Abstract
The TAZ transcription co-activator promotes cell proliferation and epithelial-mesenchymal transition. TAZ is inhibited by the Hippo tumor suppressor pathway, which promotes TAZ cytoplasmic localization by phosphorylation. We report here that TAZ protein stability is controlled by a phosphodegron recognized by the F-box protein β-TrCP and ubiquitylated by the SCF/CRL1β-TrCP E3 ligase. The interaction between TAZ and β-TrCP is regulated by the Hippo pathway. Phosphorylation of a phosphodegron in TAZ by LATS primes it for further phosphorylation by CK1ϵ and subsequent binding by β-TrCP. Therefore, the Hippo pathway negatively regulates TAZ function by both limiting its nuclear accumulation and promoting its degradation. The phosphodegron-mediated TAZ degradation plays an important role in negatively regulating TAZ biological functions.
Keywords: Breast Cancer, E3 Ubiquitin Ligase, Protein Degradation, Protein Phosphorylation, Transcription Coactivators, Hippo Pathway, TAZ
Introduction
How tissue and organ size are controlled by coordinating cell proliferation and apoptosis remains a fundamental question in biology. Genetic screens in Drosophila over the last several years have delineated a new signaling pathway, the Hippo pathway that regulates organ size by controlling both cell proliferation and apoptosis (1–3). Warts, the first identified component of the Hippo pathway, is a Ser/Thr kinase and functions together with its regulatory subunit, Mob, to inhibit tissue growth (4). Acting upstream, Hippo (a Ste-20 kinase family member) and its binding partner Sav phosphorylate and activate Warts (5, 6). Through a yet to be defined biochemical mechanism, Merlin and expanded, two ERM (ezrin moesin radixin) family members, stimulate the Hippo pathway and are regulated by Fat, a transmembrane protocadherin family member (7–10). Acting downstream of the Hippo pathway is a transcriptional co-activator Yorkie, which regulates the expression of a number of genes, including cyclin E and Diap1 involved in cell proliferation and apoptosis, respectively (11). Hence, the Hippo pathway regulates organ size by controlling cell numbers.
The Hippo pathway is conserved from Drosophila to mammals. In mammalian cells, LATS1/2 and MST1/2 are the homologues of Drosophila Warts and Hippo, respectively (12). YAP, the mammalian homologue of Drosophila Yorkie, has been demonstrated to be phosphorylated and inhibited by LATS (13). TAZ, first identified as a 14-3-3-binding protein, shares ∼50% sequence identity with YAP and has also been shown to function as a transcriptional co-activator downstream of the Hippo pathway (14, 15). TAZ is involved in the development of multiple organs such as lung, fat, muscle, bone, limb, and heart as well as many cellular processes including stem cell differentiation, cell proliferation, epithelial mesenchymal transition (EMT)4 (15–21). Taz knock-out mice develop two severe abnormalities: polycystic kidney disease and emphysema (22, 23).
TAZ has also been implicated in human tumorigenesis. TAZ is inhibited by the Hippo pathway, which contains well-established human tumor suppressor NF2, the mammalian homologue of Drosophila Merlin (15). In addition, other genes in the Hippo pathway, such as WW45 and Mob, are known to be mutated in human cancer cell lines (24, 25). Overexpression of TAZ in MCF10A cells can promote cell proliferation, EMT, and oncogenesis (15, 21, 26). Notably, elevated TAZ expression is observed in more than 20% of breast cancers, especially invasive ductal carcinomas (21). Together, these findings suggest a potential oncogenic activity of TAZ and the importance to control the level of TAZ for the normal development and tumor suppression.
We previously showed that the activity of TAZ is inhibited by LATS kinase (15, 21). There are four HXRXXS LATS phosphorylation motifs in TAZ. We have demonstrated that phosphorylation of TAZ at Ser-89 by LATS promotes its interaction with 14-3-3, resulting in cytoplasmic sequestration and functional inhibition of TAZ. S89A mutation confers TAZ a partial resistance to the inhibition by the Hippo pathway (15). Interestingly, mutation of Ser-311 in another HXRXXS motif also confers TAZ a partial resistance to LATS inhibition (15). The functional significance and mechanism underlying the phosphorylation of Ser-311 and other HXRXXS motifs in TAZ regulation are not clear. This study is directed toward the mechanism of phosphorylation in regulating TAZ ubiquitylation and degradation.
In this report, we showed that phosphorylation of TAZ Ser-311 by LATS primes subsequent phosphorylation on Ser-314 in the phosphodegron by CK1ϵ and recruitment of the SCFβ-TrCP E3 ubiquitin ligase, thus leads to TAZ ubiquitylation and degradation. This provides a mechanism of temporal regulation of TAZ by the Hippo pathway.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with10% fetal calf serum (HyClone), 100 units/ml penicillin, and streptomycin (Invitrogen). MCF10A cells were maintained in DMEM/F-12 medium (Invitrogen) supplemented with 5% horse serum (Invitrogen), 20 ng/ml epidermal growth factor, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 100 ng/ml cholera toxin, 100 units/ml penicillin, and streptomycin (Invitrogen). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) or calcium phosphate method. Cells were harvested at 24 h post-transfection for protein analyses. To establish stable TAZ-expressing cells, pBabe-TAZ retroviruses were generated and used to infect MCF10A cells or NIH3T3 and stable pools were selected in puromycin (1 μg/ml)-containing media for 5 days.
Western Blotting Analysis
Protein lysates were prepared by lysing MCF10A cells in a buffer containing 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Nonidet P-40, 1 mm EDTA, 1 mm PMSF, 25 mm NaF, and mixture protease inhibitors (Roche). Cell lysate (40 μg) was resolved by SDS-PAGE, followed by Western blotting analysis. Antibodies of Flag (cat. A00170 from GenScript or cat. A2220 from Sigma), TAZ (cat. 560235 from BD), HA(F7) (Santa Cruz Biotechnology, SC7392), c-Myc(9E10) (Santa Cruz Biotechnology, SC40), and β-actin (13E5, cat. 4970; Cell Signaling) were purchased commercially. PhosphoTAZ antibody was raised in rabbits using synthetic phosphopeptide as an antigen.
For immunoprecipitation experiments, 500 μg of cell lysate was incubated with anti-Flag M2-agarose for 2h at 4 °C. Beads were washed three times with lysis buffer and centrifuged at 5,000 × g for 5 min between each wash. Protein was eluted from beads with 50 μl of Laemmli sample buffer (Bio-Rad). Lysates were resolved on 8% or 10% SDS-PAGE gels and transferred onto nitrocellulose (Bio-Rad) for Western blotting.
Immunoprecipitation and Kinase Assay
293T cells were transfected with HA-LATS2. 36-h post-transfection, cells were lysed with lysis buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 50 mm NaF, 1.5 mm Na3VO4, protease inhibitor mixture (Roche), 1 mm DTT, 1 mm PMSF), and immunoprecipitated with anti-HA antibody. The immunoprecipitates were washed three times with lysis buffer, followed once with washing buffer (40 mm HEPES, 200 mm NaCl) and once with kinase assay buffer (30 mm HEPES, 50 mm potassium acetate, 5 mm MgCl2). The immunoprecipitated LATS2 was subjected to kinase assays in the presence of 500 μm cold ATP, and 1 μg of His-TAZ, which was expressed and purified from Escherichia coli, as a substrate. The reaction mixtures were incubated at 25 °C for 50 min and terminated with SDS sample buffer. Phosphorylation was detected by either autoradiograph or Western blotting with the phosphoTAZ specific antibody.
In Vitro Ubiquitin Ligation Assays
Myc-tagged CUL1/Skp1/Roc1/β-TrCP1 plasmids were cotransfected into 293T cells, and purified by anti-Myc antibody (Santa Cruz Biotechnology). His-tagged TAZ protein was purified in E. coli. For in vitro ubiquitin ligation assays, TAZ protein was mixed with Myc-CUL1/Skp1/Roc1/β-TrCP1 in 30 μl of ubiquitin ligation buffer (50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 2 mm NaF, 2 mm ATP, 10 nm okadaic acid, 0.6 mm DTT, 12 μg of bovine ubiquitin, 1 μg of FLAG-tagged ubiquitin (Sigma), 60 ng of E1, 500 ng of E2), incubated at 37 °C for 1 h, then terminated by boiling at 99 °C with SDS sample buffer for 5min. Western blotting with anti-FLAG antibody was performed to examine the ubiquitin ladders.
Wound Healing Assay
Monolayer cells were wounded with a sterile plastic tip. Cell migration was observed by microscopy 16 h later.
Colony Formation Assay
Colony formation assay was performed with NIH3T3 fibroblasts. TAZ mutant stable NIH3T3 (5 × 103) cells were seeded on 6-well plates and maintained in DMEM supplemented with 10% fetal bovine serum for 2–3 weeks. Cells were fixed with 10% acetic acid and 10% methanol, and then colonies were stained with 0.005% crystal violet.
RESULTS
TAZ Stability Is Regulated by the 26 S Proteasome
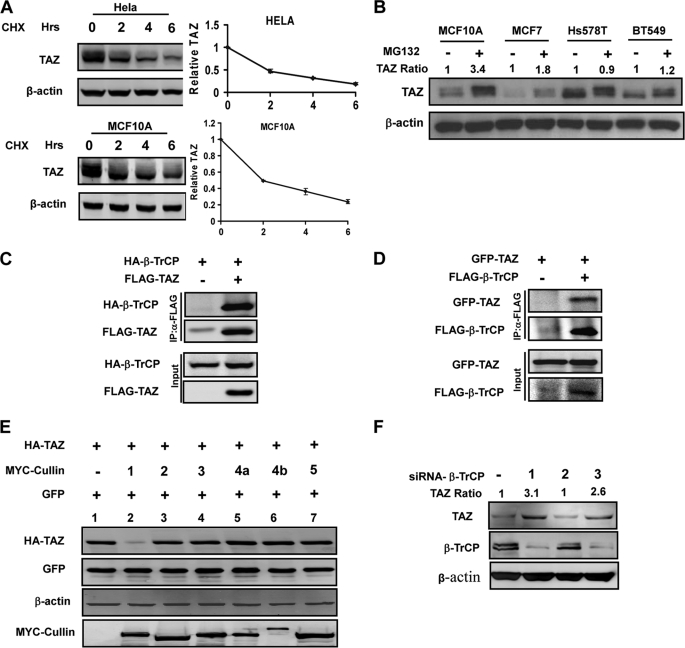
Elevated TAZ protein levels in invasive ductal breast cancers led us to examine the regulation of TAZ protein stability. To test this, we treated HeLa and MCF10A cells with a protein synthesis inhibitor, cycloheximide (CHX), and measured the half-life of endogenous TAZ. We found that TAZ is an unstable protein with a half-life less than 2 h (Fig. 1A). Treatment of several additional cell lines by MG132 also increased the steady state levels of TAZ protein albeit by varying degrees (Fig. 1B). The differential sensitivity of TAZ protein levels to MG132 treatment is likely due to the difference in TAZ stability in those cell lines. Collectively, our data suggest that TAZ protein stability is regulated by the proteasome pathway.
FIGURE 1.
TAZ is an unstable protein associated with β-TrCP. A, TAZ is an unstable protein. Both HeLa and MCF10A cells were treated CHX (20 μg/ml) for indicated times. Endogenous TAZ protein levels were determined. Relative TAZ levels were quantified by the ratio of TAZ to actin. B, MG132 increases TAZ protein levels in multiple cell lines. Cells were treated with either solvent DMSO or 10 μm MG132. Cell lysates were analyzed by Western blot (WB). Relative TAZ levels were normalized by actin and quantified by the ratio between with and without MG132 treatment. C and D, TAZ binds to β-TrCP. HA-β-TrCP was co-transfected with FLAG-TAZ into HEK293T cells as indicated. β-TrCP and TAZ associations were examined by reciprocal co-IP as indicated. E, Cullin-1 expression decreases TAZ protein levels. TAZ was co-transfected with different cullins as indicated. The steady state level of TAZ was determined by WB. F, β-TrCP knockdown increases TAZ protein levels. Three β-TrCP RNAi oligos were individually transfected into 293 cells as indicated. β-TrCP and TAZ levels were determined by WB. Relative TAZ level were quantified by the ratio of TAZ to actin.
TAZ Interacts with Components of SCFβ-TrCP E3 Ligase Complex
Proteasome-mediated degradation depends on polyubiquitylation of target protein. A direct interaction between the E3 ubiquitin ligase and target protein dictates the selective polyubiquitylation of the target protein. To search for TAZ-interacting proteins, we performed mass spectrometry analysis of affinity-purified TAZ (26). One candidate TAZ-interacting protein identified in this search was β-TrCP, of which two peptides, VIVTGSSDSTVR and LVVSGSSDNTIR, were identified (supplemental Fig. S1). β-TrCP, a F-box protein, is the substrate binding subunit of the SKP1-CUL1-F-box (SCFβ-TrCP) E3 ubiquitin ligase and has been implicated in degradation of many growth promoting proteins, such as β-catenin (27). To confirm the interaction between TAZ and SCFβ-TrCP E3 ligase complex, we performed co-immunoprecipitation (co-IP) experiments and found that HA-β-TrCP could be readily pulled down by TAZ (Fig. 1C). Similarly, reciprocal co-IP experiments also showed that TAZ was co-precipitated with β-TrCP (Fig. 1D).
Tian et al. (28) had previously reported a binding of TAZ with β-TrCP and suggested that TAZ mediated the interaction of PC2, a calcium-permeable cation channel protein polycystin 2, with and degradation by SCFβ-TrCP. The authors also noted that SCFβ-TrCP affected TAZ protein levels. Given that TAZ is an unstable protein, we considered TAZ as a substrate of SCFβ-TrCP, rather than a substrate recruiter for PC2. To this end, we first examined the effect of overexpression of different cullin family members on the steady state level of TAZ. Of six cullins examined, overexpression of CUL1, but not the other five cullins, dramatically decreased TAZ protein level (Fig. 1E). We also examined the effect of β-TrCP knockdown on TAZ protein levels. Among the three siRNA tested, two significantly reduced β-TrCP protein and concomitantly increased endogenous TAZ protein levels (Fig. 1F). These data support a notion that TAZ is a substrate of SCFβ-TrCP E3 ligase, which utilizes CUL1.
Phosphorylation of TAZ Ser-311 by LATS Is Important for β-TrCP Binding
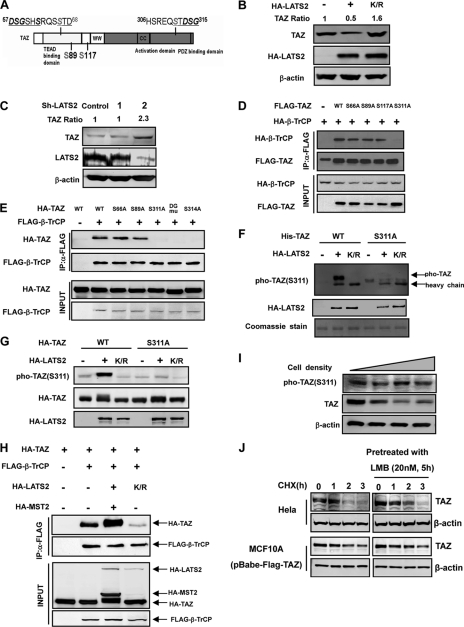
TAZ contains two putative SCFβ-TrCP recognition phosphodegrons (28), one in the N-terminal region and the other in the C-terminal (Fig. 2A). Interestingly, there are LATS phosphorylation HXRXXS motifs in close proximity to both putative phosphodegrons. We tested whether LATS may affect TAZ protein stability. We observed that expression of LATS2 wild-type (WT) significantly decreased the steady state level of endogenous TAZ while expression of the kinase-dead LATS2-K/R mutant modestly increased TAZ protein level (Fig. 2B and supplemental Fig. S2), supporting that TAZ phosphorylation by LATS promotes its degradation. To investigate the role of endogenous LATS in the regulation of TAZ stability, we used shRNA to knockdown LATS in HeLa cells and found that silencing LATS2 significantly increased endogenous TAZ protein level (Fig. 2C). These data demonstrate a role of LATS2 in promoting TAZ degradation.
FIGURE 2.
Phosphorylation of Ser-311 is important for TAZ to bind with β-TrCP. A, schematic illustration of TAZ structure showing the two putative phosphodegrons. The sequences surrounding the phosphodegrons are shown. Lats phosphorylation serine residues are indicated in gray. B, ectopic expression of LATS2 decreases the steady state level of TAZ. The indicated plasmids were co-transfected into HEK293 cells. K/R denotes the kinase inactive mutant LATS2. Relative TAZ levels were quantified by the ratio of TAZ to actin. C, LATS2 knocking down increases endogenous TAZ protein level. LATS2 or unrelated control shRNA was transfected into 293 cells, and endogenous TAZ protein level was examined. LATS2 knockdown efficiency was determined by WB. Relative TAZ levels were quantified by the ratio of TAZ to actin. D, mutation of the LATS phosphorylation site S311A disrupts the interaction between TAZ and β-TrCP. β-TrCP was co-transfected with TAZ WT or different mutants. FLAG-TAZ was immunoprecipitated and the associated HA-β-TrCP was detected by HA WB. The TAZ S311A mutant showed weak interaction with β-TrCP. E, mutations of the C-terminal phosphodegron disrupt the binding between TAZ and β-TrCP. Experiments were similar to those in panel D. Different TAZ mutants used in the transfection are indicated. F, Ser-311 is phosphorylated by LATS in vitro. His-TAZ and S311A mutant were expressed in E. coli and purified. HA-LATS2 was immunoprecipitated from transfected 293T cells and used to phosphorylate the purified His-TAZ in vitro. Phosphorylation of TAZ was detected by pTAZ (Ser-311) antibody. His-TAZ was shown by Coomassie Blue staining. G, expression of LATS enhances TAZ Ser-311 phosphorylation in transfected cells. TAZ WT or S311A mutant was co-transfected with WT or kinase inactive (K/R) mutant of LATS2. Phosphorylation of the co-transfected TAZ was detected by the pTAZ(311) antibody. H, MST/LATS co-transfection increases the interaction between TAZ and β-TrCP. Indicated plasmids were co-transfected into HEK293 cells. FLAG-β-TrCP was immunoprecipitated with FLAG antibody and the co-precipitated HA-TAZ was detected by HA WB. I, TAZ protein level decreases with cell density. MCF10A cells were cultured from 30% density to confluent. TAZ protein levels were determined by WB. J, leptomycin B treatment results in a detectable increase of TAZ stability. Both Hela and Flag-TAZ expressed MCF10A cells were pretreated with or without 20 nm leptomycin B for 5 h, followed by treatment with CHX (20 μg/ml) for indicated times. Endogenous or Flag-TAZ protein levels were determined.
The binding between β-TrCP and its substrate proteins strongly depends on the phosphorylation of target proteins. We then tested the role of the four putative LATS phosphorylation sites (Ser-66, Ser-89, Ser-117, and Ser-311) in the binding of TAZ with β-TrCP. Individual phosphorylation mutants, S66A, S89A, S117A, and S311A, were co-transfected with β-TrCP into HEK293 cells. Co-IP experiments showed that S66A, S89A, and S117A mutants had little effect on β-TrCP binding (Fig. 2D). In contrast, S311A mutation substantially diminished the interaction between TAZ and β-TrCP. These results suggest that the C-terminal phosphodegron plays an important role in mediating TAZ binding with β-TrCP and is regulated by the phosphorylation at Ser-311.
Ser-314, situated in close proximity to the LATS phosphorylation site Ser-311, is predicted to play a critical role in mediating the binding of TAZ to β-TrCP based on previous structure and function studies on the interaction between β-TrCP and other phosphodegrons (29). To further test the role of the C-terminal phosphodegron in β-TrCP binding, we constructed TAZS314A mutant and TAZD313A/G315A (referred to as DG hereafter) double mutant, and examined their binding with β-TrCP. Consistent with previous observation (28), we found that both S314A and DG mutants disrupted the interaction between TAZ and β-TrCP (Fig. 2E), demonstrating the functional importance of the C-terminal phosphodegron in mediating the binding of TAZ with β-TrCP.
To confirm that Ser-311 in TAZ is indeed phosphorylated by LATS in vivo, we generated Ser-311 phosphospecific antibody. In vitro phosphorylation was performed to test the specificity of the antibody. Our data showed that incubation with LATS2 WT but not K/R mutant led to a dramatic increase of Ser-311 phosphorylation as detected by the phosphoSer-311 antibody (Fig. 2F). S311A mutant abolished the reactivity by the phosphoSer-311 antibody, supporting the specificity of this antibody. Taking the advantage of this reagent, we examined TAZ phosphorylation by LATS in the cell. We observed a basal level of Ser-311 phosphorylation and a substantial increase of Ser-311 phosphorylation when LATS2 WT, but not K/R mutant, was co-transfected (Fig. 2G). As expected, S311A abolished recognition by the phosphoantibody. In addition, we tested the effect of MST/LATS on the interaction between TAZ and β-TrCP. MST2/LATS2 co-transfection significantly increased the interaction between TAZ and β-TrCP, whereas co-transfection of LATS2 K/R decreased TAZ interaction with β-TrCP (Fig. 2H). Taken together, these results demonstrate that phosphorylation of Ser-311 in TAZ is important for its binding with β-TrCP.
The Hippo pathway has been implicated in cell contact inhibition. We determined TAZ protein levels of cells at different density. As expected, TAZ protein levels decreased at high cell density while the TAZ Ser-311 phosphorylation, as detected by the phosphoantibody, was not significantly decreased (Fig. 2I), indicating that the relative phosphorylation of TAZ Ser-311 was increased at high cell density. These results are consistent with a role of the Hippo pathway in regulating TAZ Ser-311 phosphorylation and stability.
TAZ is localized in both nucleus and cytoplasm. Lats dependent phosphorylation promotes cytoplasmic localization. We examined if TAZ stability is affected by subcellular localization. Leptomycin B, which blocks nuclear export, was used to treat cells and TAZ stability was determined. We found that leptomycin B treatment resulted in a detectable increase of TAZ stability both in Hela and MCF10A cells (Fig. 2J). These data suggest that the nuclear form, and possibly the unphosphorylated form, of TAZ might be more stable.
Casein Kinase I Cooperates with LATS to Phosphorylate TAZ and Regulate β-TrCP Binding
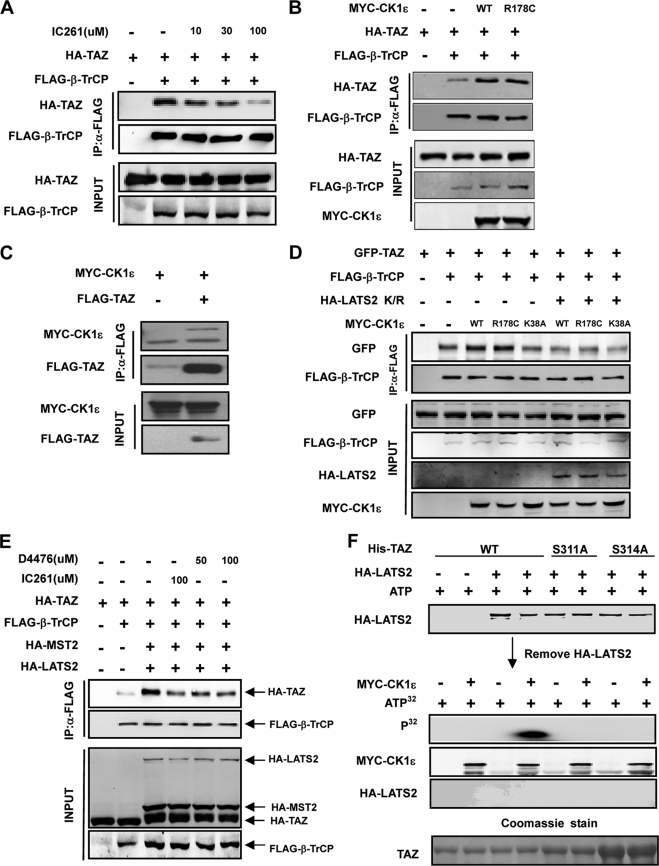
Ser-314 in the phosphodegron is important in mediating TAZ binding with β-TrCP, but does not fit LATS recognition consensus and therefore, is unlikely to be phosphorylated by LATS (Fig. 2A). We speculate that TAZ phosphorylation at Ser-311 by LATS promotes the subsequent phosphorylation of Ser-314 by another kinase. Casein kinase 1 (CK1), recognizes substrate phosphorylation often requires the priming phosphorylation at position -3. We tested IC261, a CK1ϵ/δ specific inhibitor (30), and found that IC261 strongly blocked the interaction between TAZ and β-TrCP (Fig. 3A). We directly tested the function of CK1ϵ by co-transfection and found that co-expression of CK1ϵ or a constitutive active mutant CK1ϵR178C (31) significantly increased the interaction between TAZ and β-TrCP (Fig. 3B). We also examined whether CK1ϵ could bind with TAZ in co-IP and found that TAZ was readily detected in CK1ϵ immunocomplex (Fig. 3C). Together the above data indicate that CK1ϵ/δ may be responsible for Ser-314 phosphorylation.
FIGURE 3.
Casein kinase I cooperates with LATS to regulate the interaction between TAZ and β-TrCP. A, IC261 treatment disrupts the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected and cells were treated with or without IC261, which is a CKIϵ/δ specific inhibitor. The interaction between TAZ and β-TrCP was analyzed by co-IP followed by WB. B, CK1ϵ promotes the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected. The association between TAZ and β-TrCP was examined by co-IP. R178C is an active mutant of CKIϵ. C, association between CK1ϵ and TAZ. CKIϵ was co-transfected with or without TAZ. co-IP was performed to examine the interaction between TAZ and CKIϵ. D, kinase inactive LATS2 mutant blocks the interaction between TAZ and β-TrCP induced by CK1ϵ. The indicated plasmids were co-transfected into HEK293 cells. Interaction between TAZ and β-TrCP was analyzed by co-IP. E, CK1ϵ activity is required for Mst2/LATS2 to stimulate the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected, and cells were treated with or without IC261 or D4476. The interaction between TAZ and β-TrCP was analyzed by co-IP. F, phosphorylation of TAZ S314 by CKIϵ requires LATS2 priming phosphorylation. His-TAZ WT or mutants were expressed and purified from E. coli. The purified TAZ was incubated with HA-LATS2 immunoprecipitated from transfected HEK293T cells, in the presence of cold ATP. HA-LATS2 was removed from the kinase reaction. The prime phosphorylated His-TAZ was then incubated with Myc-CK1ϵ immunoprecipitated from transfected HEK293T cells, in the presence of radioactive ATP. Phosphorylation of TAZ by CKIϵ was detected by incorporation of 32P. His-TAZ input was shown by Coomassie Blue staining (bottom panel).
We then investigated the interdependent relationship of LATS and CK1ϵ on promoting the interaction between TAZ and β-TrCP. Expression of the LATS K/R mutant significantly blocked the stimulating effect of CK1ϵ on the interaction between TAZ and β-TrCP (Fig. 3D). Furthermore, using IC261, we found that inhibition of CK1ϵ/δ decreased the TAZ binding to β-TrCP even in the presence of LATS co-expression (Fig. 3E). Treatment with D4476, another CK1 inhibitor (32), similarly inhibited the interaction between TAZ and β-TrCP, supporting the importance of CK1ϵ activity for MST/LATS to promote TAZ binding with β-TrCP (Fig. 3E).
To directly test the sequential phosphorylation model, we performed in vitro kinase assay. The purified His-tagged recombinant WT and mutant TAZ were incubated with immunoprecipitated LATS2 in the presence of cold ATP and then LATS2 was removed. The efficient removal of HA-LATS2 from the first kinase reactions was confirmed by HA Western blot (Fig. 3F). Consistently, no 32P incorporation was detected if CK1ϵ was not added in the second kinase reaction. The LATS2-phosphorylated TAZ was then phosphorylated by immunoprecipitated CK1ϵ in the presence of [32P]ATP, followed by autoradiography. We observed that CK1ϵ could potently phosphorylate TAZ in vitro if TAZ was prior-treated with LATS2 (Fig. 3F). Without pre-phosphorylation by LATS2, however, TAZ could not be phosphorylated by CK1ϵ. Furthermore, both S311A and S314A mutants abolished TAZ phosphorylation by CK1ϵ (Fig. 3F). We conclude from these results that TAZ phosphorylation by CK1ϵ at Ser-314 is dependent on a prior phosphorylation at Ser-311 by LATS.
LATS and CK1 Promotes TAZ Degradation
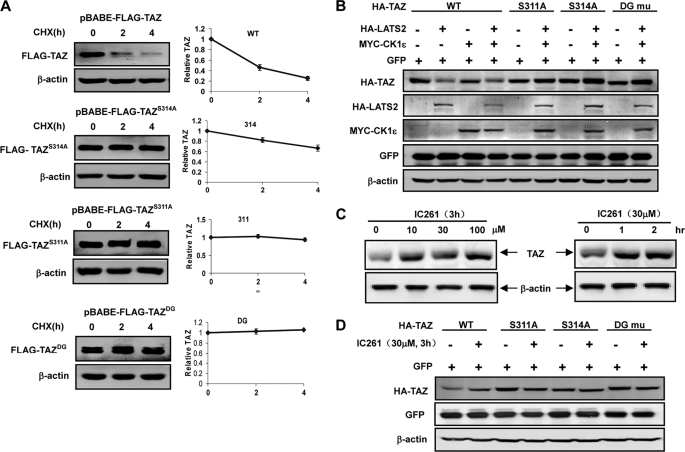
To test the functional significance of Ser-311 phosphodegron in TAZ stability regulation, we established stable MCF10A cell pools expressing TAZ WT, S311A, S314A, and DG, and determined the TAZ protein stability. We observed that TAZ WT rapidly disappeared when protein synthesis was inhibited (Fig. 4A). In contrast, both S311A and S314A mutants were dramatically stabilized (Fig. 4A). Similarly, DG mutation also stabilized TAZ. Interestingly, we observed the similar phenomena in C3H10T1/2 cells stably expressing TAZWT, TAZS311A, TAZS3114A, and TAZDG (supplemental Fig. S3). Consistently, ectopic expression of LATS2 and/or CK1ϵ decreased the steady state level of TAZWT, but had little effect on that of TAZS311A, TAZS314A, or TAZDG mutants (Fig. 4B). The protein level of the co-transfected GFP was not affected. These results further support the importance of the C-terminal phosphodegron in regulating TAZ degradation in response to LATS and CK1ϵ. Consistently, treatment of cells with IC261 also increased endogenous TAZ protein levels (Fig. 4C). Furthermore, IC261 treatment increased the steady state levels of TAZ WT, but had little effect on that of S311A, 314A, or DG mutant (Fig. 4D). Collectively, we conclude that LATS and CK1ϵ promote TAZ degradation via phosphorylation of the C-terminal phosphodegron.
FIGURE 4.
LATS and CK1 promote TAZ degradation via the C-terminal phosphodegron. A, Ser-311 and Ser-314 are important for TAZ degradation. MCF10A cells stably expressing TAZWT, TAZS311A, TAZS3114A, and TAZDG were chased with CHX treatment as indicted. TAZ stability was determined by WB. Relative TAZ levels were quantified by the ratio of TAZ to actin. B, Ser-311 and Ser-314 are required for TAZ destabilization by LATS and CK1ϵ. TAZ WT and different mutants were co-transfected with or without LATS and/or CK1ϵ as indicated. TAZ expression level was determined by WB. The co-transfected GFP and endogenous actin were included as controls. C, CK1 inhibitor increases endogenous TAZ protein levels. HeLa cells were treated with or without IC261 at different doses and time points. WB was performed to determine TAZ expression levels along the actin control. D, C-terminal phosphodegron is required for TAZ stabilization by CK1 inhibitors. The indicated plasmids were transfected into 293T cells. Treatments with or without the IC261 were indicated. TAZ expression level was determined by WB.
TAZ Ubiquitylation Is Promoted by LATS2, CK1ϵ, and SCFβ-TrCP
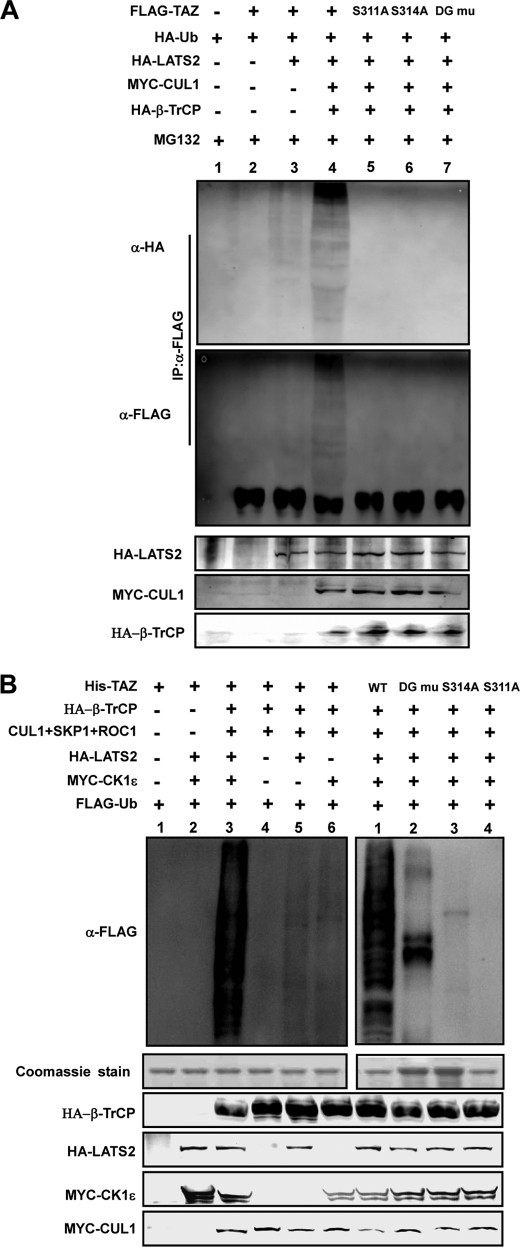
We next determined the effect of Ser-311 phosphorylation by LATS on TAZ ubiquitylation in vivo. Prior to the analysis of TAZ ubiquitylation, cells were treated with MG132, to enrich ubiquitylated proteins. Co-expression of TAZ with LATS, β-TrCP, and CUL1 resulted in the detection of characteristic incremental ladders, indicative of polyubiquitylated species (Fig. 5A). In fact, co-expression of LATS2 alone also induced a small, but detectable, increase of ubiquitylated TAZ (comparing lanes 2 and 3, Fig. 5A). Omitting β-TrCP and CUL1 resulted in a substantial decrease of polyubiquitylated TAZ (compare lanes 3 and 4), demonstrating the dependence of TAZ polyubiquitylation on SCFβ-TrCP. TAZ mutants of S311A, S314A, and DG all reduced the polyubiquitylation to nearly undetectable low level, consistent with the functional dependence of TAZ ubiquitylation on the phosphorylation by LATS and CK1ϵ and the integrity of C-terminal phosphodegron. The high molecular weight ladders were dramatically diminished in the absence of MG132 (supplemental Fig. S4), demonstrating polyubiquitylation and proteasome-dependent degradation of TAZ in vivo.
FIGURE 5.
LATS2 and CK1ϵ promote TAZ ubiquitylation by SCFβ-TrCP. A, LATS and SCFβ-TrCP E3 ligase promotes TAZ ubiquitylation depending on the C-terminal phosphodegron. FALG-TAZ was co-transfected with various plasmids as indicated. FLAG-TAZ was immunoprecipitated and ubiquitylation of the precipitated TAZ was determined by WB for the co-transfected HA-ubiquitin or FLAG-TAZ. TAZ S311A, S314A, and DG mutants were also tested for in vivo ubiquitylation. B, in vitro ubiquitylation of TAZ by SCFβ-TrCP E3 ligase requires LATS and CK1ϵ. HA-LATS2 and MYC-CK1ϵ were immunopre cipitated from transfected 293T cells. In vitro ubiquitylation assays were performed using purified His-TAZ as a substrate in the presence of various proteins as indicated. Experiments with different TAZ mutants were also performed (right panel). Ubiquitylation of TAZ was detected by FLAG antibody for FLAG-Ub. His-TAZ input was shown by Coomassie Blue staining. Other components were determined by WB as indicated.
In vitro ubiquitylation experiments were performed to directly test the role of LATS2, CK1ϵ, the phosphodegron, and SCFβ-TrCP complex in TAZ ubiquitylation. Purified TAZ (from E. coli) was incubated with various purified proteins (from transfected mammalian cells) as indicated in Fig. 5B. We found that efficient TAZ ubiquitylation required the presence of all components of SCF E3 ligase complex, LATS, and CK1ϵ (Fig. 5B). When either LATS or CK1ϵ was omitted in the reactions, TAZ ubiquitylation was considerably reduced. These data provide direct biochemical evidence that LATS and CK1ϵ are key regulators of TAZ ubiquitylation by SCFβ-TrCP. S311A, S314A, and DG all significantly reduced in vitro TAZ ubiquitylation (Fig. 5B). These results support a model that the sequential phosphorylation of Ser-311 and Ser-314 by LATS and CK1ϵ is essential for SCFβ-TrCP-mediated TAZ ubiquitylation and degradation.
The C-terminal Phosphodegron Regulates TAZ Biological Functions
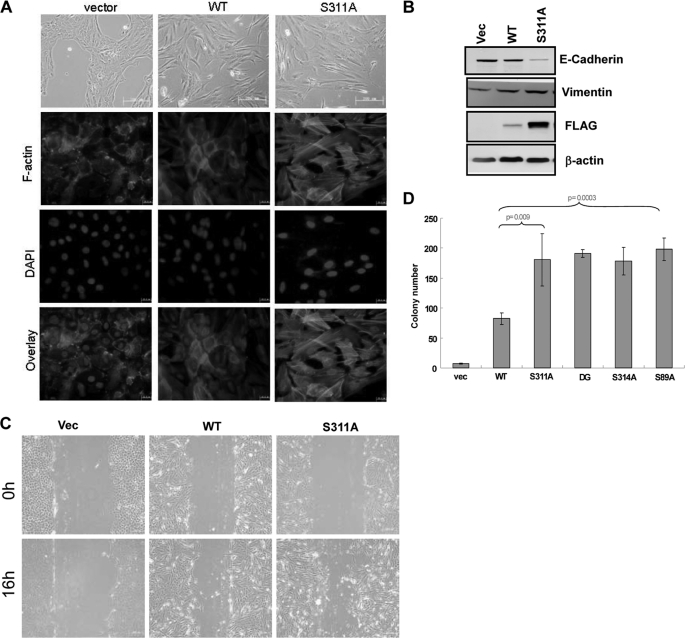
TAZ is known to induce EMT and promote cell proliferation. To determine the functional significance of C-terminal phosphodegron, we established MCF10A cells stably expressing different TAZ mutants. The control MCF10A cells grew in cluster, typical for epithelial cells, while the TAZ-expressing cells displayed a loss of cell-cell contact and were scattered (Fig. 6A). TAZ expression also induced stress fiber formation. We found that TAZ-S311A was more potent than the wild type TAZ to promote EMT morphological changes in MCF10A cells (Fig. 6A). Western blotting for EMT markers also supports that the phosphorylation mutant TAZ caused a stronger induction of vimentin, a marker for mesenchymal cells, and a stronger reduction of E-cadherin, a marker for epithelial cells (Fig. 6B). We also determined cell proliferation. The TAZ S311A mutant caused a slightly increase in cell proliferation than the wild-type TAZ in NIH3T3 cells (supplemental Fig. S5). Furthermore, cell migration was determined by wound healing assays. Expression of the TAZ phosphodegron mutant stimulated cell migration more potently than expression of the wild-type TAZ did (Fig. 6C). These data show that the C-terminal phosphodegron negatively regulate TAZ biological functions.
FIGURE 6.
Phosphodegron mutants promote TAZ function. A, TAZ degradation mutants induce a stronger morphological change (upper panel) and altered actin organization in MCF10A cells (lower panel). Phase-contrast images of MCF10A cells expressing vector, TAZ and TAZS311A are shown. Cells were stained with rhodamine-conjugated phalloidin. B, TAZ degradation mutants are more potent in inducing EMT in MCF10A cells. Cell lysates from MCF10A cells expressing vector, TAZ and TAZS311A were probed for epithelial marker and mesenchymal marker as indicated. C, TAZ degradation mutants stimulate cell migration. MCF10A cells expressing vector, TAZ and TAZS311A were analyzed for migration by a wound healing assay. D, transformation of NIH 3T3 cells by TAZ degradation mutants. 5 × 103 NIH3T3 cells stably expressing vector, TAZ and TAZS311A were cultured in soft agar for 14 days before colonies were counted. Colonies were then visualized by crystal violet staining and counted.
We also investigated the activity of TAZ phosphodegron mutant in promoting transformation. Untransformed NIH3T3 cells could not form colonies in monolayer culture while expression of TAZ could transform NIH3T3 cells. We found that the TAZ phosphodegron mutants were significantly more active in stimulating NIH3T3 transformation than the wild-type TAZ (Fig. 6D). Together, the above data demonstrate a key role of the phosphodegrons in regulating the physiological function of TAZ.
DISCUSSION
In this report, we show that TAZ protein stability is controlled by C-terminal phosphodegron that is phosphorylated by LATS and CK1, then recognized by the F-box protein, β-TrCP, and subsequent ubiquitylated by the SCFβ-TrCP E3 ligase. Our study reveals a molecular mechanism of the Hippo pathway in regulating TAZ degradation in addition to regulating TAZ subcellular localization.
As a transcription co-activator, TAZ must enter the nucleus in order to promote gene expression. We previously demonstrated that the Hippo pathway inhibits TAZ by promoting cytoplasmic localization via Ser-89 phosphorylation, which generates a 14-3-3 binding site (15). This provides a mechanism of spatial separation of TAZ by sequestering it away from its nuclear target transcription factors, such as TEAD (26, 33). In this study we revealed another mechanism, SCFβ-TrCP-mediated ubiquitylation and 26 S proteasome-dependent degradation, for the regulation of TAZ by the Hippo pathway via protein stability control.
We found that LATS phosphorylates TAZ on Ser-311, which primes TAZ for phosphorylation on Ser-314 by CK1ϵ. Phosphorylation of Ser-314 produces a functional phosphodegron that is recognized by β-TrCP, resulting in subsequent TAZ ubiquitylation and degradation. In contrast to the spatial regulation of TAZ via nuclear-cytoplasmic shuttling that is reversible and probably represents a short-term inhibition in response to the LATS activation, the SCFβ-TrCP-mediated ubiquitylation provides a mechanism of irreversible inhibition and is more likely employed by the cells for a long-term growth arrest.
One noticeable difference between these two mechanisms is that LATS-mediated Ser-89 phosphorylation alone is sufficient to promote cytoplasmic retention of TAZ, while SCFβ-TrCP-mediated ubiquitylation requires sequential phosphorylation of the C-terminal phosphodegron by both LATS and CK1. Only when both LATS and CK1 are activated, can TAZ degradation be triggered. We speculate that such a requirement of sequential phosphorylation sets a high stringency before the irreversible degradation can occur.
Our data show that activation of the C-terminal phosphodegron requires the sequential phosphorylation by LATS and CK1. CK1 kinase recognizes unique substrate specificity as p(S/T)-X(1–2)-(S/T), which requires preceding phosphorylation of residue at the -2 or -3 position of the target residue. The coordinated phosphorylation of the C-terminal phosphodegron by LATS and CK1 provides a biochemical mechanism for signal integration. TAZ ubiquitylation cannot be initiated unless both LATS and CK1 are active. LATS kinase is activated by high cell density while the signal that is mediated by CK1ϵ to destabilize TAZ remains to be determined. Consistent with our model, mutation of either the LATS phosphorylation site Ser-311 or the CK1ϵ phosphorylation site Ser-314 stabilizes TAZ protein. In fact, mutation of either Ser-311 or Ser-314 activates TAZ as the mutant TAZ is more potent than the WT in inducing EMT and transformation.
YAP is a transcription co-activator related to TAZ and also acts downstream of the Hippo pathway. YAP also contains a C-terminal phosphodegron that is regulated in a manner similar to TAZ (34). The biological functions of the two proteins are not redundant. For example, the Yap knock-out mice are early embryonic lethal while Taz knock-out mice go through normal development but develop cystic renal disease (22, 23, 35). Furthermore, elevated protein levels of both YAP and TAZ have been found in human cancers, but with clear organ and tissues specificities. For example, elevated YAP protein has been found in liver and prostate cancers while high TAZ protein is associated with breast cancer. The Hippo pathway has been shown to promote YAP degradation in a manner similar to TAZ (34), therefore, revealing a common mechanism of phosphorylation-coupled degradation in regulation of Lats targets.
In summary, we have demonstrated that TAZ stability is controlled by C-terminal phosphodegron recognized by the SCFβ-TrCP E3 ligase. Activation of the C-terminal phosphodegron depends on the sequential phosphorylation by LATS and CK1. Our studies provide new insights into the mechanism of TAZ overexpression in human cancer.
Acknowledgments
We thank the members of the Fudan MCB Laboratory for discussions throughout this study and thank the Mass Spectrometry and Systems Biology Laboratory, Institute of Molecular and Cell Biology, Republic of Singapore for TAZ MS analysis.
This work was supported, in whole or in part, by National Institutes of Health grants (to Y. X. and K.-L. G.). This work was also supported by the 985 Program and New Century Talent from the Chinese Ministry of Education (Grant No. NCET-09-0315) from the Chinese Ministry of Education, 863 (Grant No. 2006AA02A308), 973 (Grant No. 2009CB918401), NSFC (Grant No. 30600112, 30871255, 31071192), Shanghai key project (Grant No. 09JC1402300), Grant No. BK2006546, and the Shanghai Leading Academic Discipline Project, Project Number B110.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- EMT
- epithelial mesenchymal transition
- SCF
- SKP1-CUL1-F-box
- CHX
- cycloheximide.
REFERENCES
- 1.Harvey K., Tapon N. (2007) Nat. Rev. Cancer 7, 182–191 [DOI] [PubMed] [Google Scholar]
- 2.Saucedo L. J., Edgar B. A. (2007) Nat. Rev. Mol. Cell Biol. 8, 613–621 [DOI] [PubMed] [Google Scholar]
- 3.Pan D. (2007) Genes Dev. 21, 886–897 [DOI] [PubMed] [Google Scholar]
- 4.Udan R. S., Kango-Singh M., Nolo R., Tao C., Halder G. (2003) Nat. Cell Biol. 5, 914–920 [DOI] [PubMed] [Google Scholar]
- 5.Wu S., Huang J., Dong J., Pan D. (2003) Cell 114, 445–456 [DOI] [PubMed] [Google Scholar]
- 6.Wei X., Shimizu T., Lai Z. C. (2007) EMBO J. 26, 1772–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamaratoglu F., Willecke M., Kango-Singh M., Nolo R., Hyun E., Tao C., Jafar-Nejad H., Halder G. (2006) Nat. Cell Biol. 8, 27–36 [DOI] [PubMed] [Google Scholar]
- 8.Willecke M., Hamaratoglu F., Kango-Singh M., Udan R., Chen C. L., Tao C., Zhang X., Halder G. (2006) Curr. Biol. 16, 2090–2100 [DOI] [PubMed] [Google Scholar]
- 9.Silva E., Tsatskis Y., Gardano L., Tapon N., McNeill H. (2006) Curr. Biol. 16, 2081–2089 [DOI] [PubMed] [Google Scholar]
- 10.Bennett F. C., Harvey K. F. (2006) Curr. Biol. 16, 2101–2110 [DOI] [PubMed] [Google Scholar]
- 11.Huang J., Wu S., Barrera J., Matthews K., Pan D. (2005) Cell 122, 421–434 [DOI] [PubMed] [Google Scholar]
- 12.Edgar B. A. (2006) Cell 124, 267–273 [DOI] [PubMed] [Google Scholar]
- 13.Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai F., Marignani P. A., Sarbassova D., Yagi R., Hall R. A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L. C., Yaffe M. B. (2000) EMBO J. 19, 6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. (2008) Mol. Cell. Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J. H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A., Hopkins N., Yaffe M. B. (2005) Science 309, 1074–1078 [DOI] [PubMed] [Google Scholar]
- 17.Hong J. H., Yaffe M. B. (2006) Cell Cycle 5, 176–179 [DOI] [PubMed] [Google Scholar]
- 18.Mahoney W. M., Jr., Hong J. H., Yaffe M. B., Farrance I. K. (2005) Biochem. J. 388, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami M., Nakagawa M., Olson E. N., Nakagawa O. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18034–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park K. S., Whitsett J. A., Di Palma T., Hong J. H., Yaffe M. B., Zannini M. (2004) J. Biol. Chem. 279, 17384–17390 [DOI] [PubMed] [Google Scholar]
- 21.Chan S. W., Lim C. J., Guo K., Ng C. P., Lee I., Hunziker W., Zeng Q., Hong W. (2008) Cancer Res. 68, 2592–2598 [DOI] [PubMed] [Google Scholar]
- 22.Makita R., Uchijima Y., Nishiyama K., Amano T., Chen Q., Takeuchi T., Mitani A., Nagase T., Yatomi Y., Aburatani H., Nakagawa O., Small E. V., Cobo-Stark P., Igarashi P., Murakami M., Tominaga J., Sato T., Asano T., Kurihara Y., Kurihara H. (2008) Am. J. Physiol. Renal Physiol. 294, F542–553 [DOI] [PubMed] [Google Scholar]
- 23.Hossain Z., Ali S. M., Ko H. L., Xu J., Ng C. P., Guo K., Qi Z., Ponniah S., Hong W., Hunziker W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Z. C., Wei X., Shimizu T., Ramos E., Rohrbaugh M., Nikolaidis N., Ho L. L., Li Y. (2005) Cell 120, 675–685 [DOI] [PubMed] [Google Scholar]
- 25.Tapon N., Harvey K. F., Bell D. W., Wahrer D. C., Schiripo T. A., Haber D. A., Hariharan I. K. (2002) Cell 110, 467–478 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Liu C. Y., Zha Z. Y., Zhao B., Yao J., Zhao S., Xiong Y., Lei Q. Y., Guan K. L. (2009) J. Biol. Chem. 284, 13355–13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 28.Tian Y., Kolb R., Hong J. H., Carroll J., Li D., You J., Bronson R., Yaffe M. B., Zhou J., Benjamin T. (2007) Mol. Cell. Biol. 27, 6383–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlicky S., Tang X., Willems A., Tyers M., Sicheri F. (2003) Cell 112, 243–256 [DOI] [PubMed] [Google Scholar]
- 30.Behrend L., Milne D. M., Stöter M., Deppert W., Campbell L. E., Meek D. W., Knippschild U. (2000) Oncogene 19, 5303–5313 [DOI] [PubMed] [Google Scholar]
- 31.Lowrey P. L., Shimomura K., Antoch M. P., Yamazaki S., Zemenides P. D., Ralph M. R., Menaker M., Takahashi J. S. (2000) Science 288, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rena G., Bain J., Elliott M., Cohen P. (2004) EMBO Rep. 5, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan S. W., Lim C. J., Loo L. S., Chong Y. F., Huang C., Hong W. (2009) J. Biol. Chem. 284, 14347–14358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. (2010) Genes Dev. 24, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morin-Kensicki E. M., Boone B. N., Howell M., Stonebraker J. R., Teed J., Alb J. G., Magnuson T. R., O'Neal W., Milgram S. L. (2006) Mol. Cell. Biol. 26, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]