Abstract
In this study, we tested the efficacy of increasing liver glycogen synthase to improve blood glucose homeostasis. The overexpression of wild-type liver glycogen synthase in rats had no effect on blood glucose homeostasis in either the fed or the fasted state. In contrast, the expression of a constitutively active mutant form of the enzyme caused a significant lowering of blood glucose in the former but not the latter state. Moreover, it markedly enhanced the clearance of blood glucose when fasted rats were challenged with a glucose load. Hepatic glycogen stores in rats overexpressing the activated mutant form of liver glycogen synthase were enhanced in the fed state and in response to an oral glucose load but showed a net decline during fasting. In order to test whether these effects were maintained during long term activation of liver glycogen synthase, we generated liver-specific transgenic mice expressing the constitutively active LGS form. These mice also showed an enhanced capacity to store glycogen in the fed state and an improved glucose tolerance when challenged with a glucose load. Thus, we conclude that the activation of liver glycogen synthase improves glucose tolerance in the fed state without compromising glycogenolysis in the postabsorptive state. On the basis of these findings, we propose that the activation of liver glycogen synthase may provide a potential strategy for improvement of glucose tolerance in the postprandial state.
Keywords: Diabetes, Glucose Metabolism, Glycogen, Glycogen Synthase, Liver
Introduction
The liver responds to an increase in blood glucose concentration in the postprandial state by net uptake of glucose and conversion to glycogen, which is subsequently mobilized in the postabsorptive state to maintain blood glucose homeostasis. Various attempts have been made to improve blood glucose homeostasis through the modulation of the expression or activity of proteins involved in the control of liver glycogen metabolism. Glucokinase (GK)3 catalyzes the first step in hepatic glucose metabolism and exerts high control on liver glycogen synthesis (1). Previous studies using either transgenic models (2, 3) or adenoviral vectors targeting the liver (4) demonstrated improved glucose tolerance and/or a lowering of blood glucose in basal conditions. However, GK overexpression also increases flux through glycolysis, and in some circumstances this leads to hypertriglyceridemia (4).
An alternative approach to modulating liver glycogen metabolism without stimulating glycolysis and triglyceride formation is through modifying the enzymes that are involved exclusively in glycogen synthesis and degradation or regulatory proteins that may affect the activity of the former, such as protein targeting to glycogen (PTG) (5). Overexpression of PTG in the liver by means of an adenoviral vector increases glucose tolerance without perturbing lipid homeostasis (6). However, a limitation of this experimental model is that it leads to the progressive accumulation of glycogen because PTG promotes the inactivation of glycogen phosphorylase (GP) also during fasting, and consequently there is negligible depletion of glycogen in the postabsorptive state and during a prolonged fast. Similarly, GL targeting subunit overexpression in the livers of streptozotocin-induced diabetic rats causes a large increase in liver glycogen stores but only a transient decrease in blood glucose levels. The glycemia-reducing effect can be prolonged in time by using a truncated version of the scaffolding proteins GM and GL, termed GMΔC, which does not compromise the response to glycogenolytic signals (7–9).
Another approach is the use of inhibitors of GP, which promote the dephosphorylation of the enzyme (conversion of GPa to GPb) and the subsequent activation of glycogen synthase (GS) (10, 11). GP inhibitors reduce blood glucose in animal models of diabetes and improve glucose tolerance in the postprandial state. An alternative strategy has been to selectively modify the interaction between GPa and the glycogen-targeting protein GL by mutating the terminal GPa binding domain or by using small molecules that prevent the interaction of GPa with GL (12). In this way, the negative control exerted by GPa on LGS activation is released without altering the activation state of GPa.
A common end point of all of the above strategies is that they aim to induce the activation of liver GS (LGS) through indirect mechanisms. However, to date, the strategy of directly activating LGS, independently of the rate of glucose phosphorylation or the activation state of GP, to regulate blood glucose homeostasis has not been addressed.
To this end, we examined the impact of the adenovirus-mediated overexpression of wild-type LGS and a constitutively active LGS variant (13) on livers of healthy rats. In addition, we generated liver-specific transgenic mice expressing the activated mutant LGS. Using these approaches, we have demonstrated the relevance of the phosphorylation state of LGS in the control of blood glucose homeostasis and in the regulation of hepatic glycogen storage.
EXPERIMENTAL PROCEDURES
Preparation of Recombinant Adenoviruses
Recombinant adenoviruses encoding for the bacterial enzyme β-galactosidase (β-gal), wild-type Rattus norvegicus LGS (WT LGS) (14), or a constitutively active Rattus norvegicus LGS variant mutated at phosphorylation sites 2 and 3b (activated mutant LGS) (13) were amplified and purified for injection into animals, following procedures described previously (15).
Animal Studies
All procedures were approved by the Barcelona Science Park's Animal Experimentation Committee and were carried out in accordance with the European Community Council Directive and National Institutes of Health guidelines for the care and use of laboratory animals.
Rat Studies
Male Wistar rats (Charles River Laboratories) weighing 200–250 g were housed for 1 week before any procedure and were allowed free access to water and standard laboratory chow (Harlan Tekland Laboratory diet 7001). After procedures, the rats were caged individually under a standard 12-h light/12-h dark cycle to allow monitoring of food and water intake. Two experimental protocols were performed. In the first, rats were anesthetized with 2% isofluorane (Isoba vet, Schering Plough) and infused with 1 × 1012 particles of activated mutant LGS-, WT LGS-, or β-gal-encoding purified adenoviruses. 96 h after adenovirus administration, animals were either fasted for 18 h or allowed to continue to feed ad libitum. In the second protocol, animals were infused with the same titer of adenovirus, and after 96 h, they were fasted for 18 h. This was followed by an intraperitoneal glucose injection (intraperitoneal glucose tolerance test (IPGTT); 2 g/kg body weight). Blood samples from the tail vein were collected immediately before administration of the bolus and at the time intervals indicated in order to measure circulating glucose concentrations. In all procedures, tissue samples were obtained from anesthetized animals (sodium thiopental (Tiobarbital Braun), 0.1 g/kg body weight intraperitoneally), rapidly snap-frozen in liquid nitrogen, and stored at −80 °C for further analysis.
Transgenic Mouse Generation and Studies
The Mus musculus Gys2 cDNA sequence (clone ID 5051685, pCMV-SPORT6 vector, Invitrogen) previously mutated at sites 2 and 3b (Ser → Ala mutations, by site-directed mutagenesis using the following primers: for site 2, GCCGCTCCTTGCCGGTGACATCCCTTG (sense) and CAAGGGATGTCACCGGCAAGGAGCGGC (antisense); site 3b, GCTTTAAGTATCCCAGGCCCTCCGCAGTACCACC (sense) and GGTGGTACTGCGGAGGGCCTGGGATACTTAAAGC (antisense), respectively) was subcloned between the intron II of the rabbit β-globin gene and the rabbit β-globin and SV40 polyadenylation signals of the pSG5 plasmid (a generous gift from Dr. P. Chambon, Université Louis Pasteur). This fragment was then subcloned into the EcoRV site of the p2335-1 plasmid (a generous gift from Dr. K. Khono, Nara Institute of Science and Technology), which contains the mouse albumin enhancer/promoter, generating the palbpSG5MmLGS-2 + 3b vector. A 5-kb NotI/SalI digestion fragment was excised, microdialyzed, and microinjected into the pronuclei of fertilized mouse eggs (C57BL/6J × C57BL/6J) at the Mouse Mutant Core Facility, Institute for Research in Biomedicine (Barcelona, Spain). Embryos were implanted into pseudopregnant foster females (ICR), and transgenic pups were identified. DNA samples from tail clips of subsequent litters were screened by PCR with primers (forward, ATCCCCCGGGCTGCAGGAAT; reverse, GCACGTTGCCCAGGAGCTGT) that amplified a 638-bp fragment of the transgene. The transgene was maintained on the C57BL/6J background throughout the study. Transgenic and wild-type mice were allowed free access to a standard chow diet and water and maintained on a 12-h/12-h light/dark cycle under specific pathogen-free conditions in the Animal Research Center at the Barcelona Science Park. After weaning at 3 weeks of age, tail clippings were taken for genotyping by PCR. Glucose tolerance tests were carried out using 20-week male mice after fasting by injecting 2 g/kg glucose intraperitoneally. Glucose levels were measured from tail bleeds at 0, 5, 15, 30, 60, 90, and 120 min. For the determination of liver glycogen content and LGS activity and expression, fed and 18 h-fasted male mice were given a lethal dose of anesthesia (sodium thiopental (Tiobarbital Braun), 0.2 g/kg body weight intraperitoneally), and tissues were rapidly snap-frozen in liquid nitrogen and stored at −80 °C for further analyses.
Enzyme Activity and Metabolite Determination
To measure GS activity, tissue samples (100 mg) were added to 1 ml of ice-cold homogenization buffer containing 10 mm Tris-HCl (pH 7), 150 mm KF, 15 mm EDTA, 15 mm 2-mercaptoethanol, 0.6 m sucrose, 1 mm benzamidine, 1 mm phenylmethanesulfonyl fluoride, 25 nm okadaic acid, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 10 μg/ml pepstatin and were then homogenized (Polytron) at 4 °C. GS activity was measured in whole homogenates in the absence or presence of 6.6 mm glucose 6-phosphate, representing active or total activity, respectively (16). The −/+ glucose 6-phosphate activity ratio is an estimation of the activation state of the enzyme.
Electrophoresis and Immunoblotting
Immunoreactivity was determined by resolving homogenates (20 μg of protein) by 10% SDS-PAGE. The protein was transferred onto a nitrocellulose membrane and probed with the following antibodies: a rabbit antibody against rat LGS (17), a mouse antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma), and a mouse antibody against actin (Sigma). Secondary antibodies conjugated to horseradish peroxidase against rabbit (GE Healthcare) or mouse (DakoCytomation) immunoglobulins were used. Immunoreactive bands were visualized using an ECL Plus kit (GE Healthcare), following the manufacturer's instructions.
Blood Parameters
Blood glucose was measured using a HemoCue glucose analyzer (HemoCue AB, Angelholm, Sweden). Plasma aspartate-aminotransferase activity was measured spectrophotometrically by standard techniques (HORIBA ABX) adapted to a COBAS Mira analyzer (Roche Applied Science). Only animals with blood plasma aspartate aminotransferase concentrations lower than 200 units/liter (indicative of the absence of virus-induced liver damage) were used for further study (18). Plasma lactate (HORIBA ABX), triglycerides (Sigma), and 3-hydroxybutyrate (BHBA; Sigma) concentrations were measured spectrophotometrically by standard techniques adapted to a COBAS Mira analyzer. Plasma non-esterified fatty acids (NEFAs; Wako) were measured by colorimetric determination adapted to a Freedom Evo Tekan analyzer. Plasma insulin levels were measured by immunoassay (Spi Bio).
RNA Purification and Retrotranscription
Total RNA was isolated from rat liver tissue by homogenizing (Polytron) 100 mg of the sample in 1 ml of TRIzol (Invitrogen). After centrifugation at 12,000 × g for 5 min, 0.2 ml of chloroform was added to the supernatant, and it was then centrifuged again at 12,000 × g for 15 min at 4 °C to separate it into two phases. Adding 0.5 ml of isopropyl alcohol to the aqueous phase then precipitated total RNA. After an incubation of 10 min at room temperature, samples were centrifuged at 12,000 × g for 10 min at 4 °C. Pellets were washed with 1 ml of 70% ethanol and centrifuged at 7500 × g for 5 min at 4 °C. The desiccated pellets were resuspended in 100 μl of RNase-free water. 5 μg of total RNA from each sample was reverse-transcribed for 50 min at 42 °C in a 15-ml reaction volume using 200 units of SuperScript III reverse transcriptase (SuperScript First-strand Synthesis System for RT-PCR, Invitrogen) in the presence of 50 ng of random hexamers.
Quantitative Real-time PCR
PCR tests were performed following the standard real-time PCR protocol of the ABI Prism 7900 Detection System, together with the appropriate ready-made TaqMan primer/probe sets (Applied Biosystems) at the Genomic Unit of Core Scientific Services at the University of Barcelona. Each sample was analyzed from triplicate wells. The temperature profile consisted of 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Data were analyzed with the 2ΔΔCt method using 18 S rRNA as endogenous control.
Glycogen Analysis
Liver glycogen content was determined by an amyloglucosidase-based assay (19). To assess glycogen branching, the method described by Krisman (20) was used.
Electron Microscopy Analysis
Rat liver biopsies were fixed in 2.5% glutaraldehyde solution for 24 h, rinsed in Sorensen's phosphate buffer, and postfixed in 1% osmium tetroxide in Sorensen's phosphate buffer. The fixed tissue was dehydrated in an ascending series of graded ethanol solutions. It was then rinsed with propylene oxide, embedded in Eponate 12, and polymerized at 60 °C for more than 48 h. Thin sections were prepared with a diamond knife on an Leica Ultracut UCT ultratome at the Electron Microscopy Unit of the Core Scientific Services at the University of Barcelona, and sections were then collected on copper grids. Sections were then stained with 2% uranyl acetate in water and lead citrate solution. Cell structure was assessed by transmission electron microscopy JEM-1010 (Jeol).
Immunocytofluorescence
Liver biopsies were fixed in a 4% paraformaldehyde solution for 24 h. Cryoprotection was done by increasing saccharose gradients up to 30% in PBS solution. Liver was then frozen in the presence of water-soluble glycols and resins (Tissue-Tek O.C.T. compound, Sakura). Cryostat sections (10 μm, Leica CM1900) were washed with PBS and permeabilized for 20 min with 0.2% (v/v) Triton X-100 (in PBS). After 10 min of blocking with 3% BSA (w/v in PBS), incubation with the primary antibody against rat total LGS (in PBS) was done for 12 h at 4 °C. After washing with PBS, sections were incubated with the secondary antibody (Texas Red-conjugated donkey and rabbit IgG, Jackson) for 2 h at room temperature. They were then washed with PBS and mounted onto glass slides using Mowiol (Sigma).
Statistical Analysis
Data are expressed as mean ± S.E. Statistical significance was determined by unpaired Student's t test using Microsoft Excel (version XP; Microsoft Corp., Redmond, WA). Statistical significance was assumed at p ≤ 0.05.
RESULTS
Overexpression of WT LGS and Activated Mutant LGS in Rat Liver
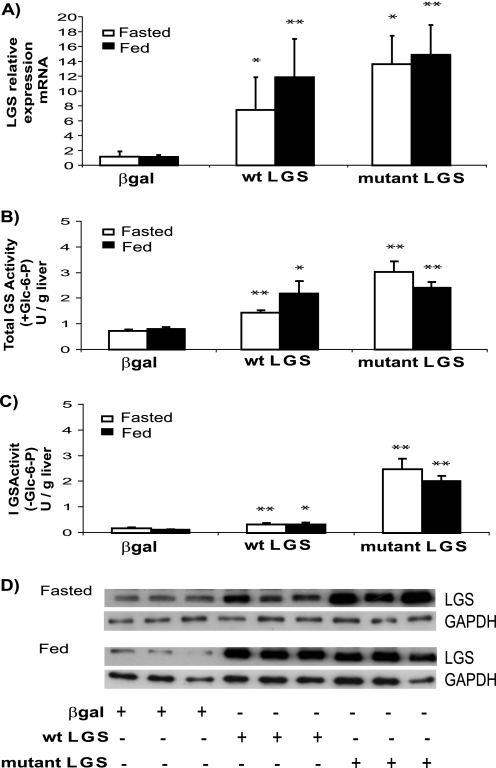
Rats were injected with adenovirus encoding for either WT LGS or a constitutively active LGS mutant form (mutations Ser → Ala at 2 and 3b phosphorylation sites, activated mutant LGS), and the control group was injected with adenovirus coding for β-gal. After 96 h postinjection, animals were subdivided into two groups, one of which was submitted to an 18-h fast while the other was allowed to feed ad libitum. The efficiency of adenovirus transfection in liver was confirmed by the significant increase in mRNA expression (Fig. 1A) and LGS immunoreactivity and total GS activity (Fig. 1, B and D) in both the WT LGS and activated mutant LGS groups compared with the control. Active GS (measured in the absence of glucose 6-phosphate) was only moderately increased in the group overexpressing WT LGS but was markedly increased in the activated mutant LGS-overexpressing group (Fig. 1C). There was no detectable immunoreactivity to LGS in adipose tissue, pancreas, kidney, testes, lung, or skeletal muscle (data not shown), thereby confirming preferential transgene delivery to liver, in agreement with previous studies (21, 22).
FIGURE 1.
Effects of the overexpression of WT LGS or activated mutant LGS on hepatic GS activity in rat liver. A, RT-PCR analysis of LGS mRNA levels in livers of rats overexpressing β-gal, WT LGS, or a constitutively active mutant LGS form (mutant LGS). B, total GS activity (units (U)/g of liver) of liver homogenates from fasted (white bars) or fed (black bars) rats overexpressing β-gal, WT LGS, or mutant LGS. C, GS activity (units/g of liver) calculated in the absence of glucose 6-phosphate. In all cases (A–C), data represent the mean ± S.E. (error bars) of the following: seven fasted and seven fed β-gal-overexpressing rats; five fasted and six fed WT LGS-overexpressing rats; and five fasted and five fed mutant LGS-overexpressing rats. * or **, statistical difference for comparisons with β-gal group at the same metabolic state with p < 0.05 or p < 0.005, respectively. D, representative Western blot analysis of three liver extracts of fasted or fed rats with antibodies against LGS or GAPDH as a load control. In all cases, 20 μg of protein were analyzed per lane.
Effects on Liver Glycogen in Fasted and Fed Rats
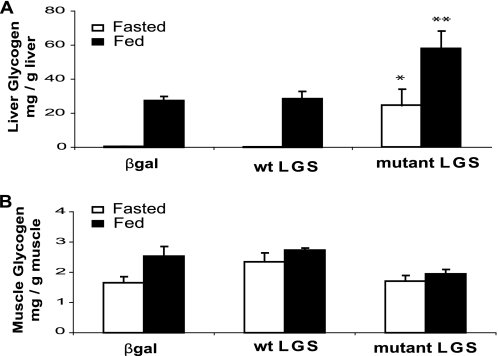
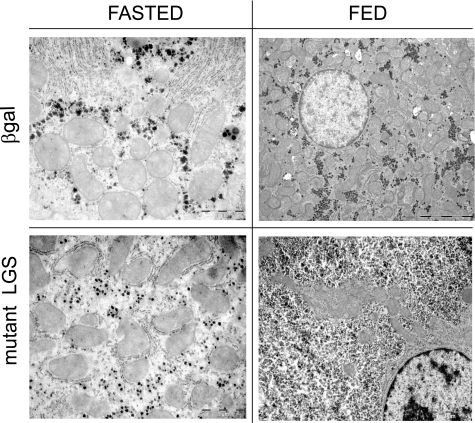
Liver glycogen content in the fed rats was enhanced ∼2-fold in the activated mutant LGS group but was unchanged in the WT LGS one (Fig. 2A), despite similar levels of total GS activity and protein in the two groups (Fig. 1, B and D). In the fasted state, the glycogen content of the activated mutant LGS-overexpressing group was decreased compared with the fed state, thereby indicating net mobilization of liver glycogen. Transmission electron microscopy confirmed a higher cytoplasmic glycogen content in the livers of rats overexpressing the activated mutant LGS compared with controls but did not show any other structural changes (Fig. 3). Furthermore, glycogen isolated from liver of the former group was normally branched (Table 1). Additionally, there was no difference in muscle glycogen content between the experimental and control groups (Fig. 2B).
FIGURE 2.
Effects of the overexpression of WT LGS and activated mutant LGS on glycogen content in rat liver and muscle. A, liver glycogen content (mg/g of liver) measured in rats overexpressing β-gal, WT LGS, or mutant LGS. B, muscle glycogen content (mg/g of muscle) determined in the same animals. In all cases, data represent the mean ± S.E. (error bars) of the following: seven fasted and seven fed β-gal-overexpressing rats; five fasted and six fed WT LGS-overexpressing rats; and five fasted and five fed mutant LGS-overexpressing rats. * or **, statistical difference for comparisons with β-gal group at the same metabolic state with p < 0.05 or p < 0.005, respectively.
FIGURE 3.
Effects of activated mutant LGS overexpression on ultracellular structure as shown by electron microscopy analysis of rat liver sections. Cellular ultrastructure analysis by electron microscopy of liver biopsies from the rats overexpressing β-gal or the constitutively active mutant form of LGS, fasted for 18 h or fed ad libitum. Scale bar, 5 μm (fed rats) or 1 μm (fasted rats).
TABLE 1.
Degree of branching of liver glycogen isolated from fed rats
Glycogen samples isolated from the livers of fed rats were complexed with iodine, and spectra were recorded to measure the degree of branching of the accumulated glycogen. Results are expressed as the mean ± S.E. of four β-gal- overexpressing rats, four WT LGS-overexpressing rats, four activated mutant-LGS-overexpressing rats. Commercial rabbit liver glycogen and commercial corn amylopectin were used as controls for high and low degree of branching, respectively.
| Polysaccharide | λmax (20) |
|---|---|
| β-gal liver glycogen | 490 ± 2 |
| WT LGS liver glycogen | 493 ± 2 |
| Mutant LGS liver glycogen | 502 ± 7 |
| Rabbit liver glycogen | 491 ± 2 |
| Starch amylopectin | 563 ± 4a |
a Statistical difference for comparisons with mutant LGS group with p < 10−6.
Effects on Blood Parameters in Fasted and Fed Rats
We examined the impact of LGS expression on the concentration of glucose and other metabolites in the blood. In the fed state, blood glucose concentrations were slightly decreased in the activated mutant LGS group but not in the WT LGS group, whereas plasma triglycerides and other blood metabolites were unchanged (Table 2); nor was plasma insulin altered with respect to controls. In the fasted state, there were no differences in glycemia; however, the concentration of plasma BHBA was significantly decreased in the activated mutant LGS group.
TABLE 2.
Blood parameters in rats overexpressing β-gal, WT LGS, or activated mutant LGS
After sacrificing the animals, blood samples were taken to measure the metabolites and hormones indicated. Glucose was measured in whole blood, whereas the rest of the parameters were determined in plasma. In all cases, data represent the mean ± S.E. of the following: seven fasted and seven fed β-gal-overexpressing rats; five fasted and six fed WT LGS-overexpressing rats; and five fasted and five fed mutant LGS-overexpressing rats. ND, not determined; TG, triglycerides.
| Fasted |
Fed |
|||||
|---|---|---|---|---|---|---|
| β-gal | WT LGS | Mutant LGS | β-gal | WT LGS | Mutant LGS | |
| Glucose (mg/dl) | 68 ± 3 | 73 ± 4 | 84 ± 8 | 124 ± 5 | 115 ± 6 | 96 ± 3a |
| Insulin (ng/ml) | 0.4 ± 0.2 | ND | 0.5 ± 0.2 | 1.4 ± 0.3 | ND | 1.1 ± 0.4 |
| TG (mg/dl) | 99 ± 12 | 71 ± 8 | 104 ± 15 | 146 ± 23 | 120 ± 21 | 183 ± 46 |
| Lactate (mg/dl) | 35 ± 4 | 26 ± 5 | 28 ± 1 | 45 ± 7 | 67 ± 19 | 55 ± 9 |
| NEFAs (mmol/liter) | 0.12 ± 0.02 | ND | 0.10 ± 0.01 | 0.02 ± 0.01 | ND | 0.06 ± 0.03 |
| BHBA (mg/dl) | 19 ± 4 | 23 ± 1 | 8 ± 3a | 2.2 ± 0.6 | 4.3 ± 0.9 | 3.3 ± 0.3 |
a Significant differences relative to β-gal-overexpressing rats, with p < 0.05.
Expression of GP, GK, PEPCK, and GLUT2 in Fasted and Fed Rats
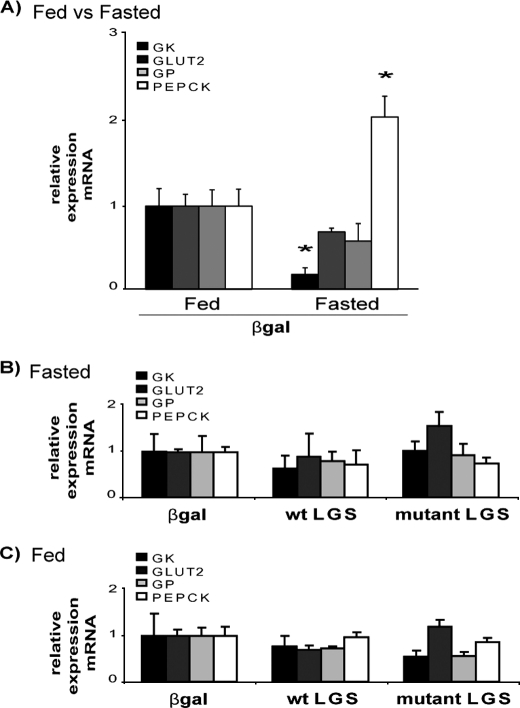
We tested whether the overexpression of LGS and/or the increased accumulation of glycogen caused secondary changes in the expression of key players of hepatic glucose metabolism. The mRNA levels of GK, GLUT2, PEPCK, and GP were determined by quantitative real-time PCR. Although fasting caused the expected decrease in GK expression and increase in PEPCK expression (23) (Fig. 4A), there were no further significant differences in the mRNA levels of these genes caused by activated mutant LGS overexpression when compared with the other experimental groups in the same nutritional state (Fig. 4, B and C).
FIGURE 4.
Effects of the overexpression of WT LGS and activated mutant LGS on glucokinase, GLUT2, glycogen phosphorylase, and phosphoenolpyruvate carboxykinase expression levels in rat liver. A, RT-PCR analysis of liver GK, GLUT2, GP, and PEPCK mRNA levels in livers of fed and fasted rats overexpressing β-gal; data are relative to the fed β-gal group. B, RT-PCR analysis in fasted overexpressing β-gal, WT LGS, or mutant LGS rats; data are relative to the fasted β-gal group of rats. C, RT-PCR analysis in fed overexpressing β-gal, WT LGS, or mutant LGS rats; data are relative to the fed β-gal group of rats. In all cases, relative expression levels were calculated with the 2ΔΔCt method using 18 S rRNA as endogenous control, and data represent the mean ± S.E. (error bars) of the following: seven fasted and seven fed β-gal-overexpressing rats; five fasted and six fed WT LGS-overexpressing rats; and five fasted and five fed mutant LGS-overexpressing rats. In A the asterisk indicates significant difference, with p < 0.05.
Intraperitoneal Glucose Tolerance Test
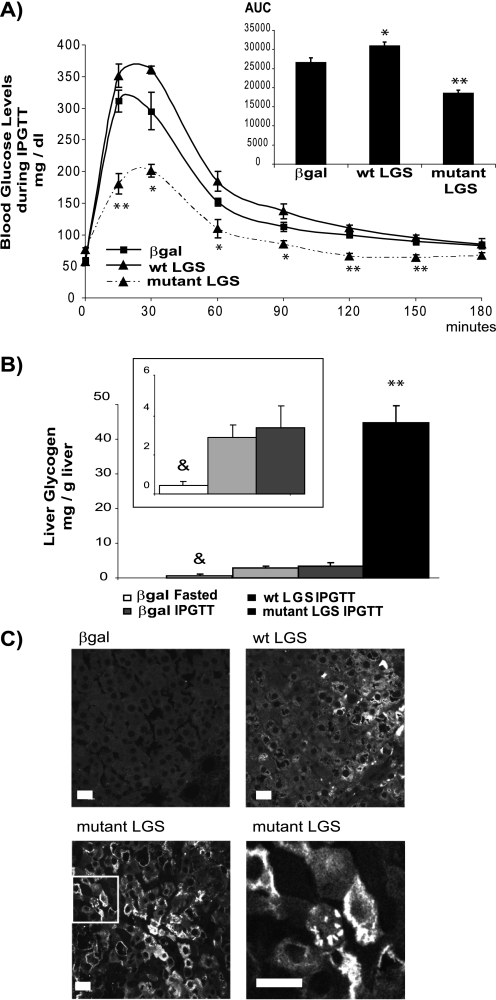
To further study the effects of WT or activated mutant LGS overexpression on blood glucose homeostasis, we performed an IPGTT to a new group of rats. 96 h after postadenoviral injection, rats were subjected to an 18-h fast and were then given an intraperitoneal glucose load (2 g/kg body weight). Blood glucose concentration determined between 15 min and 3 h after the glucose load was markedly diminished in the activated mutant LGS group compared with the β-gal and WT LGS groups, with a decrease in area under the curve of 30% (Fig. 5A). Liver glycogen content of β-gal or WT LGS-overexpressing rats showed a marked increase at the final point of the IPGTT (180 min) compared with that for β-gal-overexpressing fasted rats (Fig. 5B, inset). Overexpression of activated mutant LGS resulted in a much larger increase in the storage of this polymer compared with the other groups (Fig. 5B). Similarly, only activated mutant LGS-overexpressing animals showed significantly improved glucose tolerance. Several blood parameters were measured upon ending the test. There was no difference in the concentrations of insulin, lactate, or triglycerides between the experimental groups and the controls (Table 3). However, plasma BHBA concentrations were significantly decreased in the activated mutant LGS group, similar to the changes observed in the fasted activated mutant LGS-overexpressing rats (Table 2).
FIGURE 5.
Intraperitoneal glucose tolerance test. A, rats overexpressing β-gal, WT LGS, or activated mutant LGS were fasted for 18 h before receiving an intraperitoneal glucose bolus of 2 g/kg body weight. Tail vein blood samples were taken, and glucose concentrations were measured at the times indicated after the glucose bolus. The area under the curve was measured for each experimental group (AUC, inset). B, liver glycogen content (mg/g of liver) determined at the starting point (β-gal-fasted) and at the end point of the IPGTT (180 min). The inset shows a lower scale graph. In all cases, data are mean ± S.E. (error bars) for seven fasted β-gal-overexpressing rats and nine β-gal-, six WT LGS-, and five mutant LGS-overexpressing animals from the IPGTT. C, liver samples taken after the IPGTT were processed for immunofluorescence analysis with an antibody against LGS. Representative confocal microscopy images of liver sections from rats overexpressing β-gal, WT LGS, or activated mutant LGS. Laser intensity was adjusted so that the endogenous LGS signal (β-gal-overexpressing animals) was hardly observable. Lower right panel, magnification of the mutant LGS image (area inside the box) to show the aggregated, peripheral distribution of LGS. Scale bar, 20 μm. In A, the single or double asterisks indicate those time points at which blood glucose concentrations were significantly lower in rats overexpressing mutant LGS than in rats overexpressing β-gal, with p < 0.05 or p < 0.005 respectively; in B the double asterisk denotes statistical difference for comparisons with the β-gal IPGTT group with p < 0.005, and the ampersand denotes statistical difference between the fasted β-gal group and the β-gal IPGTT group, with p < 0.005.
TABLE 3.
Blood parameters after the IPGTT in rats overexpressing β-gal, WT LGS, or activated mutant LGS
At the end of the IPGTT (180 min), blood samples were taken in order to measure the metabolites and hormones indicated. Glucose was measured in whole blood, whereas the rest of the parameters were determined in plasma. Data are expressed as the mean ± S.E. for nine β-gal-, six WT LGS-, and five mutant LGS-overexpressing rats.
| β-gal | WT LGS | Mutant LGS | |
|---|---|---|---|
| Glucose (mg/dl) | 83 ± 6 | 91 ± 6 | 67 ± 4 |
| Insulin (ng/ml) | 2.3 ± 1 | 2.7 ± 1 | 2.8 ± 1 |
| Triglycerides (mg/dl) | 52 ± 10 | 47 ± 14 | 63 ± 8 |
| Lactate (mg/dl) | 27 ± 7 | 35 ± 3 | 40 ± 11 |
| BHBA (mg/dl) | 11 ± 2 | 13 ± 1 | 4 ± 1a |
a Significant differences relative to β-gal-overexpressing rats, with p < 0.05.
Intracellular Distribution of LGS after the IPGTT
Previous in vitro studies reported that the incubation of isolated hepatocytes with glucose activates LGS but also causes its translocation from a homogeneous cytoplasmic distribution to the cell periphery (17, 24). In the present study, we studied the subcellular distribution of LGS in all of the experimental groups after the IPGTT by means of immunofluorescence (Fig. 5C). There was a clear localization of LGS in the cell periphery in the activated mutant LGS group compared with the β-gal-overexpressing rats.
Liver-specific Transgenic Mice Expressing the Activated Mutant LGS
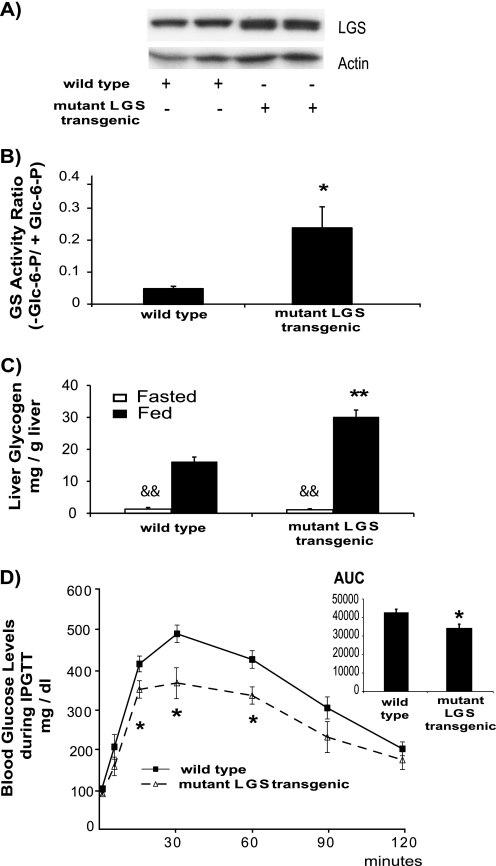
We wanted to investigate if longer (chronic) expression of the activated mutant LGS resulted in the loss of the ability to efficiently reduce blood glucose levels as a consequence of limited glycogen storage capacity. Because experiments using adenovirally transduced rats are time-limited, we undertook a completely new approach to address the issue of permanent activation of LGS as a potential strategy to chronically improve glucose tolerance. Thus, we generated transgenic mice expressing the activated mutant LGS under the control of albumin enhancer/promoter (liver-specific). Although transgenic animals expressed moderate levels of the recombinant protein as revealed by Western blot analysis (Fig. 6A; note the increased mobility due to reduced phosphorylation level), it was sufficient to drastically increase the LGS activity ratio (Fig. 6B). In addition, fed transgenic mice showed increased liver glycogen content when compared with WT animals in the same metabolic state, although this difference disappeared upon 18 h of fasting (Fig. 6C), indicating that these mice were capable of fully mobilizing their glycogen stores. Furthermore, activated mutant LGS liver-specific transgenic mice showed improved glucose tolerance when challenged with a glucose bolus (IPGTT; 2 g/kg body weight) (Fig. 6D).
FIGURE 6.
Characterization of transgenic mice expressing activated mutant LGS in liver. A, representative Western blot analysis of liver extracts of wild type and activated mutant LGS transgenic mice (two samples of each) with antibodies against LGS or actin as a load control. In all cases, 20 μg of protein were analyzed per lane. B, GS activity ratio (−glucose 6-phosphate/+glucose 6-phosphate (−Glc-6-P/+Glc-6-P)) of liver homogenates from wild type (n = 8) and mutant LGS transgenic (n = 4) mice. Data represent the mean ± S.E. (error bars). C, liver glycogen content (mg/g of liver) measured in fasted (white bars) or fed (black bars) wild type and mutant LGS transgenic mice. Data represent the mean ± S.E. of six fasted and four fed wild type mice and five fasted and four fed mutant LGS transgenic mice. D, wild type (n = 9) and mutant LGS transgenic (n = 6) mice were fasted for 18 h before receiving an intraperitoneal glucose bolus of 2 g/kg body weight. Tail vein blood samples were taken, and glucose concentrations were measured at the times indicated after the glucose bolus. The area under the curve was measured for each experimental group (AUC, inset). Data represent the mean ± S.E. The single or double asterisks denote statistical difference for comparisons with WT group at the same metabolic state with p < 0.05 or p < 0.005, respectively. The double ampersand denotes statistical difference (p < 0.005) between the fasted and fed states for each group of mice.
DISCUSSION
The liver plays a major role in the clearance of blood glucose in the postprandial state (25), and several proteins involved in the control of hepatic glucose metabolism, including GK, GP, and glycogen-targeting proteins, have been proposed as potential targets for antihyperglycemic therapy for type 2 diabetes. GK overexpression and inhibition of GP activity are both effective in improving glucose tolerance. However, there are certain critical issues associated with these strategies. GK overexpression increases flux through glycolysis, and in some circumstances, this can lead to an increase in plasma triglycerides (4). GP inhibition may have potentially negative effects on skeletal muscle function during exercise (26). Studies using glycogen-targeting proteins have shown that sustained efficacy in improving glucose tolerance can be achieved only by enhancing glycogen synthesis in the postprandial state without compromising glycogenolysis in the postabsorptive state (6, 9). Accordingly, although overexpression of PTG markedly enhances glycogen storage in isolated hepatocytes in vitro (27), it is only mildly effective at improving glucose clearance in fasted glucose-challenged rats, because glycogenolysis is markedly curtailed in the fasted state and hepatic glycogen stores are therefore nearly saturated prior to glucose loading (6). A common feature of all of the above mentioned strategies is that they lead to secondary activation of LGS. Nevertheless, the direct effects of activation of this enzyme as the primary target to modulate blood glucose homeostasis have not been addressed. We have recently shown that the activity of LGS in primary cultured hepatocytes can be modulated by expression of constitutively active forms of the enzyme (13). Thus, our objective was to determine the effects of the expression of a constitutively active form of LGS on blood glucose homeostasis in vivo.
Two key findings have emerged from this study. First, the overexpression of a constitutively active variant of LGS (Ser → Ala mutations at 2 and 3b phosphorylation sites) lowered blood glucose in the fed but not in the fasted state, and these changes were paralleled by corresponding alterations in hepatic glycogen content. Worthy of note is that the glycogen synthesized showed a normal degree of ramification. Furthermore, it also markedly enhanced glucose clearance when fasted animals were challenged with a glucose load. Importantly, overexpression of the wild-type protein had a negligible effect on hepatic glycogen storage and on blood glucose homeostasis. Consequently, this finding indicates that the amount of LGS in the normal physiological state is not limiting for hepatic glycogen synthesis and that strategies simply aiming to increase LGS protein are not likely to improve glucose homeostasis. Second, the overexpression of a constitutively active form of LGS had no effect on plasma insulin, lactate, NEFAs, or triglycerides, thereby being free of some of the negative side effects detected in other approaches. Furthermore, this strategy also prevents the increase in ketogenesis because rats expressing an activated mutant LGS had a lower concentration of BHBA in the fasted state and after a glucose challenge, when compared with controls. Changes in the plasma concentration of ketone bodies generally parallel the plasma concentration of NEFAs. However, because the NEFA levels of the rats expressing a constitutively active LGS form were similar to those of controls, we propose that intrahepatic regulation of ketogenesis by the elevated glycogen content is a plausible explanation for the lower levels of BHBA. Further experiments would be necessary to address this hypothesis. Moreover, expression of LGS (either wild-type or active form) had no effect on the expression of the main glucose metabolism-related enzymes and glucose transporters, such as GK, GP, PEPCK, and GLUT2. Interestingly, although the rats overexpressing a constitutively active form of LGS showed markedly elevated GS activity in the fed and fasted states, blood glucose concentrations were decreased only in the former state, thereby indicating that other mechanisms have an overriding role on glycogen metabolism in the fasted state.
A central result in this study is the marked improvement of glucose tolerance shown by 18 h-fasted rats when challenged with a glucose load, with a decrease in area under the curve of 30%. This can be explained almost entirely by the prominent increase in the storage of hepatic glycogen in this experimental group. In fact, the overexpressed activated mutant LGS was located at the cellular periphery, where glycogen synthesis is initiated (17, 24). Although the hepatic glycogen accumulation in rats overexpressing the activated mutant form of LGS was already higher than the other groups after an 18-h fast, this surplus of glycogen did not limit the capacity of posterior accumulation of the polysaccharide in liver. Thus, rats overexpressing this constitutively active LGS mutant had the capacity to remove the excess of glucose in blood and then deliver it to glycogen synthesis more efficiently than the other groups. However, a permanent activation of LGS could potentially cause the saturation of the liver capacity to store glycogen, thus limiting its glycemia-lowering effects (8). The results obtained with liver-specific transgenic mice chronically expressing the activated mutant LGS largely reproduce the observations from the experiments with rats: increased LGS activity and glycogen accumulation in the fed state, capacity to mobilize glycogen stores upon fasting, and improved glucose tolerance when these mice were challenged with a glucose load.
On the basis of our findings, we propose that the direct activation of LGS is an effective method to improve glucose tolerance in the postprandial state as a result of its capacity to enhance glucose storage without affecting other metabolic pathways. Therefore, our observations may provide the basis for a novel therapeutic approach to reduce hyperglycemia in diabetes.
Acknowledgments
We thank Professor P. Chambon (Université Louis Pasteur, Strasbourg, France) and Dr. K. Kohno (Nara Institute of Science and Technology, Japan) for the generous gift of the pSG5 and p2335-1 plasmids, respectively. We also thank Dr. R. Gasa, (Diabetes and Obesity Laboratory of Institut d'Investigaciones Biomediques Pi i Sunyer-Hospital Clínic, Barcelona University) for valuable assistance with the purification of the adenoviruses. We thank the Mutant Mouse Platform (Institute for Research in Biomedicine, Barcelona), the Animal Research Center (Barcelona Science Park), and the Electron Microscopy Unit of the Core Scientific Services at Barcelona University for help with the assessment and the equipment necessary to carry out this study. We are also grateful to A. Adrover, L. Babin, E. Veza, and N. Plana for technical support. We thank T. Yates for correcting the English version of the manuscript and Professor L. Agius (University of Newcastle) for helpful suggestions.
This work was supported by Ministry of Education and Science, Spain, Grants BFU2005-02253 and SAF2007-64722, Autonomous Government of Catalonia Grant 2005-SGR-00570, and a grant from the Fundación Marcelino Botín. The CIBER de Diabetes y Enfermedades Metabólicas Asociadas is an ISCIII project.
- GK
- glucokinase
- GP
- glycogen phosphorylase
- GS
- glycogen synthase
- LGS
- liver glycogen synthase
- NEFA
- non-esterified fatty acid
- BHBA
- 3-hydroxybutyrate
- PEPCK
- phosphoenolpyruvate carboxykinase
- PTG
- protein targeting to glycogen
- IPGTT
- intraperitoneal glucose tolerance test.
REFERENCES
- 1.Agius L., Peak M., Newgard C. B., Gomez-Foix A. M., Guinovart J. J. (1996) J. Biol. Chem. 271, 30479–30486 [DOI] [PubMed] [Google Scholar]
- 2.Hariharan N., Farrelly D., Hagan D., Hillyer D., Arbeeny C., Sabrah T., Treloar A., Brown K., Kalinowski S., Mookhtiar K. (1997) Diabetes 46, 11–16 [DOI] [PubMed] [Google Scholar]
- 3.Niswender K. D., Shiota M., Postic C., Cherrington A. D., Magnuson M. A. (1997) J. Biol. Chem. 272, 22570–22575 [DOI] [PubMed] [Google Scholar]
- 4.O'Doherty R. M., Lehman D. L., Télémaque-Potts S., Newgard C. B. (1999) Diabetes 48, 2022–2027 [DOI] [PubMed] [Google Scholar]
- 5.Printen J. A., Brady M. J., Saltiel A. R. (1997) Science 275, 1475–1478 [DOI] [PubMed] [Google Scholar]
- 6.O'Doherty R. M., Jensen P. B., Anderson P., Jones J. G., Berman H. K., Kearney D., Newgard C. B. (2000) J. Clin. Invest. 105, 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang R., Cao L., Gasa R., Brady M. J., Sherry A. D., Newgard C. B. (2002) J. Biol. Chem. 277, 1514–1523 [DOI] [PubMed] [Google Scholar]
- 8.Yang R., Newgard C. B. (2003) J. Biol. Chem. 278, 23418–23425 [DOI] [PubMed] [Google Scholar]
- 9.Gasa R., Clark C., Yang R., DePaoli-Roach A. A., Newgard C. B. (2002) J. Biol. Chem. 277, 1524–1530 [DOI] [PubMed] [Google Scholar]
- 10.Agius L. (2007) Best Pract. Res. Clin. Endocrinol. Metab. 21, 587–605 [DOI] [PubMed] [Google Scholar]
- 11.Treadway J. L., Mendys P., Hoover D. J. (2001) Expert. Opin. Investig. Drugs 10, 439–454 [DOI] [PubMed] [Google Scholar]
- 12.Kelsall I. R., Munro S., Hallyburton I., Treadway J. L., Cohen P. T. (2007) FEBS Lett. 581, 4749–4753 [DOI] [PubMed] [Google Scholar]
- 13.Ros S., García-Rocha M., Domínguez J., Ferrer J. C., Guinovart J. J. (2009) J. Biol. Chem. 284, 6370–6378 [DOI] [PubMed] [Google Scholar]
- 14.Gomis R. R., Ferrer J. C., Guinovart J. J. (2000) Biochem. J. 351, 811–816 [PMC free article] [PubMed] [Google Scholar]
- 15.Becker T. C., Noel R. J., Coats W. S., Gómez-Foix A. M., Alam T., Gerard R. D., Newgard C. B. (1994) Methods Cell Biol. 43, 161–189 [DOI] [PubMed] [Google Scholar]
- 16.Thomas J. A., Schlender K. K., Larner J. (1968) Anal. Biochem. 25, 486–499 [DOI] [PubMed] [Google Scholar]
- 17.García-Rocha M., Roca A., De La Iglesia N., Baba O., Fernández-Novell J. M., Ferrer J. C., Guinovart J. J. (2001) Biochem. J. 357, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An J., Muoio D. M., Shiota M., Fujimoto Y., Cline G. W., Shulman G. I., Koves T. R., Stevens R., Millington D., Newgard C. B. (2004) Nat. Med. 10, 268–274 [DOI] [PubMed] [Google Scholar]
- 19.Chan T. M., Exton J. H. (1976) Anal. Biochem. 71, 96–105 [DOI] [PubMed] [Google Scholar]
- 20.Krisman C. R. (1962) Anal. Biochem. 4, 17–23 [DOI] [PubMed] [Google Scholar]
- 21.Trinh K. Y., O'Doherty R. M., Anderson P., Lange A. J., Newgard C. B. (1998) J. Biol. Chem. 273, 31615–31620 [DOI] [PubMed] [Google Scholar]
- 22.Herz J., Gerard R. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2812–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salavert A., Iynedjian P. B. (1982) J. Biol. Chem. 257, 13404–13412 [PubMed] [Google Scholar]
- 24.Fernández-Novell J. M., Bellido D., Vilaró S., Guinovart J. J. (1997) Biochem. J. 321, 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherrington A. D. (1999) Diabetes 48, 1198–1214 [DOI] [PubMed] [Google Scholar]
- 26.Baker D. J., Greenhaff P. L., MacInnes A., Timmons J. A. (2006) Diabetes 55, 1855–1861 [DOI] [PubMed] [Google Scholar]
- 27.Berman H. K., O'Doherty R. M., Anderson P., Newgard C. B. (1998) J. Biol. Chem. 273, 26421–26425 [DOI] [PubMed] [Google Scholar]