Abstract
AMP-activated protein kinase (AMPK) β subunits (β1 and β2) provide scaffolds for binding α and γ subunits and contain a carbohydrate-binding module important for regulating enzyme activity. We generated C57Bl/6 mice with germline deletion of AMPK β2 (β2 KO) and examined AMPK expression and activity, exercise capacity, metabolic control during muscle contractions, aminoimidazole carboxamide ribonucleotide (AICAR) sensitivity, and susceptibility to obesity-induced insulin resistance. We find that β2 KO mice are viable and breed normally. β2 KO mice had a reduction in skeletal muscle AMPK α1 and α2 expression despite up-regulation of the β1 isoform. Heart AMPK α2 expression was also reduced but this did not affect resting AMPK α1 or α2 activities. AMPK α1 and α2 activities were not changed in liver, fat, or hypothalamus. AICAR-stimulated glucose uptake but not fatty acid oxidation was impaired in β2 KO mice. During treadmill running β2 KO mice had reduced maximal and endurance exercise capacity, which was associated with lower muscle and heart AMPK activity and reduced levels of muscle and liver glycogen. Reductions in exercise capacity of β2 KO mice were not due to lower muscle mitochondrial content or defects in contraction-stimulated glucose uptake or fatty acid oxidation. When challenged with a high-fat diet β2 KO mice gained more weight and were more susceptible to the development of hyperinsulinemia and glucose intolerance. In summary these data show that deletion of AMPK β2 reduces AMPK activity in skeletal muscle resulting in impaired exercise capacity and the worsening of diet-induced obesity and glucose intolerance.
Keywords: AMP-activated Kinase (AMPK), Diabetes, Energy Metabolism, Enzymes, Fatty Acid Metabolism, Gene Knockout, Glucose Transport, Glycogen, Insulin Resistance, Exercise
Introduction
The AMP-activated protein kinase (AMPK)5 is an evolutionary conserved serine/threonine protein kinase that functions as a metabolic regulatory enzyme at both the intracellular and whole body level (1, 2). As a metabolic stress-sensing enzyme, AMPK is activated through phosphorylation of Thr172 in the α-catalytic subunit by upstream kinases, liver kinase B1 (LKB1) and calcium/calmodulin-dependent kinase kinase in response to physiological processes that consume ATP (exercise) or inhibit ATP production (ischemia or hypoxia) (3). Following activation, AMPK acutely regulates lipid, protein, and carbohydrate metabolism through phosphorylation induced changes that alter enzyme activities by switching off ATP consuming anabolic pathways and switching on ATP generating catabolic pathways (4). In addition to these acute effects, AMPK regulates transcription factors to influence gene expression (4). Modulation of AMPK activity by hormones and cytokines adds a complex layer of regulation allowing energy supply and demand within a cell to be integrated with the energy requirements of the whole organism (5).
AMPK functions as an αβγ heterotrimer where the C terminus of the β isoforms (β1 and β2) contains the subunit-binding sequence that is essential for binding the γ and α subunits (6, 7). In addition to their structural role in maintaining the AMPK heterotrimer, AMPK β subunits contain an evolutionary conserved carbohydrate binding module that when bound with oligosaccharides inhibits AMPK Thr172 phosphorylation (8). Expression profiling of the AMPK β subunits has revealed that β1 is ubiquitously expressed with highest expression levels observed in the liver. We recently reported that whole body deletion of β1 (β1 KO) leads to loss of hepatic AMPK α subunit expression and consequently AMPK activity (9). The AMPK β1 KO mice showed normal development, metabolic rate, and physical activity comparable with wild type (WT) littermates. However, AMPK β1 KO mice had reduced food intake on both low and high fat diets that resulted in reduced adiposity and body mass. In addition to protection from obesity the AMPK β1 KO mice were also protected from diet-induced hyperinsulinemia, hepatic steatosis, and insulin resistance. In contrast to our AMPK β1 KO mouse phenotype, Dasgupta and Milbrandt (10) reported that deletion of β1 using a gene-trapping approach resulted in a profound brain development defect that resulted in postnatal death at day 21. Their mice express a fusion protein comprising β1(2–224)-β-galactacidase that retains the carbohydrate binding module, which may account for their development phenotype.
Studies using tissue RNA profiling (11) or AMPK β1 KO mice (9) suggest that AMPK β2 is predominately expressed in skeletal muscle. Skeletal muscle is the major tissue contributing to whole body energy expenditure and metabolism, and is the principal site of insulin-stimulated glucose uptake. It is well documented that exercise is important for the treatment of insulin resistance and prevention of Type 2 diabetes (12, 13). AMPK is activated by exercise in both rodents (14) and humans (15–17) in an intensity dependent manner (15, 17–21). In muscle the activation of AMPK is associated with increases in GLUT4 translocation and glucose uptake (22–24). AMPK activation also increases fatty acid oxidation, due to phosphorylation and inhibition of acetyl-CoA carboxylase (ACC), which subsequently reduces muscle malonyl-CoA levels and relieves inhibition of carnitine palmitoyltransferase 1 (25).
Recent studies have shown that activation of AMPK by the thienolpyridione class of drugs (26) depends on the β subunit carbohydrate binding module and are specific for the β1 isoform (27). These data suggest that targeting of the β subunits may be of therapeutic significance. Given the importance of muscle in the maintenance of metabolic homeostasis and the muscle-specific expression profile of the β2 subunit we generated mice with whole body deletion of β2 (β2 KO). We hypothesized that β2 KO mice would have reduced skeletal muscle AMPK activity and reduced exercise performance that may precipitate the development of diet-induced insulin resistance.
EXPERIMENTAL PROCEDURES
Generation of AMPK β2 KO Mice
β2 KO mice were generated on a pure C57Bl/6 background by Ozgene Pty. Ltd. (Perth, Australia) by standard homologous recombination techniques using the targeting strategy illustrated in Fig. 1A to delete exons 2–4. Amplification of cDNA from wild type and AMPK β2 knock-out mouse skeletal muscle produced the expected 837-bp wild type band and the 351-bp knock-out band. Sequencing of the AMPK β2 cDNA products confirmed that the knock-out gene encoded for exon 1 of the AMPK β2 protein and the frameshift mutation had occurred as expected and exons 5, 6, and 7 no longer encoded for the AMPK β2 protein. No evidence could be found for an in-frame exon 1-exon 6 splice variant in AMPK β2 knock-out mouse skeletal muscle as was observed for the AMPK β1 KO mice (9). In initial experiments β2 KO mice were genotyped by Southern blot using 10 μg of genomic DNA digested with EcoRI and separated on a 0.8% agarose gel overnight at 20 V. DNA was transferred to Hybond-N membrane and probed with 15 ng of a specific 3′ DNA probe labeled with [α-32P]dCTP. Subsequently, β2 KO mice were genotyped by PCR with primers designed to amplify a 1123-bp fragment in wild type (WT) mice and a 1237-bp fragment in β2 KO mice. Attempts to combine all three primers in one reaction were unsuccessful so two PCRs were performed for every sample. For all experiments homozygous β2 KO mice were generated from heterozygous intercross matings and compared with their WT littermates. All procedures were approved by the St. Vincent's Health animal ethics committee.
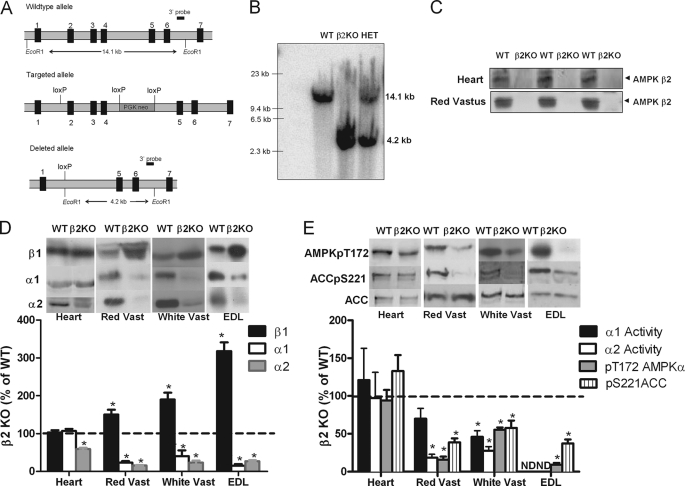
FIGURE 1.
Muscle-specific reductions in AMPK activity and subunit expression in AMPK β2 KO mice. A, gene targeting strategy for generation of β2 KO mice. B, genotyping of β2 KO mice by Southern blot. WT mice showed the expected 14.1-kb fragment (lane 1) and β2 KO mice the expected 4.2-kb fragment (lane 2). Heterozygous (HET) mice had one copy of each allele (lane 3). C, β2 protein expression in heart and red vastus (RV) of WT and β2 KO mice. D, percent of WT protein expression of AMPK β1, α1, α2 in heart, red, and white vastus and EDL muscles of β2 KO mice (representative blot above, densitometry below). E, percent of WT AMPK α1 and α2 activities and AMPK Thr172 and ACC Ser221 phosphorylation in heart, RV and WV muscles of β2 KO mice. Values are mean ± S.E., n = 4–7, ND, not determined. *, p < 0.05 compared with wild type.
Animal Experiments
Mice were housed in SPF microisolators and maintained on a 12-h light/dark cycle with lights on at 7:00 a.m. For diet studies, male mice were fed a control chow diet (4% kcal fat) for 3 weeks following weaning (until 6 weeks of age) and then maintained on this diet for 12 weeks or switched to a high-fat diet (HFD) containing 45% kcal fat (SF-01-028, Specialty Feeds, WA, Australia) for 12–22 weeks (18–30 weeks of age). For food intake studies, mice were housed individually and food weighed daily over 7 days in mice 16 weeks of age. For serum analyses of cytokines, free fatty acids, and insulin, 6-h fasted blood samples were collected retro-orbitally (∼200 μl) using a non-heparinized capillary tube and stored at −80 °C until analysis as described (28).
Hyperinsulinemic-euglycaemic Clamps
Clamps were performed in WT and β2 KO mice fed a HFD for 12 weeks, as described (9). Briefly, 3 days prior to the clamp two catheters were inserted into the right jugular vein. The clamp was conducted after a 6-h fast, which commenced at the start of the light cycle. At −60 min, a 50% dextrose solution containing d-[3-3H]glucose was infused at a constant rate (7.5 μCi/h, 0.12 ml/h) for 1 h for determination of basal glucose turnover. At 0 min, insulin diluted in saline was infused at a rate of 10 milliunits/kg/min and the glucose infusion rate was adjusted to maintain euglycemia. Once steady state was achieved, glucose-specific activity was measured in whole blood after deproteinization with BaOH and ZnSO4. Hepatic glucose production and glucose disposal rate for the basal and clamp period were calculated using Steele's equation for steady state conditions.
Glucose Uptake Assays
Extensor digitorum longus (EDL) muscles were dissected from anesthetized mice (6 mg of pentobarbital 100 g−1 body weight) and transferred to incubation flasks containing 2 ml of essential buffer (Krebs-Henseleit buffer, pH 7.4, with 2.0 mm pyruvate, 8 mm mannitol, and 0.1% BSA), gassed with 95% O2 + 5% CO2 and maintained at 30 °C as described (29, 30). For all experiments, muscles were preincubated for 15 min in this buffer. Muscles were then incubated for an additional 20 min with a similar medium in addition to containing either 2 mm AICAR (Toronto Research Chemicals Inc., ON, Canada) or 2.8 μm insulin (Actrapid®, Novo Nordisk A/S, Denmark).
For contraction experiments, EDL muscles were suspended in incubation chambers (Radnoti, CA) and contraction was induced by electrical stimulation (50 Hz, 60 V, 350-ms pulse duration, 6 tetani/min) (Grass Simulator, RI). Force produced during contraction was determined by a force transducer connected to one end of the muscle by suture and recorded on a computer using Powerlab software (AD Instruments, Colorado Spring, CO) (31). 2-Deoxy-d-glucose (2-DG) uptake was measured by replacing the existing incubation buffer with a similar buffer also containing 0.50 μCi ml−1 of 2-[2,6-3H]deoxy-d-glucose, 1 mm 2-deoxy-d-glucose, and 0.20 μCi of [1-14C]mannitol ml−1. 2-DG uptake was measured during the last 20 min of incubation. Muscle lysates were generated as described below, and radioactivity was measured by liquid scintillation counting (Tri-Carb 2000, Packard Instrument Co.).
Fatty Acid Oxidation Experiments
Isolated EDL muscles were placed in warmed (30 °C) Krebs-Henseleit buffer, pH 7.4, containing 2 mm pyruvate, 4% fatty acid-free BSA (Bovogen, VIC, Australia), and 0.5 mm palmitic acid (Sigma). After an initial incubation of 20 min, the incubation buffer was replaced with the same buffer described above supplemented with 0.5 μCi/ml of [1-14C]palmitate (Amersham Biosciences). Basal and AICAR (2 mm) stimulated fatty acid metabolism were measured over 60 min. In contraction experiments, fatty acid metabolism was measured in fused tetani of EDL (50 Hz, 60 V, 350-ms pulse duration, 6 tetani/min) over 20 min. Rates of fatty acid oxidation were determined by collecting 14CO2 produced during the intervention in benzethonium hydroxide and measuring acid soluble metabolites as described (32, 33). Radioactivity in these samples was then determined by liquid scintillation counting (Tri-Carb 2000, Packard Instrument Co).
Muscle Lactate
WT and β2 KO EDL muscles were preincubated in pre-gassed Krebs-Henseleit buffer, pH 7.4, with 2.0 mm pyruvate, 8 mm mannitol, and 0.1% BSA, at 30 °C for 10–20 min before being contracted (50 Hz, 60 V, 12 tetani/min, 350-ms pulse duration) for 5 min. Muscles were immediately frozen in liquid nitrogen and freeze-dried overnight. Freeze-dried samples were powdered and weighed, extracted by perchloric acid (70%), and neutralized by addition of 2.3 m NaHC03. Muscle extract, standard and reaction mixtures (100 mm hydrazine, 100 mm glycine, 0.5 mm NAD+) were added to a 96-well plate and background fluorescence was measured (excitation, 340 nm; emission, 460 nm). Lactate dehydrogenase (8 units/ml) (Roche Applied Sciences) was added and fluorescence was measured again after 1 h.
Treadmill Running Experiments
Prior to the exercise experiments, all mice were acclimatized to treadmill running at days −3 and −2 by placing animals on the treadmill (Columbus Instruments International, Exer4) for 10 min before running at a 10% slope for 5 min at 10 m/min and 1 min at 15 m/min as described (34). To measure exercise and endurance capacity the experimenter was blinded to the mouse genotype. For exercise capacity testing, mice (14–16 weeks of age) ran at a 10% grade at 10 m/min for 10 min. After this initial warm-up period, exercise intensity was increased by 0.5 m/min every 30 s until mice could not be prompted to continue running by stimulation of the tail. The following week mice exercise trials were conducted for endurance, which after a 10-min warm-up at 10 m/min consisted of running at 15 m/min and 0% gradient until mice could not be prompted to continue running. The next week mice were exercised at the same relative intensity (70% workload maximum) as determined from the maximal exercise intensity test. After completing 30 min or exhaustion (which ever came first) mice were euthanized by cervical dislocation and tissues were rapidly collected and snap-frozen in liquid nitrogen for analysis of AMPK activities, ACC phosphorylation, and muscle glycogen.
Protein Extraction and Immunoblotting
Tissues were homogenized in ice-cold buffer (50 mm Hepes, pH 7.4, 150 mm NaCl, 10 mm NaF, 1 mm sodium pyrophosphate, 0.5 mm EDTA, 250 mm sucrose, 1 mm dithiothreitol, 1% Triton X-100, 1 mm Na3VO4, and 1 Roche protease inhibitor tablet per 50 ml of buffer) using an electrical homogenizer. Protein content in lysates was measured by the bicinchoninic acid method (Pierce). Expression or phosphorylation of investigated proteins was determined in muscle lysates by SDS-PAGE and immunoblotting using the following primary antibodies: AMPK α1, α2, phospho-AMPK Thr172, and phospho-ACC Ser221 (as described (35)), AMPK β1 and β2 (Epitomics, Burlingame, CA), Akt, and phospho-Akt Ser473 and Thr308 (Cell Signaling Technology Inc.). Mitochondrial enzymes were detected using an antibody mixture that detects 30-kDa Complex II subunit (C2), Complex III subunit Core 2 (C3), and Complex IV Cytochrome oxidase-2 (C4) (OXPHOS AB mixture, MitoSciences, OR) as described (29). A horseradish-conjugated protein G (Bio-Rad) was used for a secondary antibody.
AMPK Activity Assay
For the determination of AMPK activity we incubated lysates with AMPK α1 and AMPK α2-specific antibody-bound protein A-agarose beads for 2 h, washed the immunocomplexes, and determined enzyme activities in the presence of 200 mm AMP using the SAMS peptide as described (36).
Lipid and Glycogen Analysis
Lipids were extracted from freeze-dried, powdered muscle using chloroform, methanol, PBS + 0.2% SDS (1:2:0.8). Diacylglycerol kinase and [32P]ATP (15 mCi/mmol of cold ATP) were added to lysates preincubated with cardiolipin/octyl glucoside, and the reaction was stopped after 2 h by the addition of chloroform:methanol (2:1). Samples were spotted onto thin-layer chromatography plates, scraped, and radioactivity determined using a liquid scintillation counter. Muscle and liver glycogen were determined using a glycogen assay kit according to the manufacturer's directions (Sigma).
Real Time Quantitative PCR
RNA was isolated using the RNeasy mini kit (Qiagen), reverse transcribed using the thermoscript RT-PCR system (Invitrogen), and analyzed via quantitative real time PCR on the Rotorgene 3000 (Corbett Research) using Assay-on-Demand gene expression kits (Applied Biosystems) following the manufacturer's recommendations. Assays were normalized using 18 S ribosomal RNA. Expression levels were calculated using the comparative critical threshold (Ct) method.
EDL Muscle Fiber Typing
Serial sections (5 μm) were cut transversely through the EDL muscle using a refrigerated (−20 °C) cryostat. Sections were stained using laminin (Sigma) and N2.261 (developed by Dr. Helen M. Blau, obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA) antibodies to assess the fiber type-specific cross-sectional area and the percentage of myosin IIa isoforms and succinate dehydrogenase (SDH) activity to determine fiber type proportions (37, 38). Digital images were obtained using an upright microscope with a camera (Axio Imager D1, Carl Zeiss, Wrek Göttingen, Germany), controlled by AxioVision AC software (AxioVision AC Rel. 4.7.1, Carl Zeiss Imaging Solutions, Wrek, Wrek Göttingen, Germany). Images were quantified using AxioVision 4.7.1 software. The optical density of succinate dehydrogenase staining was determined after 6 min of reactivity for all samples and stained sections were captured in full color using bright field light microscopy. Digitally captured images (×20 objective) with a minimum of four fields of view per muscle cross-section were analyzed. The bright field images of succinate dehydrogenase reactivity were converted post hoc to grayscale values. The mean optical density of the succinate dehydrogenase-raised signal per individual fiber was determined by averaging the optical density measured in every pixel in the cell, corrected for the mean optical density of the background stain measured in a field of view containing no muscle fibers. Succinate dehydrogenase activity was expressed as optical density.
Statistical Analysis
All data are reported as mean ± S.E. Results were analyzed using analysis of variance or Kaplan Meier statistics (Running Survival Curves) using Graphpad Prism software. A two-way repeated measure for analysis of variance was used to assess differences in body mass over time. A Tukey post hoc test was used to test for significant differences revealed by the analysis of variance. Significance was accepted at p ≤ 0.05.
RESULTS
β2 KO Mice Have Muscle-specific Reductions in AMPK Activity
Digest of genomic DNA with EcoRI produced a 14.1-kb fragment for the wild type locus and a 4.2-kb fragment for the complete Cre-deleted knock-out locus, whereas heterozygous mice contained one copy of each locus (Fig. 1, A and B). Loss of the AMPK β2 protein was confirmed by immunoblot in heart, red and white vastus muscles (Fig. 1C). AMPK β2 could not be detected by immunoblot in liver, white adipose tissue, or the hypothalamus (data not shown). Substantial compensatory increases of β1 protein were detected in red vastus (Red Vast; +50%), white vastus (White Vast; 90%), and EDL (+218%) muscle but not the heart of β2 KO mice (Fig. 1D). Despite up-regulation of the β1 isoform, AMPK α1 and α2 expression were dramatically reduced in all muscle types examined (Fig. 1D). In the heart, β2 KO mice had a 40% reduction in AMPK α2 expression but AMPK α1 expression was unchanged. AMPK α1 or α2 protein were not altered in liver, white adipose tissue or hypothalamus (data not shown). In gastrocnemius muscle from β2 KO mice, immunoprecipitation of AMPK α1 and α2 co-immunoprecipitated the β1 subunit, demonstrating that the remaining α isoforms were associated with the β1 subunit (supplemental Fig. S1).
Reductions in AMPK α expression in skeletal muscle were associated with reduced AMPK α1 and α2 activities and AMPK Thr172 and ACC Ser221 phosphorylation (Fig. 1E). The reduced heart α2 expression in β2 KO mice did not alter basal AMPK α1 and α2 activities or AMPK Thr172 and ACC Ser221 phosphorylation (Fig. 1E). Liver, white adipose tissue and hypothalamic AMPK α1 or α2 activities or AMPK Thr172 and ACC Ser221 phosphorylation were not altered in β2 KO mice (data not shown). These data indicate that whole body deletion of the β2 isoform reduces AMPK α1 and α2 protein and activity in skeletal muscle and α2 protein in the heart without altering AMPK activity in other tissues.
Skeletal Muscle from β2 KO Mice Is Insensitive to AICAR-stimulated Glucose Uptake but Not Palmitate Oxidation
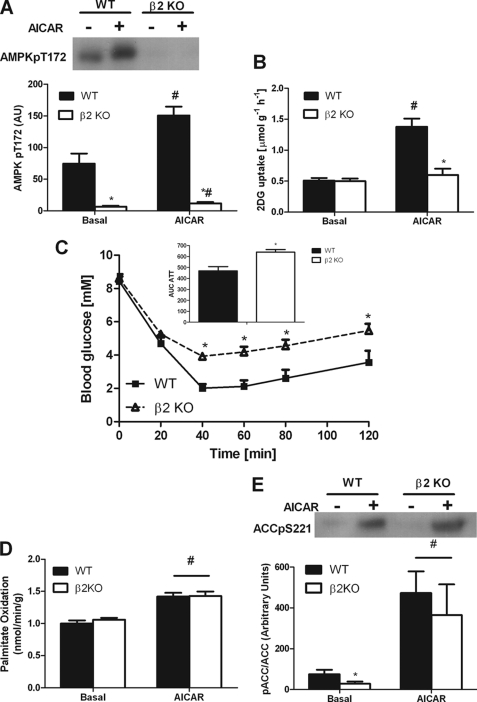
Pharmacological activation of AMPK by AICAR increases skeletal muscle glucose uptake (22, 25, 39) and this effect is dependent on AMPK α2 (24, 40) and γ3 (41). To examine whether there was a specific β isoform required for the effects of AICAR we incubated WT and β2 KO EDL muscles with and without AICAR. We found that AICAR increased AMPK Thr172 phosphorylation in EDL muscles from WT mice but this effect was markedly blunted in muscles from β2 KO mice (Fig. 2A). Basal glucose uptake was not different between WT and β2 KO mice (Fig. 2B). AICAR increased glucose uptake in EDL muscle by ∼270% in WT mice, but this effect was eliminated in β2 KO mice (Fig. 2B). Reductions in AICAR-stimulated glucose uptake did not appear to be due to reduced GLUT4 expression, which was not different between wild type and β2 KO muscle (supplemental Fig. S2). To examine whether refractiveness to AICAR was also observed in vivo, we injected mice with AICAR. Because AICAR reduces blood glucose by both suppressing hepatic glucose output and increasing skeletal muscle glucose uptake we hypothesized that given the expression profile of β2 that hepatic glucose output would be normal in β2 KO mice, whereas skeletal muscle glucose uptake would be blunted. We found that whereas AICAR reduced blood glucose levels in both WT and β2 KO mice this effect was blunted in β2 KO mice, which was consistent with the reduced AICAR-stimulated glucose uptake observed ex vivo (Fig. 2C). We also examined the effects of AICAR on fatty acid oxidation and found that in contrast to the β2-dependent effects on glucose uptake, AICAR-stimulated fatty acid oxidation (Fig. 2D) and ACC phosphorylation (Fig. 2E) were maintained in β2 KO mice. These data suggest that AICAR-stimulated glucose uptake but not fatty acid oxidation is dependent on the presence of the AMPK β2 isoform in skeletal muscle.
FIGURE 2.
Skeletal muscle of AMPK β2 KO mice have reduced glucose uptake but normal palmitate oxidation in response to AICAR. A, reduced AMPK Thr172 phosphorylation in EDL muscle of β2 KO mice both basally and following 50 min incubation with AICAR. B, β2 KO mice are insensitive to the stimulatory effects of AICAR on 2-deoxyglucose uptake in EDL muscles. C, AICAR reduces blood glucose in both WT and β2 KO mice but this reduction in glucose uptake is blunted in β2 KO mice at time points after 40 min. Inset, blood glucose area under the curve following AICAR injection. D, palmitate oxidation in isolated EDL muscle treated with or without 2 mm AICAR. E, ACC phosphorylation in isolated EDL muscle treated with or without 2 mm AICAR. Values are mean ± S.E., n = 6–15. *, p < 0.05 compared with wild type. #, p < 0.05 compared with basal.
β2 KO Mice Have Reduced Exercise Capacity
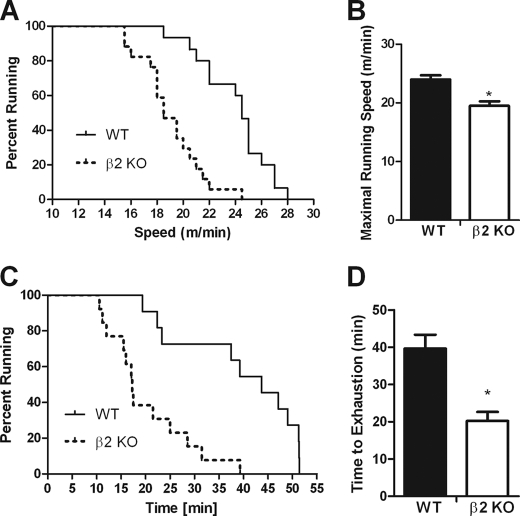
In mice, the chronic activation of skeletal muscle AMPK increases exercise capacity (42, 43), whereas the inverse is observed with reduced AMPK signaling in heart and skeletal muscle (24, 44, 45). Given the substantial reductions in skeletal muscle AMPK activity in β2 KO mice we hypothesized that these mice may have reduced exercise capacity. During an incremental progressive treadmill running exercise stress test β2 KO mice had a 20% reduction (p < 0.001) in maximal running speed (Fig. 3, A and B). As this test is predominately a measure of maximal aerobic exercise capacity we then tested the ability of the mice to perform endurance exercise over a prolonged period of time at a lower workload (0% grade and 15 m/min) and found that exercise endurance was also dramatically reduced (∼−50%) in β2 KO mice (p < 0.001) (Fig. 3, C and D).
FIGURE 3.
AMPK β2 KO mice have reduced maximal exercise capacity and endurance. A, survival plot indicating the percent of wild type and β2 KO mice running at the indicated speed during a short duration incremental VO2 max style test. B, mean maximal running speed of wild type and β2 KO mice during the short duration incremental VO2 max style test. C, survival plot indicating percent of wild type and β2 KO mice running at the indicated time during a low intensity treadmill running test (15 m/min, 0% gradient). D, mean running time of wild type and β2 KO mice during the low intensity treadmill running test (15 m/min, 0% gradient). Survival plots data are individual data points. Other figures are mean ± S.E., n = 10–15, *, p < 0.05 compared with wild type.
β2 KO Mice Have Reduced Muscle and Heart AMPK Activity at the Completion of Treadmill Exercise
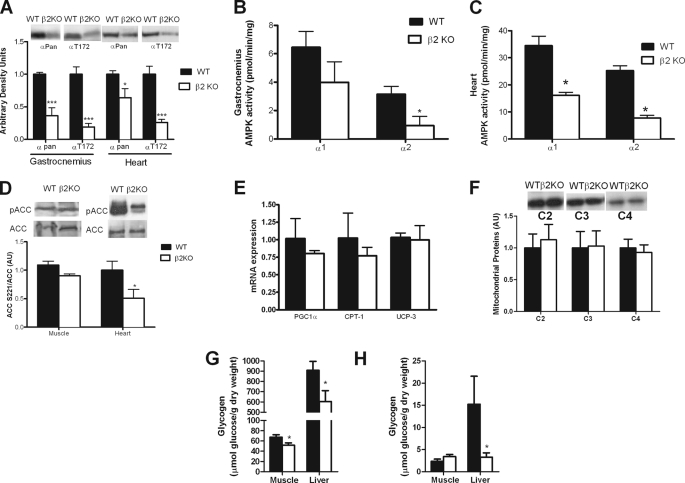
At the completion of 30 min of exercise, gastrocnemius AMPK α expression, and Thr172 phosphorylation were reduced in β2 KO mice by 64 and 81%, respectively (p < 0.001) (Fig. 4A), and this corresponded with a reduction in AMPK α2 (p = 0.033) but not AMPK α1 activity (p = 0.11) (Fig. 4B). Because AMPK α2 expression was also reduced in the heart of β2 KO mice, we measured AMPK α expression, phosphorylation and activity at the completion of exercise. Consistent with resting data showing ∼50% reduction in α2 but not α1 expression (Fig. 1A) we found that total AMPK α expression was reduced by ∼36% (Fig. 4A). However, in contrast to the resting condition where no reduction in Thr172 phosphorylation was detected, at the completion of exercise Thr172 phosphorylation (Fig. 4A) and AMPK α1 and α2 activities (Fig. 4C) were substantially reduced in β2 KO mice. We then measured ACC phosphorylation and found that it was significantly reduced in the heart but not skeletal muscle of β2 KO mice (Fig. 4D). These data suggest that a reduction in heart and muscle AMPK activity may contribute to the reduced exercise tolerance of β2 KO mice.
FIGURE 4.
AMPK β2 KO mice have lower heart and skeletal muscle AMPK activity after exercise, normal expression profile of mitochondrial markers but reduced levels of liver and muscle glycogen. Skeletal muscle and heart (A) AMPK α expression and Thr172 phosphorylation, AMPK α1 and α2 activities (B and C) and ACC phosphorylation (D) after 30 min of treadmill running at the same relative intensity (70% of maximal treadmill running speed). AMPK β2 KO mice have normal (E) mRNA and (F) protein levels of mitochondrial markers. Muscle and liver glycogen before (G) and after (H) 30 min of treadmill running at the same relative intensity. PGC1α, PPARγ co-activator 1α; CPT1, carnitine palmitoyltransferase-1; UCP3, uncoupling protein 3; C2, mitochondrial complex II; C3, mitochondrial complex III; C4, mitochondrial complex IV cytochrome oxidase-2. Data are mean ± S.E., n = 8. *, p < 0.05 compared with wild type; ***, p < 0.001 compared with wild type.
β2 KO Mice Have Normal Expression of Markers of Mitochondrial Capacity but Have Reduced Muscle and Liver Glycogen
Because oxidative phosphorylation can be limiting for exercise and AMPK α2 null mice have reduced mitochondrial content (36) and AMPK α2-kinase dead (KD) mice have reduced mitochondrial complex activity (46) we examined protein and mRNA expression of key mitochondrial enzymes. We found that mRNA of peroxisome proliferator-activated receptor γ co-activator 1α, carnitine palmitoyltransferase-1, and uncoupling protein-3 were not significantly altered in β2 KO mice (Fig. 4E). Similarly, protein expression of mitochondrial electron transport chain proteins Complex II subunit 30 kDa (C2), Complex III subunit Core 2 (C3), and Complex IV Cytochrome oxidase-2 (C4) (Fig. 4F) were unaltered in β2 KO mice relative to their WT littermates.
Glycogen in muscle and liver is an important substrate during endurance exercise. Glycogen levels have been claimed to regulate AMPK activity (47), an effect mediated through interaction with the carbohydrate binding module of the β subunit (8) (reviewed in Ref. 1). We found that both muscle (Red Vastus) and liver glycogen in β2 KO mice were reduced before exercise (Fig. 4G). After 30 min of treadmill running at the same relative workload (70% of VO2 max) liver glycogen was lower in β2 KO mice than wild type littermates (Fig. 4H). These data suggest that lower starting levels of glycogen in β2 KO mice may have contributed to the premature fatigue.
β2 KO Mice Have Normal Rates of Contraction-stimulated Glucose Uptake and Fatty Acid Oxidation but Generate Lower Muscle Force Potentially Due to Smaller Fiber Size
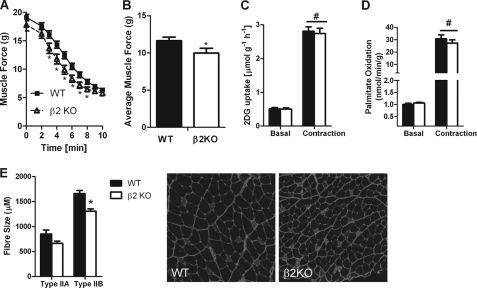
To dissect out the potential muscle-specific effects contributing to the reduced exercise capacity of β2 KO mice, we conducted experiments in isolated EDL muscles ex vivo. We found that there was a 15% reduction in muscle force production in β2 KO mice (Fig. 5, A and B). We initially hypothesized that this reduction in muscle force may be due to impaired skeletal muscle glucose uptake or fatty acid oxidation. However, contraction-stimulated glucose uptake (Fig. 5C) or fatty acid oxidation (Fig. 5D) was not altered between WT and β2 KO EDL muscles. Lactate concentrations were also similar between WT and β2 KO EDL muscles after 5 min of muscle contraction (WT = 37.5 ± 6.5 μmol/g dry weight versus β2 KO = 37.8 ± 4.9 μmol/g dry weight) consistent with normal rates of substrate utilization and/or metabolic control.
FIGURE 5.
AMPK β2 KO mice have reduced muscle function ex vivo, an effect that is not associated with reductions in glucose uptake or fatty acid oxidation but decreased muscle fiber size. A, reduced muscle force over time in isolated EDL muscles from WT and β2 KO mice contracted ex vivo. B, average muscle force in WT and β2 KO mice over a 10-min contraction period. Contraction-stimulated (C) glucose uptake and (D) fatty acid oxidation were not different between WT and β2 KO mice. E, EDL muscles from β2 KO mice have smaller Type IIA and IIB muscle fibers compared with WT littermates. Values are mean ± S.E., n = 6–15. *, p < 0.05 compared with wild type. #, p < 0.05 compared with basal.
Recent studies have suggested that reduced muscle AMPK may invoke a fiber-type switch (48) or alter muscle size (49). Therefore, to investigate whether this was the cause for the reduced muscle force during contractions we analyzed the fiber composition of EDL muscle and found that there was no difference in the ratio of type IIA (WT = 12 ± 2%, β2 KO = 10 ± 3%) to type IIB fibers (WT = 88 ± 1%, β2 KO = 90 ± 3%). However, consistent with lower force production we found that fibers from β2 KO mice were smaller than WT littermates (Fig. 5E). Taken together, these data suggest that smaller muscle fibers, but not defects in substrate oxidation, may contribute to the reduced muscle force of β2 KO mice during ex vivo muscle contractions.
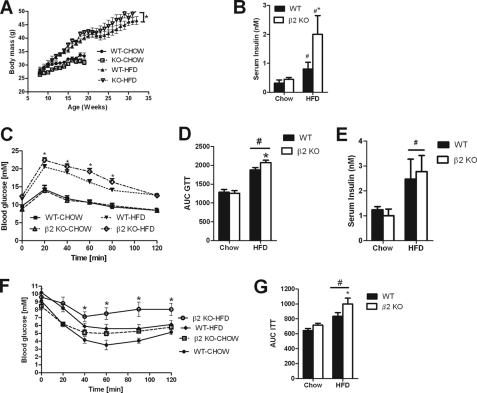
β2 KO Mice Have Increased Susceptibility to Diet-induced Skeletal Muscle Insulin Resistance
Given the reduced exercise capacity of β2 KO mice we hypothesized that they would be more susceptible to the development of obesity and whole body insulin resistance. To test this hypothesis we fed mice either a control chow diet or a HFD and monitored body mass and whole body and skeletal muscle insulin sensitivity. On a chow diet β2 mice tended to be lighter but this did not achieve statistical significance (p = 0.08, Fig. 6A). When fed a HFD over 30 weeks body mass was not significantly different at any single data point but over time β2 KO mice weight gain was greater than WT littermate controls (p < 0.001) (Fig. 6A). Caloric consumption was greater on a HFD diet compared with chow (p = 0.0125) but there was no significant difference between genotypes (Table 1). These data suggest that increased weight gain in HFD β2 KO mice may be potentially due to reduced energy expenditure and activity levels as previously reported in mice with muscle- and heart-specific overexpression of a dominant-negative AMPK (45), however, this requires further investigation.
FIGURE 6.
High-fat diet fed AMPK β2 KO mice gain more weight over time and are more susceptible to glucose intolerance and hyperinsulinemia. A, body mass over time in WT and β2 KO mice. B, hyperinsulinemia in AMPK β2 KO mice fed a HFD. C and D, impaired glucose tolerance in β2KO mice fed a HFD. E, serum insulin levels in chow and HFD-fed mice 20 min after injection with glucose (1 mg/kg). F and G, whole body insulin sensitivity following a bolus of insulin (0.75 units/kg). Values are mean ± S.E., n = 7–13. *, p < 0.05 compared with wild type. #, p < 0.05 compared with chow diet.
TABLE 1.
Caloric intake and serum measurements in chow and HFD-fed wild type and β2 KO mice 18 weeks of age
Blood was collected by retro-orbital bleed using non-heparinised capillary tubes after a 6-h fast. Values are mean ± S.E., n = 6–8.
| Chow |
HFD |
|||
|---|---|---|---|---|
| WT | β2 KO | WT | β2 KO | |
| Caloric intake (kcal/day) | 11.18 ± 0.60 | 11.40 ± 0.62 | 12.28 ± 0.84a | 13.84 ± 0.59a |
| Serum glucose (mm) | 9.12 ± 0.33 | 8.45 ± 0.41 | 10.20 ± 0.42a,b | 9.61 ± 0.43a |
| Non-esterified free fatty acids (mm) | 1.41 ± 0.07 | 1.33 ± 0.06 | 1.30 ± 0.09 | 1.24 ± 0.05 |
| Leptin (ng/ml) | 3.49 ± 0.44 | 3.55 ± 0.48 | 12.35 ± 0.14a | 11.20 ± 0.76a |
| TNFα (pg/ml) | 2.50 ± 0.96 | 2.34 ± 0.17 | 3.59 ± 0.48 | 3.26 ± 0.47 |
| IL-6 (pg/ml) | 4.53 ± 0.95 | 4.91 ± 1.30 | 5.54 ± 2.10 | 5.43 ± 1.20 |
| Resistin (ng/ml) | 1.79 ± 0.1 | 1.50 ± 0.22 | 3.4 ± 0.25a | 3.69 ± 0.44a |
a p < 0.05 compared to chow fed.
b p < 0.05 compared to WT.
In chow-fed mice, glucose, non-esterified free-fatty acids and the adipokines resistin, TNFα, IL-6 and leptin were comparable between WT and β2 KO mice (Table 1). The HFD increased serum leptin, resistin, and plasma glucose levels but there was no difference between genotypes (Table 1). Insulin levels were comparable between chow-fed WT and β2 KO mice (Fig. 6B) and as expected the HFD increased serum insulin levels, but this effect was much more pronounced in β2 KO mice (Fig. 6B). We then conducted glucose tolerance tests and found that although glucose tolerance was similar between WT and β2 KO mice when fed a chow diet (Fig. 6, C and D), when fed a HFD β2 KO mice had glucose intolerance relative to WT littermates (Fig. 6, C and D) despite similar insulin levels 20 min after the bolus of glucose (Fig. 6E). Insulin tolerance tests also demonstrated that HFD but not chow-fed β2 KO mice were insulin resistant as assessed by higher glucose levels (Fig. 6F) and greater area under the curve (Fig. 6G).
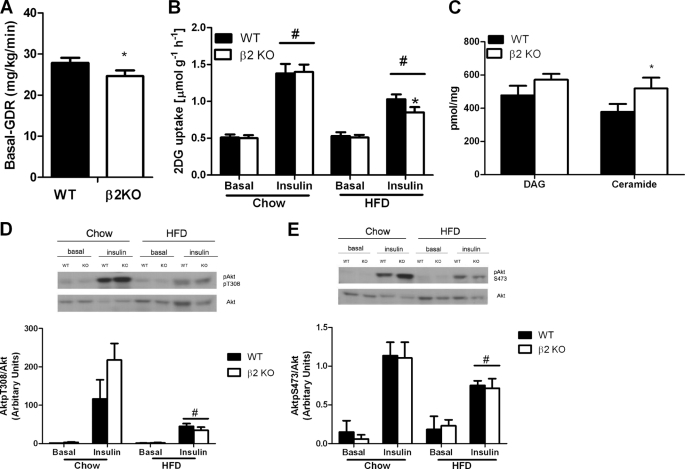
To assess whether reduced insulin sensitivity in β2 KO mice was due to peripheral or hepatic insulin sensitivity we conducted hyperinsulinemic euglycemic clamps in a cohort of WT and β2 KO mice fed the HFD for 12 weeks. Serum glucose concentrations before and during the clamp were not different between WT and β2 KO mice (Table 2). Basal glucose disposal rate was reduced in β2 KO mice (Fig. 7A). However, during insulin infusion there was no difference in glucose infusion rate, hepatic glucose output, suppression of hepatic glucose output, or glucose disposal rate, suggesting a similar degree of insulin resistance between WT and β2 KO mice (Table 2). To examine whether the reduction in basal glucose disposal rate involved changes in skeletal muscle glucose uptake we then incubated EDL muscles ex vivo with or without submaximal concentrations of insulin, and measured 2-DG uptake. We found that in the absence of insulin, 2-DG uptake was not different between WT and β2 KO mice fed either a chow or HFD (Fig. 7B). Insulin-stimulated 2-DG uptake was similar between WT and β2 KO mice fed a control chow diet (Fig. 7B) and whereas the HFD suppressed insulin-stimulated 2-DG uptake in both genotypes this effect was greater in β2 KO mice (Fig. 7B).
TABLE 2.
Body mass and hyperinsulinemic-euglycemic clamp parameters in male β2 KO mice fed a high-fat diet for 12 weeks
Values are mean ± S.E.
| WT (n = 4) | β2 KO (n = 6) | p | |
|---|---|---|---|
| Body mass (g) | 36.65 ± 1.02 | 38.48 ± 1.06 | 0.136 |
| Pre-clamp glucose (mm) | 7.48 ± 0.77 | 7.78 ± 0.55 | 0.481 |
| Clamp glucose (mm) | 6.12 ± 0.16 | 5.80 ± 0.16 | 0.065 |
| Insulin-stimulated glucose infusion rate (mg/kg/min) | 50.96 ± 5.18 | 46.05 ± 2.12 | 0.205 |
| Insulin-stimulated glucose disposal rate (mg/kg/min) | 54.07 ± 5.19 | 50.82 ± 2.12 | 0.195 |
| Hepatic glucose output (mg/kg/min) | 4.84 ± 0.45 | 4.78 ± 0.16 | 0.348 |
| % Suppression | 82.53 ± 1.68 | 80.31 ± 1.12 | 0.186 |
FIGURE 7.
High-fat diet fed AMPK β2 KO mice have reduced glucose uptake and increased levels of muscle ceramide. A, basal glucose disposal rate (GDR) during hyperinsulinemic-euglycemic clamp in HFD-fed WT and β2 KO mice (n = 5–6). B, 2-deoxyglucose uptake in isolated EDL muscles from chow and HFD-fed WT and β2 KO mice treated with or without insulin. C, diacylglycerol (DAG) and ceramide levels in tibialis anterior muscle from HFD-fed WT and β2 KO mice (n = 6). Akt (D) Thr308 and (E) Ser473 phosphorylation in isolated EDL muscles from chow and HFD-fed WT and β2 KO mice treated with or without insulin. Values are mean ± S.E., n = 7–13. *, p < 0.05 compared with wild type. #, p < 0.05 compared with chow diet.
Intramuscular lipids such as diacylglycerol and ceramide can impair basal- and insulin-stimulated glucose disposal (50), therefore we measured these lipid species in tibialis anterior muscles from mice fed a HFD. Consistent with a reduction in glucose uptake we found significantly higher levels of ceramide but not diacylglycerol in muscle from β2 KO mice (Fig. 7C). Ceramide has been shown to inhibit glucose uptake by reducing Akt Thr308 phosphorylation (50), therefore we measured phosphorylation of Akt Thr308 (Fig. 7D) and Ser473 (Fig. 7E) in basal- and insulin-treated EDL muscles from chow- and HFD-fed mice. We found that there was no difference in total Akt expression irrespective of genotype or diet (representative blot Fig. 7, D and E, and data not shown) and although the HFD suppressed insulin-stimulated Akt phosphorylation there was no difference between WT and β2 KO mice (Fig. 7, D and E).
DISCUSSION
Genetic deletion of AMPK β2 resulted in decreased AMPK α1 and α2 expression in skeletal muscle and reduced α2 expression in the heart. This reduction in AMPK α1 and α2 expression resulted in reduced AMPK Thr172 phosphorylation and AMPK α1 and α2 activities in skeletal muscle but not other tissues. These studies are in agreement with previous reports demonstrating high expression of the β2 isoform in skeletal muscle (11, 51) and recent studies from our laboratory (9) demonstrating that β1 KO mice had reduced AMPK activity in the liver, hypothalamus, and adipose tissue but not muscle. Importantly, reductions in skeletal muscle AMPK activity may have been even more substantial had there not been significant compensatory up-regulation of the β1 isoform. This is different from the β1 KO where despite the loss of greater than 90% of AMPK activity in the liver there was no compensatory up-regulation of β2 (9). These data are suggestive of either an essential role for AMPK in skeletal muscle but not liver or perhaps differential transcriptional regulation of the two β isoforms.
We demonstrate that both maximal treadmill running speed and running endurance in β2 KO mice are reduced compared with WT littermates. This reduction in exercise capacity is similar to data from AMPK α2 kinase-dead (KD) and LKB1 muscle KO mice (44, 45, 52). Both KD (53) and LKB1 muscle KO mice (54) have a greater than 95% reduction in heart AMPK α2 activity at rest. Even despite this dramatic reduction in AMPK α2 activity, recent studies suggest that KD mice have normal rates of cardiac glucose and long-chain fatty acyl-CoA uptake, heart rate, and cardiac output during treadmill exercise (46). So although heart AMPK activity is reduced in β2 KO mice during exercise we consider that this is not the primary cause of their reduced exercise capacity, however, future studies examining the role of β2 in cardiac function are certainly warranted.
A major finding of this study was the absolute requirement of the AMPK β2 subunit for AICAR-stimulated glucose uptake. This elimination of AICAR-stimulated glucose uptake was associated with a marked reduction in AMPK Thr172 phosphorylation following AICAR treatment. This observation when combined with results from previous studies showing that the AMPK α2 (40) and AMPK γ3 (41) but not α1 (40) and β1 (27) subunits are required for AICAR-stimulated glucose uptake, demonstrates that AICAR increases in glucose uptake is isoform-specific via an α2β2γ3 heterotrimer. Because AICAR is effective in lowering blood glucose in type 2 diabetics the development of drugs that target the β2 subunit containing isoforms may be effective in restoring glucose homeostasis in this population.
Despite the complete elimination of AICAR-stimulated glucose uptake in β2 KO mice contraction-stimulated glucose uptake was not impaired. We also find that AICAR and contraction-stimulated ACC phosphorylation and fatty acid oxidation were indistinguishable between WT and β2 KO mice. These findings are consistent with previous reports in α2 KO (40), γ3 KO (41), and KD mice (31). Taken together these data suggest one of three possibilities for the maintenance of contraction-stimulated glucose uptake and fatty acid oxidation: 1) β1 containing heterotrimers are capable of compensating; 2) AMPK independent pathways activated by muscle contraction such as calcium/calmodulin-dependent kinase II may be important (55, 56); or 3) because contraction-stimulated glucose uptake is reduced in muscle-specific LKB1 null mice (57) these data may indicate that AMPK-related kinases may be important for controlling contraction-stimulated glucose uptake and fatty acid oxidation. Future studies in mice with muscle-specific deletion of both β1 and β2 should help determine more directly the importance of AMPK in regulating metabolism during muscle contractions.
Because contraction-stimulated glucose uptake and fatty acid oxidation are not altered in β2 KO mouse muscle, then what is the cause for their reduced exercise capacity? Glycogen is an important substrate during exercise and β2 KO mice had lower levels of muscle and liver glycogen at rest. AMPKα2 (58) and γ3 KO mice (41) also have lower levels of muscle glycogen, however, to our knowledge, liver glycogen has not been reported. The reason for the reduced glycogen levels in β2 KO mice is not currently known but deserves further study.
We also detected that EDL muscles generated lower muscle forces, a factor that would be independent of muscle glycogen, but is in agreement with findings in KD mice (59, 60). One possibility for this reduction in muscle force could be the smaller muscle fibers of β2 KO EDL, a finding consistent with a recent report in LKB1 muscle KO mice (49). Further studies are required to determine the cause of the reduced exercise capacity and muscle force of β2 KO mice and other models of muscle AMPK deficiency.
AMPK α2 KO mice develop whole body insulin resistance, on a chow diet independent of obesity due to overactivation of the sympathetic nervous system (58). In contrast, β2 KO mice on a chow diet appear to be phenotypically normal and do not display hyperinsulinemia, insulin resistance, or glucose intolerance. These findings are in agreement with studies from KD mice (29, 61) suggesting a specific role for the α2 subunit in controlling SNS activation.
When challenged with a HFD, β2 KO mice gained more weight over time, which is a finding similar to α2 KO mice (62). However, in contrast to HFD-fed α2 KO mice β2 KOs also developed more pronounced hyperinsulinemia and glucose intolerance compared with WT littermates. This effect was associated with lower basal but not insulin-stimulated glucose disposal rates during a hyperinsulinemic-euglycemic clamp and reductions in insulin-stimulated 2-DG uptake in isolated skeletal muscles. The impaired basal glucose disposal in vivo but not in isolated muscles could reflect the importance of the β2 subunit in controlling insulin-independent glucose uptake by a cytokine such as IL-6, which has been shown to increase glucose uptake via an AMPK dependent pathway (63).
In conclusion these results demonstrate that the AMPK β2 isoform is essential for the maintenance of muscle AMPK α expression and activity. The deletion of AMPK β2 results in insensitivity to AICAR-stimulated glucose uptake and reduces exercise performance despite normal rates of contraction-stimulated glucose uptake and fatty acid oxidation. Instead the reduction in exercise performance may be related to lower starting levels of muscle and liver glycogen or muscle fiber size. Lastly, we demonstrate that β2 KO mice have increased susceptibility to weight gain and develop glucose intolerance and hyperinsulinemia when fed a HFD. Thus far, compounds that activate AMPK β1 containing complexes have been found (26, 27). Our data suggest that the selective activation of β2-containing AMPK isoforms may increase exercise capacity and glucose uptake.
Acknowledgments
We are most grateful to Catherine Economou and Dewita Kamaruddin for assistance in the mouse exercise studies and Frosa Katsis for the preparation of purified rabbit polyclonal antibodies.
This work was supported in part by grants from the Australian Research Council (to B. E. K.), National Health and Medical Research Council (to D. J. C., B. E. K., G. R. S., and M. J. W.), Natural Science and Engineering Research Council of Canada (to G. R. S.), Diabetes Australia Research Trust (to G. R. S.), and the National Heart Foundation of Australia (to B. E. K. and D. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- AMPK
- AMP-activated protein kinase
- KO
- knock-out
- ACC
- acetyl-CoA carboxylase
- HFD
- high fat diet
- 2-DG
- 2-deoxy-d-glucose
- KD
- kinase-dead
- EDL
- extensor digitorium longus
- AICAR
- aminoimidazole carboxamide ribonucleotide.
REFERENCES
- 1.Steinberg G. R., Kemp B. E. (2009) Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 2.Richter E. A., Ruderman N. B. (2009) Biochem. J. 418, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005) Cell Metabolism 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 4.Long Y. C., Zierath J. R. (2006) J. Clin. Invest. 116, 1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzamko N. L., Steinberg G. R. (2009) Acta Physiol. (OXF) 196, 115–127 [DOI] [PubMed] [Google Scholar]
- 6.Iseli T. J., Walter M., van Denderen B. J., Katsis F., Witters L. A., Kemp B. E., Michell B. J., Stapleton D. (2005) J. Biol. Chem. 280, 13395–13400 [DOI] [PubMed] [Google Scholar]
- 7.Townley R., Shapiro L. (2007) Science 315, 1726–1729 [DOI] [PubMed] [Google Scholar]
- 8.McBride A., Ghilagaber S., Nikolaev A., Hardie D. G. (2009) Cell Metab. 9, 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzamko N., van Denderen B. J., Hevener A. L., Jørgensen S. B., Honeyman J., Galic S., Chen Z. P., Watt M. J., Campbell D. J., Steinberg G. R., Kemp B. E. (2010) J. Biol. Chem. 285, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta B., Milbrandt J. (2009) Dev. Cell 16, 256–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton C., Snowden M. A., Carling D. (1998) J. Biol. Chem. 273, 12443–12450 [DOI] [PubMed] [Google Scholar]
- 12.Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M.Diabetes Prevention Program Research Group (2002) N. Engl. J. Med. 346, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuomilehto J., Lindström J., Eriksson J. G., Valle T. T., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., Salminen V., Uusitupa M. (2001) N. Engl. J. Med. 344, 1343–1350 [DOI] [PubMed] [Google Scholar]
- 14.Winder W. W., Hardie D. G. (1996) Am. J. Physiol. Endocrinol. Metab. 270, E299–304 [DOI] [PubMed] [Google Scholar]
- 15.Wojtaszewski J. F., Nielsen P., Hansen B. F., Richter E. A., Kiens B. (2000) J. Physiol. 528, 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii N., Hayashi T., Hirshman M. F., Smith J. T., Habinowski S. A., Kaijser L., Mu J., Ljungqvist O., Birnbaum M. J., Witters L. A., Thorell A., Goodyear L. J. (2000) Biochem. Biophys. Res. Commun. 273, 1150–1155 [DOI] [PubMed] [Google Scholar]
- 17.Chen Z. P., McConell G. K., Michell B. J., Snow R. J., Canny B. J., Kemp B. E. (2000) Am. J. Physiol. Endocrinol. Metab. 279, E1202-E1206 [DOI] [PubMed] [Google Scholar]
- 18.Chen Z. P., Stephens T. J., Murthy S., Canny B. J., Hargreaves M., Witters L. A., Kemp B. E., McConell G. K. (2003) Diabetes 52, 2205–2212 [DOI] [PubMed] [Google Scholar]
- 19.Wojtaszewski J. F., MacDonald C., Nielsen J. N., Hellsten Y., Hardie D. G., Kemp B. E., Kiens B., Richter E. A. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E813–822 [DOI] [PubMed] [Google Scholar]
- 20.Stephens T. J., Chen Z. P., Canny B. J., Michell B. J., Kemp B. E., McConell G. K. (2002) Am. J. Physiol. Endocrinol. Metab. 282, E688–694 [DOI] [PubMed] [Google Scholar]
- 21.Dean D., Daugaard J. R., Young M. E., Saha A., Vavvas D., Asp S., Kiens B., Kim K. H., Witters L., Richter E. A., Ruderman N. (2000) Diabetes 49, 1295–1300 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., Goodyear L. J. (1998) Diabetes 47, 1369–1373 [DOI] [PubMed] [Google Scholar]
- 23.Kurth-Kraczek E. J., Hirshman M. F., Goodyear L. J., Winder W. W. (1999) Diabetes 48, 1667–1671 [DOI] [PubMed] [Google Scholar]
- 24.Maarbjerg S. J., Jorgensen S. B., Rose A. J., Jeppesen J., Jensen T. E., Treebak J. T., Birk J. B., Schjerling P., Wojtaszewski J. F., Richter E. A. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E924–E934 [DOI] [PubMed] [Google Scholar]
- 25.Merrill G. F., Kurth E. J., Hardie D. G., Winder W. W. (1997) Am. J. Physiol. Endocrinol. Metab. 273, E1107–1112 [DOI] [PubMed] [Google Scholar]
- 26.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H. S., Frevert E. (2006) Cell Metab. 3, 403–416 [DOI] [PubMed] [Google Scholar]
- 27.Scott J. W., van Denderen B. J., Jorgensen S. B., Honeyman J. E., Steinberg G. R., Oakhill J. S., Iseli T. J., Koay A., Gooley P. R., Stapleton D., Kemp B. E. (2008) Chem. Biol. 15, 1220–1230 [DOI] [PubMed] [Google Scholar]
- 28.Watt M. J., Dzamko N., Thomas W. G., Rose-John S., Ernst M., Carling D., Kemp B. E., Febbraio M. A., Steinberg G. R. (2006) Nat. Med. 12, 541–548 [DOI] [PubMed] [Google Scholar]
- 29.Beck Jorgensen S., O'Neill H. M., Hewitt K., Kemp B. E., Steinberg G. R. (2009) Diabetologia 52, 2395–4004 [DOI] [PubMed] [Google Scholar]
- 30.Steinberg G. R., Watt M. J., Ernst M., Birnbaum M. J., Kemp B. E., Jørgensen S. B. (2009) Diabetes 58, 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzamko N., Schertzer J. D., Ryall J. G., Steel R., Macaulay S. L., Wee S., Chen Z. P., Michell B. J., Oakhill J. S., Watt M. J., Jørgensen S. B., Lynch G. S., Kemp B. E., Steinberg G. R. (2008) J. Physiol. 586, 5819–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg G. R., Dyck D. J. (2000) Am. J. Physiol. Endocrinol. Metab. 279, E1374–1382 [DOI] [PubMed] [Google Scholar]
- 33.Steinberg G. R., Bonen A., Dyck D. J. (2002) Am. J. Physiol. Endocrinol. Metab. 282, E593–600 [DOI] [PubMed] [Google Scholar]
- 34.Huijsman E., van de Par C., Economou C., van der Poel C., Lynch G. S., Schoiswohl G., Haemmerle G., Zechner R., Watt M. J. (2009) Am. J. Physiol. Endocrinol Metab. 297, E505–513 [DOI] [PubMed] [Google Scholar]
- 35.Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. (1999) FEBS Lett. 443, 285–289 [DOI] [PubMed] [Google Scholar]
- 36.Jørgensen S. B., Wojtaszewski J. F., Viollet B., Andreelli F., Birk J. B., Hellsten Y., Schjerling P., Vaulont S., Neufer P. D., Richter E. A., Pilegaard H. (2005) FASEB J. 19, 1146–1148 [DOI] [PubMed] [Google Scholar]
- 37.Koopman R., Manders R. J., Jonkers R. A., Hul G. B., Kuipers H., van Loon L. J. (2006) Eur. J. Appl. Physiol. 96, 525–534 [DOI] [PubMed] [Google Scholar]
- 38.Blanco C. E., Sieck G. C., Edgerton V. R. (1988) Histochem. J. 20, 230–243 [DOI] [PubMed] [Google Scholar]
- 39.Bergeron R., Russell R. R., 3rd, Young L. H., Ren J. M., Marcucci M., Lee A., Shulman G. I. (1999) Am. J. Physiol. Endocrinol. Metab. 276, E938–944 [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen S. B., Viollet B., Andreelli F., Frøsig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. (2004) J. Biol. Chem. 279, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 41.Barnes B. R., Marklund S., Steiler T. L., Walter M., Hjälm G., Amarger V., Mahlapuu M., Leng Y., Johansson C., Galuska D., Lindgren K., Abrink M., Stapleton D., Zierath J. R., Andersson L. (2004) J. Biol. Chem. 279, 38441–38447 [DOI] [PubMed] [Google Scholar]
- 42.Barré L., Richardson C., Hirshman M. F., Brozinick J. T., Fiering S., Kemp B. E., Goodyear L. J., Witters L. A. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E802–811 [DOI] [PubMed] [Google Scholar]
- 43.Winder W. W., Holmes B. F., Rubink D. S., Jensen E. B., Chen M., Holloszy J. O. (2000) J. Appl. Physiol. 88, 2219–2226 [DOI] [PubMed] [Google Scholar]
- 44.Thomson D. M., Porter B. B., Tall J. H., Kim H. J., Barrow J. R., Winder W. W. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E196–202 [DOI] [PubMed] [Google Scholar]
- 45.Mu J., Barton E. R., Birnbaum M. J. (2003) Biochem. Soc. Trans. 31, 236–241 [DOI] [PubMed] [Google Scholar]
- 46.Lee-Young R. S., Griffee S. R., Lynes S. E., Bracy D. P., Ayala J. E., McGuinness O. P., Wasserman D. H. (2009) J. Biol. Chem. 284, 23925–23934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wojtaszewski J. F., Jørgensen S. B., Hellsten Y., Hardie D. G., Richter E. A. (2002) Diabetes 51, 284–292 [DOI] [PubMed] [Google Scholar]
- 48.Röckl K. S., Hirshman M. F., Brandauer J., Fujii N., Witters L. A., Goodyear L. J. (2007) Diabetes 56, 2062–2069 [DOI] [PubMed] [Google Scholar]
- 49.Thomson D. M., Hancock C. R., Evanson B. G., Kenney S. G., Malan B. B., Mongillo A. D., Brown J. D., Hepworth S., Fillmore N., Parcell A. C., Kooyman D. L., Winder W. W. (2010) J. Appl. Physiol. 108, 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitz-Peiffer C., Craig D. L., Biden T. J. (1999) J. Biol. Chem. 274, 24202–24210 [DOI] [PubMed] [Google Scholar]
- 51.Chen Z., Heierhorst J., Mann R. J., Mitchelhill K. I., Michell B. J., Witters L. A., Lynch G. S., Kemp B. E., Stapleton D. (1999) FEBS Lett. 460, 343–348 [DOI] [PubMed] [Google Scholar]
- 52.Fujii N., Seifert M. M., Kane E. M., Peter L. E., Ho R. C., Winstead S., Hirshman M. F., Goodyear L. J. (2007) Diabetes Res. Clin. Pract. 77, Suppl. 1, S92–98 [DOI] [PubMed] [Google Scholar]
- 53.Russell R. R., 3rd, Li J., Coven D. L., Pypaert M., Zechner C., Palmeri M., Giordano F. J., Mu J., Birnbaum M. J., Young L. H. (2004) J. Clin. Invest. 114, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamoto K., Zarrinpashneh E., Budas G. R., Pouleur A. C., Dutta A., Prescott A. R., Vanoverschelde J. L., Ashworth A., Jovanović A., Alessi D. R., Bertrand L. (2006) Am. J. Physiol. Endocrinol. Metab. 290, E780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witczak C. A., Fujii N., Hirshman M. F., Goodyear L. J. (2007) Diabetes 56, 1403–1409 [DOI] [PubMed] [Google Scholar]
- 56.Jensen T. E., Rose A. J., Jørgensen S. B., Brandt N., Schjerling P., Wojtaszewski J. F., Richter E. A. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E1308–1317 [DOI] [PubMed] [Google Scholar]
- 57.Sakamoto K., McCarthy A., Smith D., Green K. A., Grahame Hardie D., Ashworth A., Alessi D. R. (2005) EMBO J. 24, 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viollet B., Andreelli F., Jørgensen S. B., Perrin C., Geloen A., Flamez D., Mu J., Lenzner C., Baud O., Bennoun M., Gomas E., Nicolas G., Wojtaszewski J. F., Kahn A., Carling D., Schuit F. C., Birnbaum M. J., Richter E. A., Burcelin R., Vaulont S. (2003) J. Clin. Invest. 111, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mu J., Brozinick J. T., Jr., Valladares O., Bucan M., Birnbaum M. J. (2001) Mol. Cell 7, 1085–1094 [DOI] [PubMed] [Google Scholar]
- 60.Fujii N., Hirshman M. F., Kane E. M., Ho R. C., Peter L. E., Seifert M. M., Goodyear L. J. (2005) J. Biol. Chem. 280, 39033–39041 [DOI] [PubMed] [Google Scholar]
- 61.Fujii N., Ho R. C., Manabe Y., Jessen N., Toyoda T., Holland W. L., Summers S. A., Hirshman M. F., Goodyear L. J. (2008) Diabetes 57, 2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villena J. A., Viollet B., Andreelli F., Kahn A., Vaulont S., Sul H. S. (2004) Diabetes 53, 2242–2249 [DOI] [PubMed] [Google Scholar]
- 63.Carey A. L., Steinberg G. R., Macaulay S. L., Thomas W. G., Holmes A. G., Ramm G., Prelovsek O., Hohnen-Behrens C., Watt M. J., James D. E., Kemp B. E., Pedersen B. K., Febbraio M. A. (2006) Diabetes 55, 2688–2697 [DOI] [PubMed] [Google Scholar]