] [Google Scholar]
- 20.Zhang F., Xia M., Li P. L. (2010) Am. J. Physiol. Cell Physiol. 298, C1209–C1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macgregor A., Yamasaki M., Rakovic S., Sanders L., Parkesh R., Churchill G. C., Galione A., Terrar D. A. (2007) J. Biol. Chem. 282, 15302–15311 [DOI] [PubMed] [Google Scholar]
- 22.Thai T. L., Churchill G. C., Arendshorst W. J. (2009) Am. J. Physiol. Renal Physiol. 297, F510–F516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran Q. K., Ohashi K., Watanabe H. (2000) Cardiovasc. Res. 48, 13–22 [DOI] [PubMed] [Google Scholar]
- 24.Aley P. K., Noh H. J., Gao X., Tica A. A., Brailoiu E., Churchill G. C. (2010) J. Pharmacol. Exp. Ther. 333, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tugba Durlu-Kandilci N., Ruas M., Chuang K. T., Brading A., Parrington J., Galione A. (2010) J. Biol. Chem. 285, 24925–24932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brailoiu G. C., Brailoiu E., Chang J. K., Dun N.J. (2008) Neuroscience 151, 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bräuner T., Hülser D. F., Strasser R. J. (1984) Biochm. Biophys. Acta 771, 208–216 [DOI] [PubMed] [Google Scholar]
- 28.Kojima H., Nakatsubo N., Kikuchi K., Kawahara S., Kirino Y., Nagoshi H., Hirata Y., Nagano T. (1998) Anal. Chem. 70, 2446–2453 [DOI] [PubMed] [Google Scholar]
- 29.Leikert J. F., Räthel T. R., Müller C., Vollmar A. M., Dirsch V. M. (2001) FEBS Lett. 506, 131–134 [DOI] [PubMed] [Google Scholar]
- 30.Brailoiu E., Filipeanu C. M., Tica A., Toma C. P., de Zeeuw D., Nelemans S. A. (1999) Br. J. Pharmacol. 126, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkesh R., Lewis A. M., Aley P. K., Arredouani A., Rossi S., Tavares R., Vasudevan S. R., Rosen D., Galione A., Dowden J., Churchill G. C. (2008) Cell Calcium 43, 531–538 [DOI] [PubMed] [Google Scholar]
- 32.Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., Mizote A., Rosen D., Thomas J. M., Izumi M., Ganesan A., Galione A., Churchill G. C. (2009) Nat. Chem. Biol. 5, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowman E. J., Siebers A., Altendorf K. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soares S., Thompson M., White T., Isbell A., Yamasaki M., Prakash Y., Lund F. E., Galione A., Chini E. N. (2007) Am. J. Physiol. Cell Physiol. 292, C227-C239 [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki M., Masgrau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J., Galione A. (2004) J. Biol. Chem. 279, 7234–7240 [DOI] [PubMed] [Google Scholar]
- 36.Jardin I., Ben Amor N., Bartegi A., Pariente J. A., Salido G. M., Rosado J. A. (2007) Biochem. J. 401, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey V., Chuang C. C., Lewis A. M., Aley P. K., Brailoiu E., Dun N. J., Churchill G. C., Patel S. (2009) Biochem. J. 422, 503–512 [DOI] [PubMed] [Google Scholar]
- 38.Félétou M. (2009) Br. J. Pharmacol. 156, 545–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brailoiu G. C., Brailoiu E., Parkesh R., Galione A., Churchill G. C., Patel S., Dun N. J. (2009) Biochem. J. 419, 91–97 [DOI] [PubMed] [Google Scholar]