Abstract
NBCe1-A and AE1 both belong to the SLC4 HCO3− transporter family. The two transporters share 40% sequence homology in the C-terminal transmembrane region. In this study, we performed extensive substituted cysteine-scanning mutagenesis analysis of the C-terminal region of NBCe1-A covering amino acids Ala800–Lys967. Location of the introduced cysteines was determined by whole cell labeling with a membrane-permeant biotin maleimide and a membrane-impermeant 2-((5(6)-tetramethylrhodamine)carboxylamino) ethyl methanethiosulfonate (MTS-TAMRA) cysteine-reactive reagent. The results show that the extracellular surface of the NBCe1-A C-terminal transmembrane region is minimally exposed to aqueous media with Met858 accessible to both biotin maleimide and TAMRA and Thr926–Ala929 only to TAMRA labeling. The intracellular surface contains a highly exposed (Met813–Gly828) region and a cryptic (Met887–Arg904) connecting loop. The lipid/aqueous interface of the last transmembrane segment is at Asp960. Our data clearly determined that the C terminus of NBCe1-A contains 5 transmembrane segments with greater average size compared with AE1. Functional assays revealed only two residues in the region of Pro868–Leu967 (a functionally important region in AE1) that are highly sensitive to cysteine substitution. Our findings suggest that the C-terminal transmembrane region of NBCe1-A is tightly folded with unique structural and functional features that differ from AE1.
Keywords: Anion Transport, Membrane Proteins, Protein Conformation, Protein Structure, Sodium Transport
Introduction
The intra- and extracellular pH in mammals must be finely tuned within narrow limits to maintain normal biochemical and physiological processes. The bicarbonate buffering system (HCO3− /CO32−/CO2) is arguably the most important buffer system in mammalian acid-base homeostasis. The SLC4 membrane transporter family that mediates bicarbonate transport in various cell types plays a critical role in this regard (except SLC4A11, which does not transport HCO3−) (1, 2). All SLC4 HCO3− transporters share high amino acid sequence homology but vary in their dependence/transport of Na+ and/or Cl−. Specifically, Na+-dependent HCO3− cotransport, Na+-independent Cl−/HCO3− exchange, and Na+-dependent Cl−/HCO3− exchange activity are mediated by various members of the SLC4 family (1, 2).
Of the known SLC4 transporters, the structural and functional importance of the C-terminal transmembrane region of the anion exchanger AE1 (encoded by the SLC4A1 gene-encoded variant) has been extensively analyzed. AE1 is abundantly expressed in erythrocytes and an N-terminal truncated form in the kidney, where it performs the 1:1 electroneutral exchange of Cl− for HCO3− across the plasma membrane (3). In erythrocytes, AE1 dramatically increases the capacity of blood to carry CO2. In the basolateral membrane of α-intercalated cells in the renal collecting duct, AE1 plays an important role in transcellular bicarbonate absorption (1). AE1 consists of two domains: an N-terminal cytoplasmic domain that interacts with the cytoskeleton and a C-terminal transmembrane domain that transports anions independently. The membrane domain of AE1 is proposed to have 13 transmembrane segments (TM)2 with two reentrant loops in the C-terminal region (3). Residues in TM 8 and TM 13–14 have been identified that are involved in forming the AE1 substrate translocation pathway (4, 5). The transport function of AE1 is sensitive to the inhibition by several chemical reagents including 4, 4′-diisothiocyanato-2, 2′-stilbenedisulfonate (DIDS). The covalent DIDS reactive sites in human AE1 have been mapped to Lys539 in TM 5 and Lys851 in TM 12 (3, 6).
The C-terminal transmembrane region of AE1 is implicated in the anion translocation process (5). Chemical probing (7), mutagenesis analysis (3), and methylation studies (6, 8) all highlight the functional importance of TM 8, 12, 13 (4, 5) and residue Lys851 in AE1. Topology analysis showed that the last two TMs in AE1 are shorter than a standard TM and are composed of only 16 amino acids each connected by a small extracellular loop (3). Functional studies suggested that the small extracellular loop participates in forming the anion selectivity filter and several flanking residues in TM 12 and 13 are involved in forming the ion binding site of AE1 (5).
The electrogenic Na+-HCO3− cotransporter 1 (NBCe1-A) is an SLC4A4 gene-encoded variant that is expressed in the basolateral membrane of the renal proximal tubule cells, where it cotransports Na+ and HCO3− with a 1:3 stoichiometry from cells to blood (1). NBCe1-A is responsible for reabsorbing 60–80% of the filtered HCO3− load in the mammalian kidney. The N-terminal cytoplasmic region of NBCe1-A is functionally important, and the C-terminal transmembrane region is required for protein membrane trafficking as evidenced from truncation studies (9). We recently showed that unlike AE1, NBCe1-A has 14 TMs in its transmembrane region (10). The N-terminal transmembrane region contains 8 TMs that are homologous to AE1; however, the C terminus seems to lack the two predicted AE1 reentrant loops.
The C-terminal transmembrane region of NBCe1-A and AE1 share 40% sequence homology and appear to have certain common properties. TM 8 in NBCe1-A was reported to participate in forming the substrate translocation pore that resembles AE1 (4, 11). Mutant R881C (causing human proximal renal tubular acidosis) in NBCe1-A impairs protein membrane trafficking, and the corresponding mutation in AE1 (R808C) induces the same cellular effect (12, 13). Both NBCe1-A and AE1 have a small extracellular connecting loop between the last two TMs with high sequence homology (3, 10). More interestingly, NBCe1-A and AE1 share a common functional inhibitor, DIDS, and the predicted DIDS binding sites in NBCe1-A (Lys559 in TM 5 and Lys924 in TM 13) mirror that in AE1 (1, 14).
Taken together, the current literature is ambiguous regarding whether the C-terminal transmembrane regions of NBCe1-A and AE1 share significant structural and functional properties. Given the biological importance of addressing this question for understanding the transport mechanism(s) of SLC4 HCO3− transporters, we extensively examined the properties of the C-terminal region of NBCe1-A. Our data show for the first time that SLC4 transporters, specifically NBCe1-A and AE1, differ topologically and functionally in this region. We propose that this region may contribute to their unique transport mechanisms (cotransporter versus exchanger).
EXPERIMENTAL PROCEDURES
Materials
Site-directed mutagenesis kits were from Stratagene. Biotin maleimide, BCECF-AM, DMEM, and all cell culture reagents were from Invitrogen. MTS-TAMRA was from Toronto Research Chemicals Inc. Protein A-Sepharose, streptavidin/biotinylated-horseradish peroxidase complex (streptavidin-HRP) and goat anti-rabbit IgG-conjugated horseradish peroxidase were from GE Healthcare. Igepal, polylysine, and nigericin were from Sigma. PVDF membrane was from Millipore.
Site-directed Mutagenesis
A wild type and a modified human NBCe1-A construct with 5 endogenous cysteines substituted with serines (NBCe1-A-5C−) were used as the templates for site-directed mutagenesis. 168 amino acids at the position between Ala800 and Lys967 were individually substituted with cysteine in the NBCe1-A-5C− construct. Mutagenesis was performed using the Strategene site-directed mutagenesis kit following manufacturer's instruction. The complete cDNA sequence of each mutant was verified by DNA sequencing.
Protein Expression
Mutant NBCe1-A proteins were transiently expressed in the human embryonic kidney 293 cells (HEK 293) by using Lipofectamine 2000 (from Invitrogen) transfection following the manufacturer's instruction. Cells were grown at 37 °C in a 5% CO2 atmosphere and harvested 24–48 h after transfection.
Immunocytochemistry
24 h after transfection, cells were rinsed with PBS (140 mm NaCl, 3 mm KCl, 6.5 mm Na2HPO4, 1.5 mm KH2PO4, pH 7.4) and incubated with a rabbit anti-human NBCe1-A antibody (Ab-162, 1:100 dilution in PBS). After a 15-min incubation at room temperature, cells were rinsed with PBS and further incubated with goat anti-rabbit IgG-conjugated with Cy3 (1:500 dilution in PBS, from Jackson ImmunoResearch) for 30 min at room temperature. Cells were then rinsed three times with PBS and mounted in Crystal/Mount (from Biomeda, Foster City, CA). In some experiments, transfected cells were permeabilized with 1 ml of ice-cold methanol for 2 min prior to the immunostaining. Fluorescence images were acquired by a PXL charge-coupled device camera (model CH1; Photometrics) coupled to a Nikon Microphot-FXA epifluorescence microscope.
Functional Transport Assay of NBCe1-A
HEK 293 cells grown on coated coverslips were transfected with various NBCe1-A mutant cDNAs using Lipofectamine 2000. 24 h after transfection, cells were loaded with fluorescent pH probe BCECF-AM and assayed using a microfluorometer as described previously (10, 15).
Biotin Maleimide Labeling and Immunoprecipitation
Whole cell labeling with biotin maleimide (BM) was performed as described previously (10). Biotinylated NBCe1-A proteins were immunoprecipitated by a rabbit anti-human NBCe1-A N terminus polyclonal antibody (16). For detailed protocols, see Ref. 10.
SDS-PAGE and Immunoblotting
Protein samples were resolved on 7.5% polyacrylamide gels and transferred to PVDF membranes. Biotinylated proteins were detected by incubation of blots with 1:10,000 diluted streptavidin-biotinylated horseradish peroxidase (GE Healthcare) in TBSTB buffer (TBST buffer (0.1% (v/v) Tween 20, 137 mm NaCl, 20 mm Tris, pH 7.5), containing 0.5% (w/v) bovine serum albumin). Protein expression levels were determined by probing the blot with anti-NBCe1 C terminus polyclonal antibody (17) at 1:3,000 dilutions in TBSTM buffer (TBST buffer containing 5% (w/v) nonfat milk).
MTS-TAMRA Labeling Assay
HEK 293 cells expressing mutant NBCe1-A proteins were washed three times with PBS and incubated with 100 μm MTS-TAMRA (1:1,000 dilution in PBS, pH 7.4) for 3 min on ice. The cells were then washed twice with PBS and subsequently imaged (Olympus BH2 microscope) using an excitation wavelength of 545 nm and an emission wavelength of 590 nm.
Plasma Membrane Isolation and Labeling with MTS-TAMRA
HEK 293 cells expressing mutant NBCe1-A proteins were collected, washed twice with TBS (140 mm NaCl, 10 mm Tris, pH 7.4), and resuspended in homogenization buffer (10 mm Tris, 5 mm EDTA, pH 7.4) with protease inhibitors (from Roche Applied Science). After a 30-min incubation on ice, the cells were homogenized with a Dounce homogenizer. Cell debris was removed with a low speed centrifugation (4,000 × g, 5 min, 4 °C), and the supernatant was then subjected to a high speed centrifugation for 30 min (35,000 × g, 4 °C). The membrane pellet was rinsed twice and then resuspended in 500 μl of PBS, pH 7.5, followed by adding 1 μl of MTS-TAMRA (50 mm stock in dimethyl sulfoxide), and incubated for 20 min at room temperature. The reaction was stopped by adding a 5-fold molar excess of glutathione. The membranes were pelleted, and the samples were processed for immunoprecipitation as described previously, except that the immunoprecipitated proteins were eluted with a 2× sample buffer without β-mercaptoethanol. MTS-TAMRA labeling was analyzed by a Typhoon 9410 scanner using an exciting wavelength of 545 nm and an emission wavelength of 590 nm.
Plasma Membrane Stripping
Isolated cell membranes were resuspended in 10 mm EDTA, pH 8.0, and incubated at 37 °C on a rotating shaker for 30 min. Membranes were then pelleted and washed twice with PBSCM (PBS containing 0.1 mm CaCl2 and 1 mm MgCl2, pH 7.0) prior to BM labeling. For KCl treatment, the EDTA-treated membranes were incubated with 1 m KCl at room temperature for 30 min, and then the samples were BM labeled as described above. Membrane treatment with Na2CO3 was performed as described previously (10). Membrane samples were treated with 0.1 m and 3 m Na2CO3, respectively, followed by BM labeling.
Image and Data Analysis
Films from immunoblots and biotinylation blots were scanned with a Hewlett-Packard Scanjet 5590. Scanned images were quantified with UN-SCAN-IT gelTM version 6.1 software. Biotinylation levels were calculated according to Ref. 3.
Statistical Analysis
Means ± S.E. were calculated with SigmaPlot 10 software. Statistical analysis was performed using SigmaPlot 10 software. Dunnett's t test was used to assess statistical significance with p < 0.05 considered significant.
RESULTS
Construction and Cellular Localization of Cysteine-introduced NBCe1-A Constructs
Construction of 168 Introduced Cysteines in the C-terminal Region of NBCe1-A
In our previous random cysteine mutagenesis study of NBCe1-A, we were unable to detect the two reentrant loops that were predicted based on the topology of AE1 (Fig. 1) (10, 14). To obtain a clearer picture of the folding of the C-terminal transmembrane region, we sequentially introduced 168 cysteine codons at the amino acid positions between Ala800 and Lys967 into a modified NBCe1-A construct (NBCe1-A-5C−) with 5 endogenous cytoplasmic cysteines substituted with serines (Fig. 1). 26 of the cysteine-introduced constructs were constructed previously. The cloning template, NBCe1-A-5C−, is free of endogenous reactive cysteines and is fully functional (15). Each construct contained only 1 exogenously introduced cysteine. The range of introduced cysteines covered the whole C-terminal transmembrane region of NBCe1-A, including the predicted 5 TMs, 4 connecting loops, and 23 amino acids beyond the predicted intracellular border of the last TM (10).
FIGURE 1.
Topology models of NBCe1-A and AE1. Topology model of AE1 was adapted from Ref. 3, and NBCe1-A was from Ref. 10. The branched structures represent N-linked glycosylation sites. The identified DIDS-reactive sites in AE1 and the putative DIDS-reactive sites in NBCe1-A are depicted as filled circles. The mutations in AE1 (R808C) and NBCe1-A (R881C) that impair protein membrane trafficking are also depicted. Positions of the first and the last cysteine-substituted amino acids in NBCe1-A are indicated, and the region analyzed in the present study is shaded in dark gray.
Cellular Localization of Cysteine-substituted NBCe1-A Constructs
Single amino acid substitution in NBCe1-A may cause protein intracellular retention as observed previously (10). To determine whether the substituted cysteines impair the mutant NBCe1-A plasma membrane processing, we performed immunocytochemistry assay on all of the constructs with an anti-NBCe1-A antibody (Ab-162) on intact cells. Ab-162 is a rabbit polyclonal antibody that recognizes the peptide between the two glycosylated sites in the proposed extracellular loop 3 of NBCe1-A. If the cysteine-introduced construct is processed to the plasma membrane, Ab-162 will bind to it and be detected under a fluorescent microscope after incubation with a secondary fluorescent antibody. On the contrary, if the construct is intracellularly retained, the epitope will not be available for Ab-162 binding and therefore will not be detected (Fig. 2A). Fig. 2B shows that 21 of 168 cysteine-introduced NBCe1-A constructs were not detected in the plasma membrane. Notably, mutation of any of the 5 consecutive residues in the region between Glu831 and Gly836 (except Val834) impaired NBCe1-A plasma membrane targeting, implying that this region has an important role in protein folding. Ab-162 staining in methanol-permeabilized cells showed that the 21 membrane unprocessed constructs were well expressed but fully retained intracellularly (data not shown).
FIGURE 2.
Membrane processing of cysteine-substituted NBCe1-A constructs. A, representative images of Ab-162 staining on intact and permeabilized HEK 293 cells expressing NBCe1-A constructs. HEK 293 cells expressing various NBCe1-A cysteine-substituted constructs were stained with Ab-162 for 15 min at room temperature, and fluorescence images were acquired by a PXL charge-coupled device camera coupled to a Nikon Microphot-FXA epifluorescence microscope. a, cells expressing NBCe1-A-5C−; b, cells expressing mutant S804C; c, cells expressing mutant S804C stained with Ab-162 after methanol permeation. B, summary of membrane-unprocessed cysteine-substituted NBCe1-A constructs. NBCe1-A-5C− was used as a positive control. +, positive staining; −, negative staining.
Labeling of NBCe1-A-introduced Cysteines with Biotin Maleimide
Determining the location of an introduced cysteine with cysteine-reactive reagents is a well established technique that faithfully reports the target protein structure as evidenced from crystallized lactose permease (18, 19). BM is a membrane-permeable cysteine-reactive reagent. The chemical basis for BM labeling is that it covalently reacts with an ionized sulfhydryl group in the target protein via a thioether bond to introduce a biotin group (20, 21). The incorporated biotin can then be detected by streptavidin-conjugated horseradish peroxidase on a Western blot. If an introduced cysteine is in the lipid bilayer, in a folded conformation or buried in protein-protein interactions, it will not be available for BM labeling. However, if it is aqueous exposed on the intra- or extracellular surface of the target protein, it will be labeled (21). Whole cell labeling of intact HEK 293 cells expressing various NBCe1-A cysteine-introduced constructs with BM was performed at 48 h after transfection. A single labeled endogenous cysteine residue NBCe1-A (Cys1035 in NBCe1-A-5C− background) and NBCe1-A-5C− construct were included in every labeling assay as internal positive and negative labeling controls, respectively (Fig. 3A). The biotin signal of each introduced cysteine mutant was quantified by densitometry of a BM blot and the corresponding immunoblot. Fig. 3A shows that NBCe1-A appears as two bands on the immunoblot that represent the glycosylated (higher molecular weight band) and unglycosylated (lower molecular weight band) forms of NBCe1-A. The glycosylated form of NBCe1-A is the membrane-processed and properly folded protein and therefore was chosen for the final data analysis. Data were normalized to construct C1035, which served as an internal standard and positive control in each experiment. Fig. 3B shows the region of Ala800 to Lys812 is minimally labeled, indicating that this region is not exposed to the aqueous media. In contrast, the region of Met813 to Gly828 is strongly labeled with a plateau at residues Ala819 and Pro820, indicating that it forms an aqueous exposed loop. After Gly828, BM labeling is abruptly stopped until Met858, whose labeling was ∼25% of the control. There then followed a long unlabeled region until amino acid Asp960.
FIGURE 3.
BM labeling of NBCe1-A cysteine-substituted constructs. A, representative data of BM labeling. HEK 293 cells expressing individual cysteine-substituted constructs were labeled with BM at room temperature. Cells were lysed, and NBCe1-A protein was immunoprecipitated, resolved on 7.5% SDS-PAGE, and transferred to PVDF membrane. Incorporated biotin was detected by HRP-streptavidin and ECL. Blots were stripped and probed with an anti-NBCe1 antibody to detect the amount of NBCe1-A protein in each sample. Pro, protein. B, summary of BM labeling of cysteine-substituted NBCe1-A constructs. The level of biotin incorporation in each sample was quantified by densitometry, and the signal was normalized to the amount of NBCe1-A protein present in the sample. In each experiment, the level of biotinylation was compared with C1035, whose labeling was set at 100%. Region between Val829 and Asp959 was labeled to a level similar to the background (NBCe1-A-5C−) except Met858 and therefore is not shown. Asterisks mark the constructs that were not analyzed due to lack of membrane expression. Data represent mean of three to five experiments ± S.E. (error bars).
Accessibility of NBCe1-A-introduced Cysteines to MTS-TAMRA
To determine whether the introduced cysteine mutations are on the extra- or intracellular surface of NBCe1-A, we performed whole cell labeling with a membrane impermeant sulfhydryl-specific chemical MTS-TAMRA. MTS-TAMRA carries a sulfhydryl-reactive MTS group and a highly charged tetramethylrhodamine group. If an introduced cysteine is on the extracellular surface of NBCe1-A, it will be labeled by MTS-TAMRA and detected under a fluorescent microscope (excitation 545 nm/emission 590 nm). Fig. 4A shows that cells expressing T442C (extracellular control) are labeled with MTS-TAMRA, indicating that T442C is on the extracellular surface of NBCe1-A; whereas the cells expressing C1035 (intracellular control) had no labeling, indicating it is located in the cytosol. Additionally, MTS-TAMRA has a much smaller sulfhydryl reactive group compared with BM and is able to detect introduced cysteines that are inaccessible to BM labeling as shown previously (15). HEK 293 cells individually transfected with NBCe1-A cysteine-substituted constructs were subjected to MTS-TAMRA labeling (except the 21 membrane unprocessed constructs). Fig. 4B shows, of the 168 introduced cysteines, only 5 could be labeled with MTS-TAMRA, including the previously identified M858C, T926C, and V927C. This observation demonstrates that the highly biotinylated Met813 to Gly828 region is in the cytosol, residue Met858 faces the extracellular medium, and the BM unlabeled region of Thr926–Ala929 forms an extracellular facing loop.
FIGURE 4.
Whole cell labeling with MTS-TAMRA. A, representative images of MTS-TAMRA labeling on intact cells expressing NBCe1-A constructs. HEK 293 cells expressing cysteine-substituted constructs were incubated with MTS-TAMRA in PBS and imaged at an excitation wavelength of 545 nm and emission wavelength of 590 nm. Fluorescence images were acquired by a PXL charge-coupled device camera coupled to a Nikon Microphot-FXA epifluorescence microscope. a, cells expressing mutant T442C; b, cells expressing C1035; c, cells expressing C1035 stained by Ab-162 in intact cells. B, summary of MTS-TAMRA-labeled NBCe1-A cysteine-substituted constructs. T442C and C1035 were used as an extra- and an intracellular experimental control. +, positive labeling; −, negative labeling.
Labeling of Isolated Plasma Membrane with MTS-TAMRA
The lack of detectable labeling of any residues in the Pro859–Ser925 region with BM or MTS-TAMRA on whole cell labeling was surprising. The region contains 67 residues with a stretch of highly charged amino acids (Pro888–Pro902). The corresponding stretch in AE1 (Pro815–Arg827) was labeled strongly with BM, indicating its aqueous exposure (3). Considering MTS-TAMRA has a much smaller sulfhydryl reactive group and is able to reach cysteines deep in the protein complex, we isolated the plasma membrane from HEK 293 cells expressing NBCe1-A constructs in the predicted intracellular loop (Leu885–Val906) and subjected it to MTS-TAMRA labeling. Fig. 5A shows that MTS-TAMRA labels the intracellular control Cys1035 clearly but not the negative control NBCe1-A-5C−. The TAMRA signal of each introduced cysteine mutant and the corresponding immunoblot was quantified as described previously for the BM labeling experiments. The data were normalized to the construct C1035. We define the constructs that have more than 40% labeling of C1035 as positive. Fig. 5B shows that of the 22 tested introduced cysteines, 9 were labeled with MTS-TAMRA in the isolated membrane intermittently. The results clearly demonstrated that residue Met887 marks the beginning and Arg904 marks the end of this intracellular loop.
FIGURE 5.
MTS-TAMRA labeling of isolated cell membranes. A, representative data of MTS-TAMRA labeling on isolated cell membranes expressing NBCe1-A cysteine-substituted constructs. Membranes isolated from HEK 293 cells expressing various cysteine-substituted NBCe1-A constructs were resuspended in PBS, pH 7.5, and incubated with 0.1 mm MTS-TAMRA for 20 min at room temperature. NBCe1-A proteins were immunoprecipitated, resolved on 7.5% SDS-PAGE, and transferred to PVDF membrane. Labeled samples were detected by a Typhoon Scanner 9410 at an excitation wavelength 545 nm and emission wavelength of 590 nm. B, summary of MTS-TAMRA labeling in the region of Leu885–Val906. NBCe1-A-5C− and C1035 was included in every experiment serving as internal positive and negative labeling controls. +, positive labeling; −, negative labeling; %, relative labeling to C1035. The experiment was performed at least three times.
Labeling of Chemically Stripped Plasma Membrane with Biotin Maleimide
A potential mechanism for the irregular pattern of MTS-TAMRA labeling in the region of Met887–Arg904 is that, this region is tightly associated with a cytoplasmic protein. To test this, we selected two residues, Asp894 and Arg899 which were not labeled with TAMRA, for further analyses. Plasma membranes from cells expressing Asp894 or Arg899 were isolated and treated with 10 mm EDTA, pH 8.0, 1 m KCl, 0.1 m or 3 m Na2CO3, pH 12, prior to BM labeling. Fig. 6 shows that none of these conditions exposed the endogenous cysteines in NBCe1-A-5C− to BM labeling, nor was there an effect on the BM labeling of C1035 (positive control). EDTA, high salt, 0.1 m Na2CO3 had no effect on exposing the Asp894 or Arg899 to labeling. Treatment with 3 m Na2CO3, a condition that offers combination of high salt concentration and alkalization only slightly exposed Arg899 to BM labeling, indicating this region is tightly folded.
FIGURE 6.

BM labeling of chemically stripped cell membranes. Membranes isolated from HEK 293 cells expressing NBCe1-A-5C−, C1035, D894C, or R899C were treated with 10 mm EDTA (A), 1 m KCl (B), 0.1 m Na2CO3 (C), or 3 m Na2CO3 (D) prior to BM labeling in PBSCM, pH 7.0. The assay was performed at least three times.
Functional Sensitivity of Residues in the Last Two TMs to Cysteine Substitution
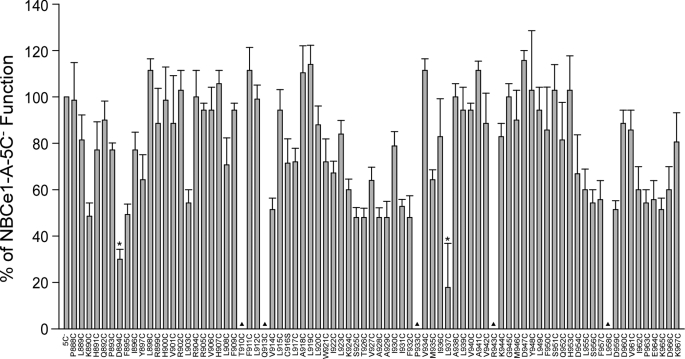
Numerous residues in the C-terminal transmembrane region of AE1 are functionally sensitive to cysteine substitution, indicating that the region has an important role in transport function (3). To assess whether the C-terminal region of NBCe1-A is functionally important, we analyzed the functional effect of cysteine substitution between amino acid Pro888 and Lys967, an indentified functionally important region in AE1 (3). A standard transport assay is shown in Fig. 7. Transfected 293 cells were first equilibrated in Na+-free HEPES solution and then exposed to Na+-free HCO3−-buffered solution. The rapid influx of CO2 quickly acidifies the intracellular pH. When the baseline was stabilized, Na+ was added into the assay solution to activate NBCe1-A, which subsequently led to an intracellular pH recovery. Fig. 8 summarizes the result of the functional assay. Data were normalized to NBCe1-A-5C−, whose transport activity was set as 100%. Of the 75 assayed cysteine-substituted constructs, only 2 (Asp894 and Leu937) had severely impaired transport function (30% of the control), whereas the rest retained at least 50% transport activity. Interestingly, Asp894 is located in the cryptic intracellular loop connecting TM 12 and 13, where it is inaccessible to BM and MTS-TAMRA labeling. Fig. 9 shows on a helical wheel plot that Leu937 is located on one surface of TM 14 clustered with three membrane unprocessed mutations (Leu955, Pro933, and Leu958).
FIGURE 7.
Representative NBCe1-A transport assay. HEK 293 cells grown on coverslips were transiently transfected with NBCe1-A, loaded with pH-sensitive probe BCECF-AM, and assayed with a microfluorometer. Cells were initially equilibrated in HEPES-buffered Na+-free solution, and then intracellular pH was decreased by perfusing cells with a CO2− HCO3−-buffered Na+-free solution. Upon addition of a 140 mm Na+-containing CO2− HCO3−-buffered solution, intracellular pH in NBCe1-A transfected cells rapidly recovered (B), but not the mock-transfected cells (A). All solutions contained 30 μm 5′-(N-ethyl-N-isopropyl) amiloride (EIPA) to block endogenous Na+/H+ exchange.
FIGURE 8.
Summary of the transport function of NBCe1-A cysteine-substituted constructs. Transport data were expressed as a percentage of NBCe1-A-5C− function. Triangles mark the uncharacterized constructs due to lack of plasma membrane expression. Asterisks mark the constructs with <30% transport activity of NBCe1-A-5C−. Error bars represent mean ± S.E. (n = 5–7).
FIGURE 9.
Helical wheel models for the regions spanning the lipid bilayer. Residues in the identified 4 TMs were plotted on helical wheels (3.6 residues/turn). The underlined amino acids mark the beginning and the end of a TM. The shaded residues represent membrane-unprocessed constructs (dark) or functionally inactive (<30%) (gray) mutations. The helical wheel is viewed from the N to the C terminus of a TM, and the sequence proceeds clockwise.
Functional Importance of Lys924 in NBCe1-A
The last extracellular loop of NBCe1-A is similar to that of AE1 both at amino acid sequence (NBCe1-A (KSTVAS) versus AE1 (KSTPAA)) and structural (both are minimally exposed) level. In AE1, the positive charge carried by amino acid Lys851 is critical for the transporter function (5, 22). To determine whether the positive charge carried by Lys924 in NBCe1-A has a functional role, we selectively substituted it with neutral (Ala, Ser, Cys), negative (Asp and Glu), and positive (Arg and His) charged amino acids. Fig. 10 shows that removal of the positive charge at the position of Lys924 impaired the transport function to 40% of the wild type, and the reversed negative charge further impaired the transport function to 30%. Substitution of Lys924 with Arg, an amino acid carrying positive charge with similar pKa, rescued the function to 70%; however, substitution with His impaired the transport function to the same level as the neutral amino acids.
FIGURE 10.
Functional effect of amino acid substitution at position of NBCe1-A-Lys924. Amino acid at position Lys924 was individually mutated to Ala, Ser, Cys, Asp, Glu, Arg, and His, and transport activity of each mutant was assayed as described in the legend to Fig. 7. Transport data were expressed as a percentage of wild type (WT)-NBCe1-A function. Error bars represent mean ± S.E. (n = 7–9). *, p < 0.05, Dunnett's t test.
DISCUSSION
In this study, we examined the topological and the functional properties of the C-terminal transmembrane region of NBCe1-A in detail. Our data demonstrate that the C-terminal transmembrane region of NBCe1-A is tightly folded containing several unique features that differ from AE1: (i) a sharp turn and cryptic connecting loop on the extracellular surface; (ii) highly exposed and cryptic cytosolic connecting loops; and (iii) a functional important positive charge carried by Lys924 (Fig. 11). Compared with AE1, the average size of the NBCe1-A C-terminal TMs is greater and more importantly, substitution of only 2 residues in this region inactivates the transport function in contrast to AE1. These observations support our previously proposed topology model of NBCe1-A and clearly demonstrate that the C-terminal region of NBCe1-A folds differently from AE1.
FIGURE 11.
New topology model of NBCe1-A. Topology of the N-terminal transmembrane region of NBCe1-A was based on our previous report (10); the C-terminal region after TM 9 was proposed in the present study. The branched structures at Asn597 and Asn617 represent N-linked glycosylation. The positions of cysteine-substituted residues in NBCe1-A are depicted as filled circles. Residues were shaded to indicate the degree of labeling with BM: open, not determined; light gray, no significant labeling; dark gray, weakly labeled; black, strongly labeled. Asterisks and triangles mark the MTS-TAMRA-labeled residues in intact cells and isolated membranes, respectively. Squares mark the residues that are membrane-unprocessed when mutated to cysteine. The N terminus (Nt) and C terminus (Ct) are marked.
Our data showed that unlike AE1, the C-terminal transmembrane region of NBCe1-A is minimally exposed to the extracellular medium with only one residue, Met858, that could be labeled weakly with BM but clearly with MTS-TAMRA. The variation in accessibility is due to the differences in the size of the sulfhydryl reactive group of MTS-TAMRA and BM. Met858 is uniquely located in between two prolines that are known to potentially introduce sharp turns in connecting loops or kinks in helices. Interestingly, substitution of either of the prolines with cysteine misfolds the mutant protein as evidenced from the immunostaining and immunoblot analysis. This suggests that Met858 is located at the junction of two TMs where a sharp rigid turn immediately reenters the following TM into the lipid bilayer. The conclusion is further supported by the observation that none of the residues around Met858 could be labeled with MTS-TAMRA. The second detected extracellular facing region (Thr926–Ala929) is only accessible to MTS-TAMRA labeling and not to BM. The sulfhydryl-reactive group of MTS-TAMRA is much smaller than that of BM and is linked to rhodamine via ∼6.7-Å distance. This enables the reagent to reach cysteines that are deeper in the protein complex/lipid bilayer. The inaccessibility of this small extracellular connecting loop to BM indicates that it resides deeper in NBCe1-A in comparison to the corresponding loop in AE1, which was ∼30% labeled with BM (3).
The sequence and structural homology of the last extracellular loop in NBCe1-A and AE1 are striking. Specifically, both transporters have a positively charged lysine residue in the region. In AE1, the positive charge carried by Lys851 is critical for the ion translocation. The underlying mechanism involves the counteracting of the partial negative helical dipole carried by TM 12 (5). Interestingly, our data showed that replacement of Lys924 in NBCe1-A with a neutral or negative charged amino acid dramatically impaired the transport activity; however, a positive charged amino acid (Arg) with similar size and pKa rescued the transport activity to 70%. Surprisingly, replacement of Lys924 with His, an amino acid that also carries positive charge, impaired transport activity to the same level as the neutral amino acids. These observations may suggest that Lys924 is located in a unique position that functions as a counter ion and is important for maintaining the proper structure of the transporter required for normal transport function, rather than counteracting the helical dipole carried by TM 13.
Labeling the predicted NBCe1-A intracellular loops with BM yielded surprising results: (i) the region of Met813–Gly828 was intensely labeled with BM but not with MTS-TAMRA, which unambiguously showed that it forms an intracellular connecting loop containing 16 amino acids; (ii) the first labeled residue in the C-terminal cytoplasmic tail is Asp960, followed by a sharp increased labeling at Pro963. This indicates that Asp960 marks the intracellular lipid/aqueous interface of TM 14, and Pro963 forms a kink at the end of the helix that highly exposes the C-terminal tail to the aqueous; (iii) unexpectedly, whole cell labeling with BM failed to detect the predicted intracellular connecting loop between TM 12 and 13 and showed no labeling of any of the substituted cysteines.
To locate this predicted intracellular loop further, we alternatively probed amino acids Leu885-His907 with MTS-TAMRA in isolated plasma membranes. The results showed that 9 residues in the region are intermittently accessible to the aqueous medium, indicating that a cryptic connecting loop is present between TM 13 and 14. We hypothesized that the inaccessibility of this cryptic loop to BM labeling may be caused by cytoskeletal or cytosolic protein interaction. However, stripping of membranes with EDTA, 1 m KCl, or 0.1 m Na2CO3 failed to expose the selected MTS-TAMRA unlabeled residues (Asp894 and Arg899) to aqueous. Moreover, treatment with 3 m Na2CO3 at pH 12, a stringent condition inducing both counterion and ionic strength, only weakly exposed Asp899 to BM labeling. These observations suggest that this loop is likely intrinsically folded within the NBCe1-A protein complex rather than loosely associated with intracellular proteins. Functional coupling of the cytoplasmic domain of NBCe1-A with the transmembrane domain was previously suggested, in that truncation at the extreme N terminus affects NBCe1-A function (9). In the present study, we showed that substitution of Asp894 (not Arg899) with cysteine impaired NBCe1-A transport function to 30%, although the mutant protein was well expressed on the membrane. Taken together, the obtained data seem to suggest that the transmembrane region of NBCe1-A interacts with its cytoplasmic domain to maintain the normal NBCe1-A transport function.
Our study clearly demonstrated that 5 TMs are present in the C-terminal region between Ala800 and Lys967. An interesting observation in these TMs is that each contains multiple residues that are structurally sensitive to cysteine substitution. Importantly, substitution of any prolines in the proposed TMs results in the misfolding of NBCe1-A, suggesting that removal of any potential kinked structure introduced by proline dramatically affects helix packing. Moreover, replacement of polar residues with cysteines produces more profound structural effects than replacement of nonpolar residues, indicating the intramolecular hydrogen bonding contributes significantly to the stabilization of the cotransporter structure. On helical wheel plots, the mutation sensitive residues are clustered on one surface of each of the TMs, revealing that these surfaces may be involved in helix packaging in the transmembrane region of NBCe1-A. Interestingly, 5 mutation-sensitive residues are located at the beginning of TM 11 sequentially, which suggests this stretch of amino acids serves as the internal topogenic signal that guides TM 11 to fold back into the lipid bilayer. Our data show that TM 11 contains 28, TM 14 contains 30, and TM 12 and TM 13 are likely to contain 28 and 21 residues, respectively. These longer than average TMs contain several proline and glycine residues which may indicate they are kinked or twisted at different angles in the lipid bilayer.
More than 10 residues in the C-terminal region of AE1 are functionally sensitive to cysteine substitution (3), indicating that the region plays a critical role in substrate translocation. Our functional assay revealed that only 2 residues in the corresponding region in NBCe1-A are highly sensitive to cysteine substitution: Asp894 in the cryptic intracellular loop and Leu937 in the last TM. The C-terminal region of AE1 is considered to be highly flexible in that it undergoes significant conformational changes during the substrate transport cycle (5). Our results suggest that the C-terminal region of NBCe1-A is more rigid based on the following observations: (i) 21 amino acids are structurally sensitive to mutation (cause protein misfolding), (ii) longer TMs may be kinked or twisted in the lipid bilayer, (iii) no reentrant loops are present in the region, (iv) only one residue in the TM 14 is functionally sensitive to substitution. We propose that unlike AE1, the longer TMs in the C-terminal region of NBCe1-A may participate in forming a scaffold to accommodate the substrate translocation site of the transporter, similar to that in the sodium-coupled leucine transporter or sodium-coupled glucose transporter 1 (19, 23).
This work was supported, in whole or in part, by National Institutes of Health Grants DK077162 and DK058563.
- TM
- transmembrane segment
- BCECF-AM
- 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein tetrakis (acetoxymethyl) ester
- BM
- biotin maleimide (3-(N-maleimidylpropionyl) biocytin)
- DIDS
- 4, 4′-diisothiocyanato-2, 2′-stilbenedisulfonate
- MTS-TAMRA
- 2-((5(6)-tetramethylrhodamine)carboxylamino) ethyl methanethiosulfonate.
REFERENCES
- 1.Pushkin A., Kurtz I. (2006) Am. J. Physiol. Renal Physiol. 290, F580–F599 [DOI] [PubMed] [Google Scholar]
- 2.Boron W. F., Chen L., Parker M. D. (2009) J. Exp. Biol. 212, 1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Q., Lee D. W., Casey J. R. (2003) J. Biol. Chem. 278, 3112–3120 [DOI] [PubMed] [Google Scholar]
- 4.Tang X. B., Kovacs M., Sterling D., Casey J. R. (1999) J. Biol. Chem. 274, 3557–3564 [DOI] [PubMed] [Google Scholar]
- 5.Zhu Q., Casey J. R. (2004) J. Biol. Chem. 279, 23565–23573 [DOI] [PubMed] [Google Scholar]
- 6.Okubo K., Kang D., Hamasaki N., Jennings M. L. (1994) J. Biol. Chem. 269, 1918–1926 [PubMed] [Google Scholar]
- 7.Kawano Y., Okubo K., Tokunaga F., Miyata T., Iwanaga S., Hamasaki N. (1988) J. Biol. Chem. 263, 8232–8238 [PubMed] [Google Scholar]
- 8.Jennings M. L. (1982) J. Biol. Chem. 257, 7554–7559 [PubMed] [Google Scholar]
- 9.McAlear S. D., Liu X., Williams J. B., McNicholas-Bevensee C. M., Bevensee M. O. (2006) J. Gen. Physiol. 127, 639–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q., Kao L., Azimov R., Newman D., Liu W., Pushkin A., Abuladze N., Kurtz I. (2010) J. Biol. Chem. 285, 13416–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlear S. D., Bevensee M. O. (2006) J. Biol. Chem. 281, 32417–32427 [DOI] [PubMed] [Google Scholar]
- 12.Toye A. M., Parker M. D., Daly C. M., Lu J., Virkki L. V., Pelletier M. F., Boron W. F. (2006) Am. J. Physiol. Cell Physiol. 291, C788–C801 [DOI] [PubMed] [Google Scholar]
- 13.Quilty J. A., Reithmeier R. A. (2000) Traffic 1, 987–998 [DOI] [PubMed] [Google Scholar]
- 14.Boron W. F. (2006) J. Am. Soc. Nephrol. 17, 2368–2382 [DOI] [PubMed] [Google Scholar]
- 15.Zhu Q., Azimov R., Kao L., Newman D., Liu W., Abuladze N., Pushkin A., Kurtz I. (2009) J. Biol. Chem. 284, 8918–8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bok D., Schibler M. J., Pushkin A., Sassani P., Abuladze N., Naser Z., Kurtz I. (2001) Am. J. Physiol. Renal Physiol. 281, F920–F935 [DOI] [PubMed] [Google Scholar]
- 17.Tatishchev S., Abuladze N., Pushkin A., Newman D., Liu W., Weeks D., Sachs G., Kurtz I. (2003) Biochemistry 42, 755–765 [DOI] [PubMed] [Google Scholar]
- 18.Frillingos S., Sahin-Tóth M., Wu J., Kaback H. R. (1998) FASEB J. 12, 1281–1299 [DOI] [PubMed] [Google Scholar]
- 19.Abramson J., Smirnova I., Kasho V., Verner G., Kaback H. R., Iwata S. (2003) Science 301, 610–615 [DOI] [PubMed] [Google Scholar]
- 20.Karlin A., Akabas M. H. (1998) Methods Enzymol. 293, 123–145 [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q., Casey J. R. (2007) Methods 41, 439–450 [DOI] [PubMed] [Google Scholar]
- 22.Jennings M. L. (1985) Annu. Rev. Physiol. 47, 519–533 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]