Abstract
Both the use of non-steroidal anti-inflammatory drugs (NSAIDs), such as indomethacin, and infection with Helicobacter pylori are major causes of gastric ulcers. Although some clinical studies suggest that infection with H. pylori increases the risk of developing NSAID-induced gastric lesions, the molecular mechanism governing this effect is unknown. We recently found that in cultured gastric cells, expression of endoplasmic reticulum (ER) chaperones (such as 150-kDa oxygen-regulated protein (ORP150) and glucose-regulated protein 78 (GRP78)) is induced by NSAIDs and confers protection against NSAID-induced apoptosis, which is important in the development of NSAID-induced gastric lesions. In this study we have found that co-culture of gastric cells with H. pylori suppresses the expression of ER chaperones. This suppression was regulated at the level of transcription and accompanied by a reduction in the level of activating transcription factor 6 (ATF6), one of the transcription factors for ER chaperone genes. In vivo, inoculation of mice with H. pylori suppressed the expression of ER chaperones at gastric mucosa both with and without administration of indomethacin. Inoculation with H. pylori also stimulated formation of indomethacin-induced gastric lesions and mucosal cell death. In addition, we found that heterozygous ORP150-deficient mice are sensitive to the development of indomethacin-induced gastric lesions and mucosal cell death. The results of this study suggest that H. pylori exacerbates NSAID-induced gastric lesions through suppression of expression of ER chaperones, which stimulates NSAID-induced mucosal cell death.
Keywords: Apoptosis, Cell Death, Chaperone Chaperonin, ER Stress, Gene Expression
Introduction
The balance between aggressive and defensive factors determines whether gastric ulcers develop. The gastric mucosa is challenged by a variety of aggressive factors, and of these, both non-steroidal anti-inflammatory drugs (NSAIDs)2 and Helicobacter pylori are major causes of gastric lesions. Therefore, an important question is whether infection with H. pylori increases the risk of developing NSAID-induced gastric lesions (in other words, if eradication of H. pylori would reduce the risk of developing NSAID-induced gastric lesions). Recent clinical studies suggest that infection with H. pylori increases the risk of developing NSAID-induced gastric lesions (1–4); however, some studies have shown the opposite effect (5, 6). Animal models could be useful to address this issue. For example, some reports have demonstrated that NSAID-induced gastric lesions in mongolian gerbils are exacerbated by infection with H. pylori (7–9), although the molecular mechanism governing this exacerbation is unclear.
An inhibitory effect of NSAIDs on cyclooxygenase (COX) activity and the resulting decrease in the gastric level of prostaglandins (PGs), especially PGE2, was believed to be the only explanation for the gastric side effects of NSAIDs because PGE2 is a strong protective factor for gastric mucosa (10). However, the increased incidence of gastrointestinal lesions and the decrease in PG levels induced by NSAIDs are not always linked with each other. For example, it has been shown that higher doses of NSAIDs are required for producing gastric lesions than are required for inhibiting COX at the gastric mucosa (11), suggesting that there are additional mechanisms involved in the development of NSAID-induced gastric lesions. We have recently demonstrated that NSAIDs induce apoptosis in cultured gastric mucosal cells and at gastric mucosa in a manner independent of COX inhibition (12–16) and have suggested that both COX inhibition (measured as a decrease in the gastric PGE2 level) and gastric mucosal apoptosis are required for the formation of NSAID-induced gastric lesions (16–18). Therefore, protection against gastric mucosal apoptosis is important for protecting gastric mucosa against the formation of NSAID-induced lesions. As for the molecular mechanism governing this apoptosis, we have proposed the following pathway. Permeabilization of cytoplasmic membranes by NSAIDs stimulates Ca2+ influx and increases intracellular Ca2+ levels, which in turn induces the endoplasmic reticulum (ER) stress response (12, 13, 19, 20). In the ER stress response, an apoptosis-inducing transcription factor, C/EBP homologous transcription factor (CHOP), is induced. We have previously shown that CHOP is essential for NSAID-induced apoptosis (13). CHOP induces expression of p53 up-regulated modulator of apoptosis and the resulting activation of Bax, mitochondrial dysfunction, and the activation of caspases and apoptosis (18, 21).
The ER stress response is induced by accumulation of unfolded proteins in the ER, a process involving three types of ER transmembrane proteins: protein kinase and site-specific endoribonuclease (IRE1), protein kinase R-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (22–24). ER stressors phosphorylate PERK, which in turn phosphorylates eukaryotic initiation factor-2α, leading to the activation of ATF4 expression (25). In the presence of ER stressors, p90-ATF6 (full-length ATF6) is translocated from the ER to the Golgi apparatus, where it is sequentially cleaved by site-1 protease (S1P) and site-2 protease (S2P) into p50-ATF6 (24). Activation of IRE1 causes frame switch splicing of X box-binding protein 1 (XBP-1), which produces the active (spliced) form of XBP-1 (26). All of ATF4, p50-ATF6, and XBP-1 specifically activate the transcription of ER stress response-related genes. ER stress response-related proteins contain not only CHOP but also ER chaperones (such as 150-kDa oxygen-regulated protein (ORP150) and glucose-regulated protein 78 (GRP78)) that confer protection against ER stressors by refolding unfolded proteins in the ER. We have recently reported that up-regulation of expression of GRP78 and ORTP150 by NSAIDs protects gastric cells from NSAID-induced apoptosis in vitro (19, 20), suggesting that ER chaperones are defensive factors for gastric mucosa. Although it has been reported that expression of ER chaperones is induced with the development of gastric lesions by water-immersion stress (27), there is no direct (such as genetic) evidence supporting the notion that ER chaperones are defensive factors for gastric mucosa.
The identification of H. pylori in the human stomach has changed the diagnosis and treatment of gastric diseases because it is now clear that H. pylori plays an important role in various gastric diseases, such as chronic gastritis, peptic ulcers, and gastric cancers (28, 29). This idea is supported by clinical results that eradication of H. pylori significantly decreases the risk of these gastric diseases (2, 30, 31). Infection with H. pylori damages gastric mucosa through production of ammonia and cytotoxic proteins such as vacuolating cytotoxin A (VacA) and cytotoxin-associated gene A (CagA) and induction of host inflammatory responses (32–36). In addition to these mechanisms, it is also possible that H. pylori damages gastric mucosa through decreasing the expression of host defensive factors for gastric mucosa. It was recently reported that H. pylori inhibit the expression of heat shock proteins and mucin, both of which are major defensive factors for gastric mucosa (35, 37). However, the effect of H. pylori on other defensive factors, including ER chaperones, has not been tested. In this study we found that co-culture of gastric cells with H. pylori in vitro decreases the expression of ER chaperones, and we suggest that this suppression is mediated by the degradation of ATF6. We also show that inoculation of mice with H. pylori not only suppresses the expression of ER chaperones at gastric mucosa but also exacerbates NSAID-induced gastric lesions and mucosal cell death. We have also found that heterozygous ORP150-deficient mice are sensitive to the development of NSAID-induced gastric lesions and mucosal cell death. The results of this study suggest that H. pylori exacerbates NSAID-induced gastric lesions through suppression of expression of ER chaperones and the resulting stimulation of NSAID-induced mucosal cell death.
EXPERIMENTAL PROCEDURES
Chemicals and Animals
RPMI 1640 and Helicobacter selection agar were obtained from Nissui Pharmaceutical Co (Osaka, Japan). Aneropack Bikouki was from Mitsubishi Gas Chemical (Tokyo, Japan). Horse serum was from Invitrogen. Paraformaldehyde, epoxomycin, cycloheximide, pepstatin A, and fetal bovine serum (FBS) were from Sigma. E-64-d was from the Peptide Institute Inc. (Tokyo, Japan). Indomethacin was obtained from Wako Co. (Osaka, Japan). Brain heart infusion was from Difco. Antibodies against GRP78, ATF4, ATF6, actin, lamin, the N-terminal region of Bax (Bax N20), and CHOP were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against GRP94, GRP58, and protein disulfide isomerase were from StressGen (San Diego, CA). An antibody against cytochrome c was from BD Biosciences, and that against connexin 43 was from Invitrogen. An antibody against GFP was from Clontech (Mountain View CA), and that against ORP150 was from our laboratory stocks (38). Terminal deoxynucleotidyltransferase was obtained from TOYOBO (Osaka, Japan). Biotin 14-ATP, Alexa Fluor 488 goat anti-rabbit (or anti-mice) immunoglobulin G, Alexa Fluor 594 goat anti-rat immunoglobulin G, Lipofectamine (TM2000), Dynabeads Protein G, and Alexa Fluor 488 conjugated with streptavidin were purchased from Invitrogen. Mounting medium for immunohistochemical analysis (VECTASHIELD) was from Vector Laboratories (Burlingame, CA). 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was from Dojindo (Kumamoto, Japan). The RNeasy kit and HiPerFect transfection reagents were obtained from Qiagen (Valencia, CA), the first-strand cDNA synthesis kit was from Takara (Kyoto, Japan), and the iQ SYBR Green Supermix was from Bio-Rad. The Dual Luciferase Assay System was from Promega (Southampton, UK). Mice heterozygous for a truncated/inactivated mutant form of ORP150 (ORP150+/−) and their wild-type counterparts (ORP150+/+) (6–8 weeks of age) were prepared as described previously (38, 39). The experiments and procedures described here were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Bethesda, MD) and were approved by the Animal Care Committee of Kumamoto University.
H. pylori Inoculation and Gastric Damage Assay
H. pylori inoculation was done as described previously (40) with some modifications. H. pylori strain ATCC43504 (CagA+ and VacA+) (a gift from Dr. Oguma, Okayama University) was cultured in a brain heart infusion broth containing 10% horse serum at 37 °C under a microaerophilic atmosphere. H. pylori were also cultured on brain heart infusion agar supplemented with heat-inactivated 7% horse blood or on Helicobacter selection agar under a gas-pack jar with an Anaeropack Bikouki. Mice were orally inoculated with H. pylori at a dose of 2.0 × 108 H. pylori/animal/0.5 ml of PBS every second day for 6 days (total 3 times). H. pylori lysates were prepared by sonication of cells in PBS. The protein concentration of lysates was determined by the Bradford method (41).
The gastric ulcerogenic response was examined as described previously (18), with some modifications. H. pylori-inoculated or non-inoculated mice (1 day after the final inoculation of H. pylori) fasted for 6 h were orally administered with indomethacin (10 mg/kg, 10 ml 1% methylcellulose/kg). Twelve hours later, the animals were sacrificed, after which their stomachs were removed, and the areas of gastric mucosal lesions were measured by an observer unaware of the treatment they had received. Calculation of the scores involved measuring the area of all the lesions in square millimeters and summing the values to give an overall gastric lesion index.
Cell Culture
AGS and MKN45 are human adenocarcinoma gastric cell lines. Cells were cultured in RPMI1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air with 5% CO2 at 37 °C.
Transfections were carried out using Lipofectamine (TM2000) according to the manufacturer's instructions. Cells were used for experiments after a 24-h recovery period. Transfection efficiency was determined in parallel plates by transfection of cells with a pEGFP-N1 control vector. Transfection efficiencies were greater than 80% in all experiments. The plasmid pCMVshort-EGFP-ATF6α (a gift from Dr. Mori, Kyoto University) (42) was transfected into AGS cells, according to the manufacturer's protocols.
siRNA Targeting of Genes
The siRNAs for ATF6 and ATF4 and nonspecific siRNA were purchased from Qiagen. AGS cells were transfected with siRNA using HiPerFect transfection reagent according to the manufacturer's instructions.
Pulse-Chase Analysis
A pulse-chase experiment was carried out with 0.1 mCi/ml Expre35S35S protein labeling mix (PerkinElmer Life Sciences) as described previously (43), with some modifications. Cells were labeled with [35S]methionine and [35S]cysteine in methionine- and cysteine-free RPMI1640 medium for 30 min. To chase labeled proteins, cells were washed with fresh complete medium three times and incubated in fresh complete medium with or without H. pylori. ATF6 was immunoprecipitated with its antibody and separated by SDS-polyacrylamide gel electrophoresis followed by autoradiography (Fuji BAS 2500 imaging analyzer).
Immunostaining
Plasmids (pCMVshort-EGFP-ATF6α) were transfected into AGS cells, and cells were co-cultured with H. pylori in the Lab-Tek II chamber slide system (Nalge Nunc International, Rochester, NY). Cells were fixed in 4% paraformaldehyde for 20 min, blocked with goat serum for 15 min, then incubated for 12 h with antibody against GRP94 in the presence of 2.5% bovine serum albumin before finally being incubated for 2 h with Alexa Fluor 594 goat anti-rat IgG. Samples were mounted with VECTASHIELD. Images were captured on a confocal laser-scanning fluorescence microscope (FLUOVIEW FV500-IX-UV, Olympus).
RT-PCR and Real-time RT-PCR Analyses
Real-time RT-PCR was performed as previously described with some modifications (44). Total RNA was extracted from gastric tissues or cultured cells using an RNeasy kit according to the manufacturer's protocol. Samples (2.5 μg RNA) were reverse-transcribed using a first-strand cDNA synthesis kit. Synthesized cDNA was used in real-time RT-PCR (Chromo 4 instrument; Bio-Rad) experiments using iQ SYBR GREEN Supermix and analyzed with Opticon Monitor Software. Specificity was confirmed by electrophoretic analysis of the reaction products and by the inclusion of template- or reverse transcriptase-free controls. The cycle conditions were 2 min at 50 °C followed by 10 min at 90 °C and finally 45 cycles of 95 °C for 30 s and 63 °C for 60 s. To normalize the amount of total RNA present in each reaction, actin cDNA was used as an internal standard.
Primers were designed using the Primer3 website and are listed as forward and reverse, respectively. Human primers were: atf4 (5′-tcaaacctcatgggttctcc-3′) and (5′-gtgtcatccaacgtggtcag-3′); atf6, (5′-ctccgagatcagcagaggaa-3′) and (5′-aatgactcagggatggtgct-3′); chop, (5′-tgcctttctcttcggacact-3′) and (5′-tgtgacctctgctggttctg-3′); grp78, (5′-tagcgtatggtgctgctgtc-3′) and (5′-tttgtcaggggtctttcacc-3′); orp150, (5′-gaagatgcagagcccatttc-3′) and (5′-tctgctccaggacctcctaa-3′); xbp-1, (5′-aaacagagtagcagcgcagactg-3′) and (5′-ggatctctaaaactagaggcttggtg-3′); xbp-1 (u), (5′-agcactcagactacgtgcac-3′) and (5′-ccagaatgcccaacaggata-3′); actin, (5′-ggacttcgagcaagagatgg-3′) and (5′- agcactgtgttggcgtacag-3′). Mouse primers were: atf6 (5′-catcaaaagctcctcggttc-3′) and (5′-gggtcgtctctgtggttgtt-3′); grp78, (5′-gcttccgataatcagccaac-3′) and (5′-gcaggaggaattccagtca-3′); orp150, (5′-cagactgaagaggcgaaacc-3′) and (5′-ttcctgttcaggtccagctc-3′); chop, (5′-acagaggtcacacgcacatc-3′), and (5′-gggcactgaccactctgttt-3′); gapdh, (5′-tgcctttctcttcggacact-3′ and (5′-tgtgacctctgctggttctg-3′).
For regular RT-PCR, we used an initial denaturation step of 94 °C for 1 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 20 s, and elongation at 72 °C for 1 min. A final elongation step at 72 °C for 10 min completed the RT-PCR. The amplified PCR products were separated by 3% agarose gel electrophoresis and then visualized with ethidium bromide.
Immunoblotting Analysis
Total protein and nuclear protein extracts were prepared as described previously (20). The protein concentration of each sample was determined by the Bradford method (41). Samples were applied to polyacrylamide SDS gels and subjected to electrophoresis, after which the proteins were immunoblotted with appropriate antibodies.
Histological, Immunohistochemical, and Terminal Deoxynucleotidyltransferase-mediated Biotinylated UTP Nick End Labeling (TUNEL) Analyses
Gastric tissue samples were fixed in 4% buffered paraformaldehyde and embedded in paraffin before being cut into 4-mm sections.
For histological examination (hematoxylin and eosin (H&E) staining), sections were stained first with Mayer's hematoxylin and then with 1% eosin alcohol solution. Samples were mounted with Malinol and inspected with the aid of an Olympus BX51 microscope.
For immunohistochemical analysis, sections were blocked with 2.5% goat serum for 10 min, incubated for 12 h with antibody against ORP150 or GRP78 in the presence of 2.5% bovine serum albumin, and finally incubated for 2 h with Alexa Fluor 488 goat anti-rabbit immunoglobulin G in the presence of DAPI (5 mg/ml). Samples were mounted with VECTASHIELD and inspected using fluorescence microscopy (Olympus BX51).
For TUNEL assays, sections were incubated first with proteinase K (20 mg/ml) for 15 min at 37 °C, then with terminal deoxynucleotidyltransferase and biotin 14-ATP for 1 h at 37 °C and finally with Alexa Fluor 488 conjugated with streptavidin for 1 h. Samples were mounted with VECTASHIELD and inspected using fluorescence microscopy (Olympus BX51).
Luciferase Assay
The pGL-3/ERSE (ER stress response element) plasmid, which was constructed by inserting ERSE (5′-ccaatcagaaagtggcacg-3′) just upstream of the luciferase gene (45), was kindly provided by Dr. Gotoh (Kumamoto University). The pGL-3/grp78pro plasmid, which was constructed by inserting the human grp78 promoter (from −304 to +7 region) into the same region (46), was generously provided by Dr. Mori (Kyoto University).
The luciferase assay was performed as described previously (47). Cells were transfected with 1 μg of one of the Photinus pyralis luciferase reporter plasmids (pGL-3 or its derivatives) and 0.125 μg of the internal standard plasmid bearing the Renilla reniformis luciferase reporter (pRL-SV40). P. pyralis luciferase activity in cell extracts was measured using the Dual Luciferase Assay System and then normalized for R. reniformis luciferase activity.
Statistical Analysis
Two-way analysis of variance followed by the Tukey test or the Student's t test for unpaired results was used to evaluate differences between more than three groups or between two groups, respectively. Differences were considered to be significant for values of p < 0.05.
RESULTS
Effect of H. pylori on Expression of ER Chaperones in Vitro
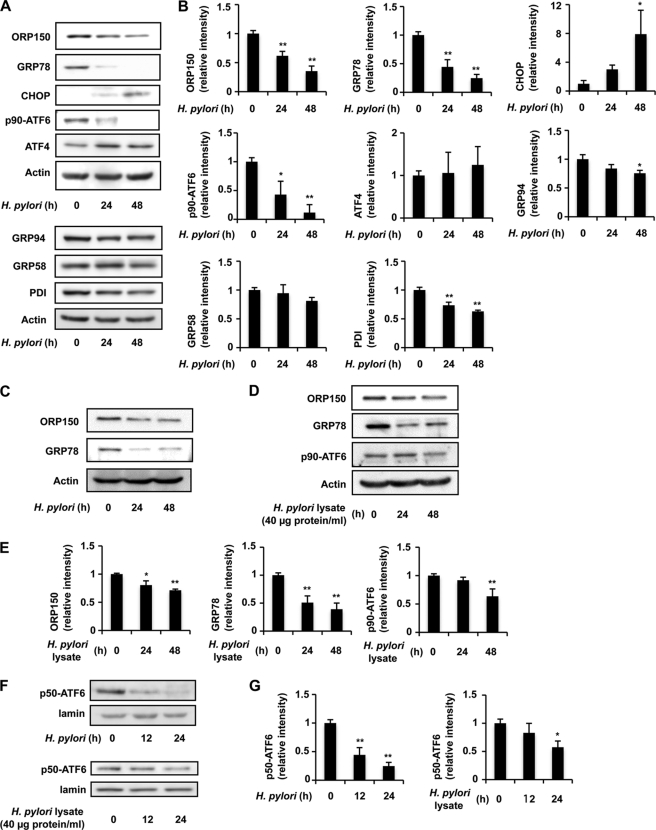
It was previously reported that co-culture of AGS cells with H. pylori at a bacteria:cell ratio of 200:1 causes partial induction of apoptosis (48, 49). In the current study we examined the expression of ER chaperones under these conditions and, using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, determined cell viability to be 80% after a 24-h incubation of AGS cells with H. pylori (data not shown). As shown in Fig. 1, A and B, treatment of cells with H. pylori decreased the levels of ORP150 and GRP78. Similar results were observed in another gastric cell line (MKN45) (Fig. 1C). A slight reduction of levels of other ER chaperones (such as GRP94 and protein disulfide isomerase but not GRP58) was also observed in cells treated with H. pylori (Fig. 1, A and B). We also found that treatment of AGS cells with H. pylori cell lysates also decreased the levels of ORP150 and GRP78; however, the extent of these decreases was not as apparent as that observed with H. pylori (Fig. 1, D and E). Unlike the ER chaperones, the level of CHOP was increased by co-culture of cells with H. pylori (Fig. 1, A and B).
FIGURE 1.
Down-regulation of expression of ER chaperones by H. pylori. AGS (A, B, and D–G) or MKN-45 (C) cells were co-cultured with H. pylori at a bacteria:cell ratio of 200:1 (A–C, F, and G) or with H. pylori lysates (D–G) for the indicated periods. Whole cell extracts (A, C, and D) or nuclear extracts (F) were analyzed by immunoblotting with an antibody against ORP150, GRP78, CHOP, ATF6, ATF4, GRP94, GRP58, protein disulfide isomerase (PDI), actin, or lamin. The intensity of each band in three independent experiments (one of them is shown in A, D, and F) was determined and expressed relative to the control (B, E, and G, respectively). Values are the mean ± S.D. (n = 3). **, p < 0.01; *, p < 0.05.
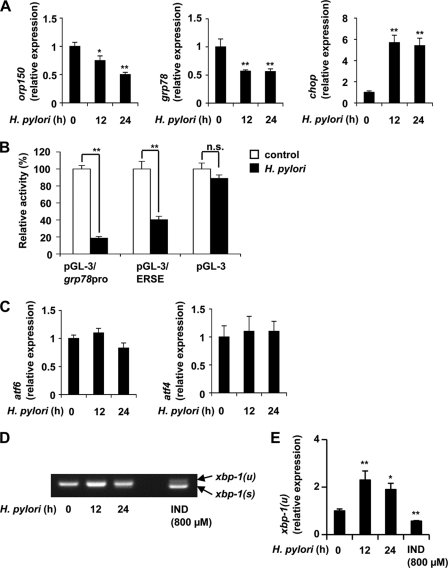
Real-time RT-PCR analysis revealed that the H. pylori-dependent down-regulation of expression of ORP150 and GRP78 and up-regulation of expression of CHOP was also observed at the level of mRNA (Fig. 2A). We also performed a luciferase reporter assay using a reporter plasmid where the promoter of the grp78 gene was inserted upstream of the luciferase gene (pGL-3/grp78pro). As shown in Fig. 2B, co-culture of cells with H. pylori decreased the luciferase activity in cells with pGL-3/grp78pro but not in those with the control vector (pGL-3). These results suggest that co-culture of cells with H. pylori inhibits the transcription of ER chaperone genes.
FIGURE 2.
Inhibitory effects of H. pylori on transcription of ER stress response-related genes. AGS cells were co-cultured with H. pylori at a bacteria:cell ratio of 200:1 for the indicated periods (A and C–E) or treated with indomethacin (IND) for 24 h (D and E). A, C, and E, the relative expression of each gene was monitored by real-time RT-PCR using a specific primer for each gene. Values normalized to the actin gene are expressed relative to the control sample. B, AGS cells were co-transfected with pRL-SV40 (internal control plasmid carrying the R. reniformis luciferase gene) and pGL-3 or its derivatives (pGL-3/grp78pro and pGL-3/ERSE) and cultured for 24 h. Cells were then co-cultured with or without H. pylori at a bacteria:cell ratio of 200:1 for 24 h, and P. pyralis luciferase activity was measured and normalized for R. reniformis luciferase activity. The 100% value of the P. pyralis luciferase activity is 5.4 × 106, 7.4 × 105, or 2.4 × 104 units for pGL-3/grp78pro, pGL-3/ERSE, or pGL-3, respectively. D, RT-PCR was performed with total RNAs and primer sets for detecting the un-spliced (xbp-1(u)) and spliced (xbp-1(s)) forms of xbp-1 mRNA, which were separated by agarose gel electrophoresis. Values are the mean ± S.D. (n = 3). **, p < 0.01; *, p < 0.05; n.s., not significant.
Mechanism for Suppression of Expression of ER Chaperones by H. pylori
To understand the molecular mechanism governing suppression of expression of ER chaperones by H. pylori, we first examined the effect of H. pylori on the level of ER stress response-related transcription factors (ATF6, ATF4, and XBP-1). Co-culture of cells with H. pylori decreased the level of ATF6 protein (p90-ATF6) but not atf6 mRNA (Figs. 1, A and B, and 2C). We also found that the level of p50-ATF6 in nuclear extracts decreased after co-culture of cells with H. pylori (Fig. 1, F and G). On the other hand, the levels of ATF4 protein and atf4 mRNA did not alter after co-culture with H. pylori (Figs. 1, A and B, and 2C). Furthermore, although treatment of cells with indomethacin increased the level of the spliced form (active form) of xbp-1 mRNA, treatment of cells with H. pylori did not result in a similar observation (Fig. 2D). We also found that treatment of cells with indomethacin or H. pylori decreases or increases, respectively, the level of un-spliced form (inactive form) of xbp-1 mRNA by real-time RT-PCR analysis (Fig. 2E). These results suggest that ATF6, rather than ATF4 and XBP-1, is responsible for suppression of expression of ER chaperones by H. pylori.
To confirm this idea, we performed a luciferase assay using a reporter plasmid where the ATF6 binding consensus sequence, ERSE, was inserted upstream of the luciferase gene (pGL-3/ERSE). As shown in Fig. 2B, treatment of cells with H. pylori decreased the luciferase activity in cells with pGL-3/ERSE, suggesting that the transcriptional activity of ATF6 decreased after treatment of cells with H. pylori. This is consistent with the observation that the level of p50-ATF6 decreased in H. pylori-treated cells (Fig. 1, F and G).
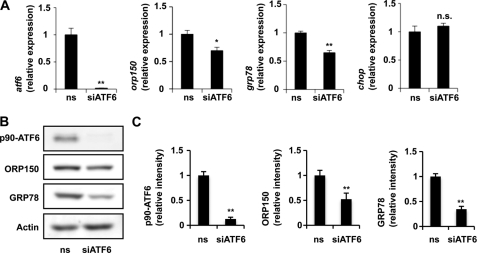
For further confirmation of the idea that ATF6 plays an important role in H. pylori-dependent suppression of expression of ER chaperones, we examined the effect of siRNA for ATF6 on the expression of ER chaperones. As shown in Fig. 3, transfection of cells with siRNA for ATF6 suppressed expression of not only ATF6 but also ORP150 and GRP78, affecting both mRNA and protein levels. The transfection did not affect the expression of chop mRNA (Fig. 3A). On the other hand, transfection of cells with siRNA for ATF4 suppressed expression of ATF4 but not that of ORP150 and GRP78 (supplemental Fig. S1). The results in Fig. 3 suggest that the reduction in the level of ATF6 is partly involved in the H. pylori-dependent suppression of expression of ER chaperones but not for induction of expression of CHOP.
FIGURE 3.
Effect of siRNA for ATF6 on expression of ER chaperones. AGS cells were transfected with siRNA for ATF6 (siATF6) or nonspecific siRNA (ns) and were incubated for 48 h (A) or 72 h (B). The mRNA (A) and protein (B and C) expression was monitored and expressed as described in the legends of Figs. 1 and 2. Values are the mean ± S.D. (n = 3). **, p < 0.01; *, p < 0.05; n.s., not significant.
We also examined the effect of H. pylori cell lysates on levels of p90-ATF6 and p50-ATF6. As shown in Fig. 1, D–G, levels of p90-ATF6 and p50-ATF6 were slightly decreased by treatment of cells with H. pylori cell lysates, but the decrease occurred more slowly than that of ORP150 and GRP78.
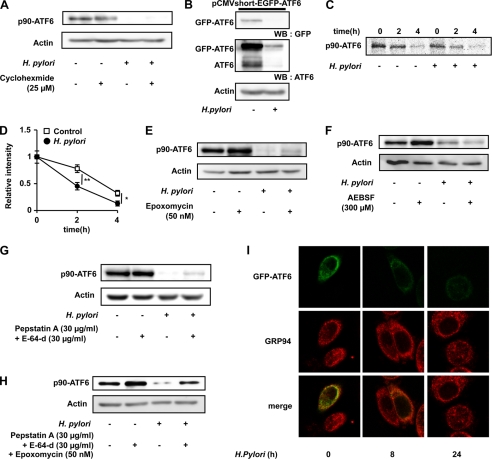
As described above, atf6 mRNA expression was not affected by H. pylori (Fig. 2C). Thus, either suppression of translation or post-translational modification of ATF6 (such as degradation by proteases) may be responsible for the observed reduction in the level of ATF6 after co-culture of cells with H. pylori. To address this issue, we first examined the effect of H. pylori on the level of ATF6 in cells pretreated with cycloheximide, an inhibitor of protein synthesis. As shown in Fig. 4A, the H. pylori-dependent decrease in the level of ATF6 was observed even in cells pretreated with cycloheximide. We also found that the H. pylori-dependent decrease in the level of ATF6 was observed for GFP-ATF6, whose expression is regulated by the strong cytomegalovirus promoter (Fig. 4B). We also examined the effect of H. pylori on the stability of p90-ATF6 by the pulse-chase experiment. As shown in Fig. 4, C and D, the labeled p90-ATF6 disappeared more rapidly in the presence of H. pylori treatment than its absence. These results suggest that post-translational modification of ATF6, such as protein degradation, is responsible for the lower level of ATF6 observed after treatment of cells with H. pylori.
FIGURE 4.
Mechanism for the H. pylori-dependent decrease in the level of ATF6. A and E–H, AGS cells were pre-incubated with or without each drug for 1 h and further incubated with or without H. pylori at a bacteria:cell ratio of 200:1 for 24 h in the presence (E, G, and H) or absence (A and F) of the same concentration of each drug as in the preincubation step. B and I, AGS cells were transfected with pCMVshort-EGFP-ATF6α (42) and co-cultured with or without H. pylori at a bacteria:cell ratio of 200:1 for 24 h (B) or indicated periods (I). A, B, and E–H, whole cell extracts were analyzed by immunoblotting (WB) with an antibody against GFP, ATF6, or actin. C, AGS cells were pulse-labeled for 30 min with [35S]methionine and [35S]cysteine and then chased with excess amounts of cold methionine and cysteine for the indicated periods in the absence or presence of H. pylori at a bacteria:cell ratio of 200:1. Labeled proteins were extracted, immunoprecipitated with antibody against ATF6, subjected to SDS-PAGE, and autoradiographed. D, the band intensity of p90-ATF6 was determined and expressed relative to the control. I, cells were fixed, stained with antibody against GRP94, and analyzed by confocal laser-scanning fluorescence microscope (magnification, 600 times). Values are the mean ± S.D. (n = 3). **, p < 0.01; *, p < 0.05. AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride.
In addition to cleavage by S1P and S2P, it is known that ATF6 is continuously degraded by the proteasome-ubiquitin pathway (50, 51). Thus, using specific inhibitors, we examined the contribution of these systems to the H. pylori-dependent decrease in the level of ATF6. As shown in Fig. 4E, an inhibitor of the proteasome-ubiquitin system, epoxomycin, weakly suppressed the H. pylori-dependent decrease in the level of ATF6. On the other hand, an inhibitor of S1P, 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), did not affect the level of ATF6 in the presence of H. pylori (Fig. 4F). Furthermore, we found that inhibitors of lysosomal proteases (pepstatin A (an inhibitor of aspartate proteases) and E-64-d (an inhibitor of cysteine protease)) also weakly suppressed the H. pylori-dependent decrease in the level of ATF6 (Fig. 4G). Interestingly, combination of epoxomycin and inhibitors of lysosomal proteases resulted in clear suppression of the H. pylori-dependent decrease in the level of ATF6 (Fig. 4H). The results in Fig. 4 suggest that H. pylori decreases the level of ATF6 partly through modulation of its degradation by the proteasome-ubiquitin and lysosomal systems.
We also examined the effect of H. pylori on subcellular localization of ATF6 using GFP-ATF6. As shown in Fig. 4I, GFP-ATF6 co-localized with GRP94 (ER marker). Although the level of GFP-ATF6 was decreased, the localization of ATF6 was not clearly affected by treatment of cells with H. pylori (Fig. 4I).
Effect of H. pylori on the Gastric Ulcerogenic Response and Expression of ER Chaperones Induced by Indomethacin in Mice
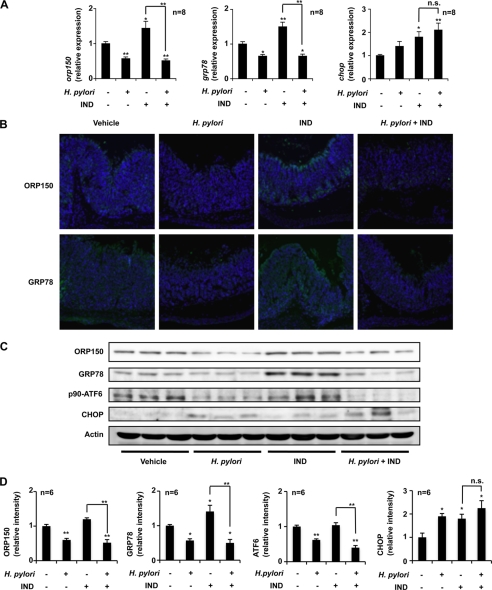
To evaluate the in vivo relevance of our in vitro observation that H. pylori suppress the expression of ER chaperones, we first examined the effect of oral inoculation of H. pylori on the expression of ER chaperones at gastric mucosa. We monitored by real-time RT-PCR analysis the mRNA expression of ER chaperones and CHOP after administration of indomethacin and/or H. pylori in gastric tissues. Oral inoculation of H. pylori to mice suppressed the background (without indomethacin administration) expression of orp150 and grp78 mRNAs but not chop mRNA (Fig. 5A). Indomethacin administration up-regulated the expression of orp150, grp78, and chop mRNAs, whereas the expression of orp150 and grp78 mRNAs but not that of chop mRNA was significantly suppressed by prior administration of H. pylori (Fig. 5A). Immunohistochemical analyses also demonstrated that oral inoculation with H. pylori decreased the levels of ORP150 and GRP78 at gastric mucosa in both the presence and absence of indomethacin administration (Fig. 5B). We consider that the staining of ORP150 and GRP78 in Fig. 5B is specific, because no positive staining was observed without a primary antibody (supplemental Fig. S2). We also performed immunoblotting analysis, and suppression of the gastric expression of ORP150 and GRP78 by inoculation with H. pylori in both the presence and absence of indomethacin treatment was confirmed (Fig. 5, C and D). Indomethacin-induced expression of GRP78 and CHOP was also confirmed (Fig. 5, C and D). Furthermore, we found that the gastric level of p90-ATF6 was decreased by inoculation with H. pylori in both the presence and absence of indomethacin treatment (Fig. 5, C and D). We also found that there is a tendency that inoculation with H. pylori decreases the levels of ORP150 and GRP78 in the presence of indomethacin treatment in the small intestine but not other organs (supplemental Fig. S3A).
FIGURE 5.
Effect of H. pylori on expression of ER chaperones at gastric mucosa. H. pylori were orally inoculated into mice (C57/BL6) at a dose of 2.0 × 108 H. pylori/animal every second day for 6 days (total 3 times). One day after the final inoculation, H. pylori-inoculated or control mice were orally administered 10 mg/kg of indomethacin (IND), and their stomachs were removed after 12 h. A, total RNA was extracted and subjected to real-time RT-PCR using a specific primer for each gene. Values normalized to the gapdh gene are expressed relative to the control sample. B, sections of gastric tissues were subjected to immunohistochemical analysis with an antibody against ORP150 or GRP78 and DAPI staining (magnification, 200 times). C and D, protein expression was monitored and expressed as described in the legend of Fig. 1. Values are given as the mean ± S.E. **, p < 0.01; *, p < 0.05; n.s., not significant.
The observation that inoculation with H. pylori reduces the gastric expression of ER chaperones suggests that this inoculation stimulates protein aggregation in ER in gastric cells. To address this idea, we examined the effect of H. pylori inoculation on the gastric level of connexin 43, which is known to be degraded by ER-associated degradation when it is aggregated in the ER (52, 53). As shown in supplemental Fig. S3, B and C, the gastric level of connexin 43 was decreased by H. pylori inoculation, suggesting that H. pylori stimulate aggregation of the protein in ER.
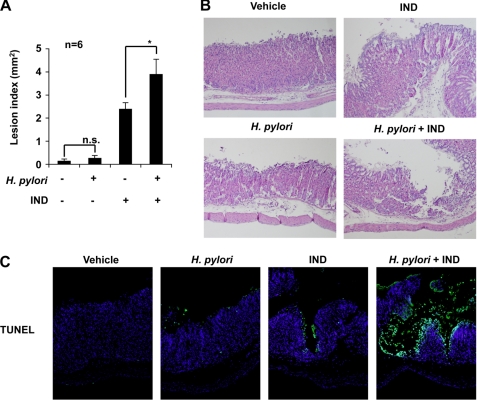
The effect of pre-administration of H. pylori on the development of gastric lesions after oral administration of indomethacin was examined. Administration of indomethacin produced gastric lesions, and this lesion production was significantly enhanced by pre-administration of H. pylori (Fig. 6A). Administration of H. pylori alone did not significantly produce gastric lesions under the conditions used (Fig. 6A). Histological analysis with H&E staining also supported the notion that indomethacin-produced gastric lesions are exacerbated by pre-administration of H. pylori (Fig. 6B).
FIGURE 6.
Effect of H. pylori on indomethacin-induced gastric lesions and mucosal cell death. Mice (C57/BL6) were administered H. pylori and indomethacin (IND), as described in the legend of Fig. 5. A, the stomach was scored for hemorrhagic damage. Values are the mean ± S.E. *p < 0.05; n.s., not significant. B, sections of gastric tissues were subjected to H&E staining (magnification, 200 times). C, sections of gastric tissues were subjected to TUNEL assay and DAPI staining (magnification, 200 times). n.s., not significant.
As mentioned above, gastric mucosal cell death plays an important role in the formation of NSAID-induced gastric lesions. We, therefore, examined the effect of pre-administration of H. pylori on this process. The level of gastric mucosal cell death was determined by TUNEL assay. An increase in the number of TUNEL-positive cells was observed after indomethacin administration, and this increase was more apparent in mice pre-administered with H. pylori than in control mice (Fig. 6C). We also examined the effect of H. pylori on indomethacin-induced expression of ORP150, GRP78, and p90-ATF6 or apoptosis in vitro. Treatment of cells with H. pylori decreased the levels of these proteins and increased apoptotic cells in both the presence and absence of indomethacin treatment (supplemental Fig. S4, A and B). These results suggest that H. pylori exacerbate indomethacin-induced gastric lesion formation by stimulating indomethacin-induced gastric mucosal cell death.
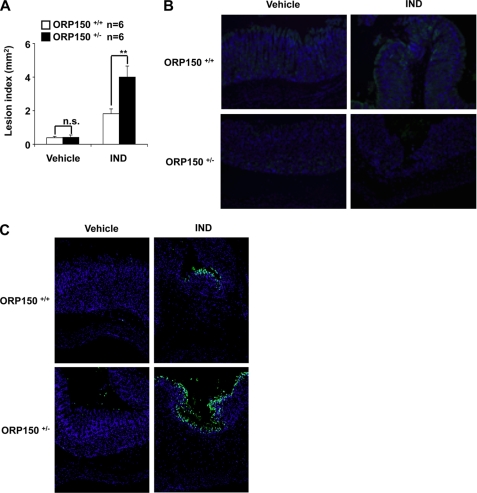
The results in Figs. 5 and 6 suggest that H. pylori exacerbates indomethacin-induced gastric lesion formation through down-regulation of expression of ER chaperones. To test this idea using a genetic approach, the development of gastric lesions after oral administration of indomethacin was compared between heterozygous ORP150-deficient mice (ORP150−/+) and wild-type mice (ORP150+/+). Indomethacin-induced gastric lesions were significantly worse in heterozygous ORP150-deficient mice than in wild-type controls (Fig. 7A). ORP150 deficiency did not affect the background level of gastric lesions (Fig. 7A). Immunohistochemical analyses confirmed that the level of ORP150 in gastric mucosa was lower in heterozygous ORP150-deficient mice than wild-type mice in both the presence and absence of indomethacin administration (Fig. 7B). These results show that ORP150 plays an important role in protecting gastric mucosa against indomethacin-induced lesions.
FIGURE 7.
Indomethacin-induced gastric lesions and mucosal cell death in heterozygous ORP150-deficient mice. Heterozygous ORP150-deficient mice (ORP150+/+) and wild-type mice (ORP150+/+) were orally administered with 10 mg/kg of indomethacin (IND), and their stomachs were removed after 12 h. Gastric lesions (A), expression of ORP150 (B), and mucosal apoptosis (C) were assayed as described in the legends of Figs. 5 and 6. Values are mean ± S.E. **, p < 0.01; *, p < 0.05; n.s., not significant. n.s., not significant.
We also examined the indomethacin-induced gastric mucosal cell death in heterozygous ORP150-deficient mice. Indomethacin-induced gastric mucosal cell death was more apparent in heterozygous ORP150-deficient mice than in wild-type mice (Fig. 7C). These results suggest that ORP150 protects the gastric mucosa from indomethacin-induced cell death. Combining the results in Figs. 5–7, we consider that H. pylori exacerbates indomethacin-induced gastric lesion formation partly through down-regulation of ER chaperones and the resulting stimulation of indomethacin-induced gastric mucosal cell death.
DISCUSSION
There have been contradictory reports about whether infection with H. pylori increases the risk of developing NSAID-induced gastric lesions (in other words, whether eradication of H. pylori reduces the risk of developing NSAID-induced gastric lesions) (1–6, 54). This may be due to differences in diagnostic criteria (endpoints), standards for patient recruitment, and populations used for these studies. The most we can conclude is that under certain conditions infection with H. pylori increases the risk of developing NSAID-induced gastric lesions. Thus, it is important to examine the effect of H. pylori on factors that affect the formation of NSAID-induced gastric lesions. In this study we have focused on ER chaperones and found that co-culture of gastric cells with H. pylori decreases the level of ER chaperones. This is the first observation that H. pylori affect the expression of ER stress response-related proteins. However, although we used here the transient infection model of H. pylori, the infection in humans is chronic. The H. pylori strain used in this study does not colonize mice (data not shown), and thus, studies in the future need to be done with strains that do colonize mice.
By using real-time RT-PCR and luciferase reporter assays, we have shown that the H. pylori-dependent decrease in the level of ER chaperones in vitro is regulated at the level of transcription. Of three ER stress response-related transcription factors (ATF6, ATF4, and XBP-1), only the level of ATF6 (but not the atf6 mRNA) was decreased by co-culture of cells with H. pylori, suggesting that ATF6 is involved in the H. pylori-dependent suppression of transcription of ER chaperone genes. Because the H. pylori-dependent decrease in the level of ER chaperones was observed in cells whose protein synthesis was inhibited and H. pylori decreased the stability of p90-ATF6, post-translational modification (protein degradation) of ATF6 would be involved in this process. Analysis with each inhibitor suggested that the proteasome-ubiquitin system rather than degradation by S1P is involved in this degradation of ATF6. The observation that the level of p50-ATF6 (the proteolytic product of S1P and S2P) did not increase after co-culture of cells with H. pylori further supports this notion. We also suggest that protein degradation in lysosomes is involved in this degradation of ATF6. It is known that VacA perturbs endocytic traffic at a late stage (55, 56), and as such it is possible that H. pylori affects the traffic of ATF6 to lysosomes and its degradation in lysosomes. Furthermore, because the suppression of H. pylori-dependent degradation of ATF6 by inhibitors of proteasomal and lysosomal proteases was weak, other proteases seem to be involved in this degradation.
We found that not only H. pylori themselves but also cell extracts of H. pylori suppress the expression of ER chaperones in vitro. However, the suppression of expression of ER chaperones by cell extracts of H. pylori was not as great as that induced by H. pylori themselves, and cell extracts of H. pylori did not affect the level of ATF6 so distinctly (Fig. 1, D–G). Furthermore, the decrease in level of ATF6 occurred more slowly that that of ORP150 and GRP78 in the presence of cell extracts of H. pylori (Fig. 1), suggesting that the decrease in levels of ORP150 and GRP78 observed with cell extracts of H. pylori is not due to the decrease in levels of ATF6. In other words, results suggest that the mechanism for the decrease in levels of ORP150 and GRP78 is different between H. pylori cells and cell extracts of H. pylori. We also compared the signal pathway for induction of apoptosis between H. pylori cells and cell extracts of H. pylori. As shown in supplemental Fig. S5, the decrease in Bax and increase in cytochrome c in cytosol fractions (an indicator for mitochondria-mediated apoptosis) and induction of expression of CHOP were not observed with apoptosis induced by cell extracts of H. pylori so apparently as that induced by H. pylori cells, suggesting that the signal pathway for induction of apoptosis is also different between H. pylori cells and cell extracts of H. pylori. Although siRNA for ATF6 suppressed the expression of ER chaperones, the extent of suppression was not as apparent as that seen with H. pylori. These results suggest that in addition to the mechanism described above (the ATF6-mediated mechanism), an ATF6-independent and as yet unknown mechanism that can be reproduced with cell extracts of H. pylori should also be mainly involved in the H. pylori-dependent suppression of expression of ER chaperones.
We have previously reported that suppression of expression of GRP78 and ORP150 by siRNA stimulated NSAID-induced apoptosis in cultured gastric cells (19, 20). We have also suggested that NSAID-induced apoptosis at gastric mucosa plays an important role in the formation of NSAID-induced gastric lesions (16–18). These results suggest that ER chaperones play a protective role against the development of NSAID-induced gastric lesions; however, there is no direct evidence supporting this notion. In this study we have shown that heterozygous ORP150-deficient mice display phenotypes sensitive to indomethacin-induced gastric mucosal cell death and gastric lesion formation. This is the first genetic evidence that an ER chaperone is protective against NSAID-induced gastric lesion formation. This result also suggests that inducers of ER chaperones may be therapeutically beneficial against NSAID-induced gastric lesions, as is the case for heat shock proteins inducers (18, 57).
The in vitro observation that expression of ER chaperones is suppressed by H. pylori suggests that H. pylori would suppress the expression of GRP78 and ORP150 at the gastric mucosa and stimulate NSAID-induced gastric mucosal cell death and lesion formation. In fact, we have shown that pre-inoculation of mice with H. pylori not only suppresses the expression of ER chaperones but also stimulates NSAID-induced cell death and gastric lesion formation.
We also showed in vitro that co-culture of gastric cells with H. pylori up-regulates the expression of CHOP, suggesting that this up-regulation is involved in the H. pylori-dependent stimulation of NSAID-induced cell death. There are two possible mechanisms that could explain this up-regulation of CHOP. One is that H. pylori directly affects the expression of CHOP. However, because siRNA for ATF6 did not up-regulate the expression of CHOP, H. pylori-dependent degradation of ATF6 must not be involved. The other possibility is that this up-regulation is a result of the suppression of expression of ER chaperones, as we previously reported that suppression of expression of GRP78 and ORP150 by siRNA induces the expression of CHOP in the presence of NSAIDs (19, 20).
Although we suggest that the H. pylori-dependent exacerbation of indomethacin-induced gastric lesion formation is mediated by the suppression of expression of ER chaperones, various other mechanisms are likely to be involved in this exacerbation. For example, cytotoxic proteins produced by H. pylori, such as VacA and CagA, which are known to induce apoptosis in gastric cells (32–36), may stimulate indomethacin-induced cell death, resulting in exacerbation of indomethacin-induced gastric lesions. CagA disrupts the epithelial apical junction complex (58), which may also be involved in H. pylori-dependent exacerbation of indomethacin-induced gastric lesions. We believe that this animal model for H. pylori-dependent exacerbation of indomethacin-induced gastric lesion formation will be useful in future studies for examining the relationship between H. pylori and NSAIDs and their involvement in the production of gastric lesions.
Acknowledgments
We thank Dr. Oguma (Okayama University) and Drs. Gotoh (Kumamoto University) and Mori (Kyoto University) for generously providing the H. pylori strain or plasmids, respectively.
This work was supported by grants-in-aid of Scientific Research from the Ministry of Health, Labour, and Welfare of Japan, grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and grants-in-aid of the Japan Science and Technology Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- NSAID
- non-steroidal anti-inflammatory drug
- ATF
- activating transcription factor
- CagA
- cytotoxin-associated gene A
- CHOP
- C/EBP homologous transcription factor
- ER
- endoplasmic reticulum
- ERSE
- ER stress response element
- GRP78
- glucose-regulated protein 78
- IRE1
- protein-kinase and site-specific endoribonuclease
- PERK
- protein kinase R-like ER kinase
- ORP150
- 150-kDa oxygen-regulated protein
- PG
- prostaglandin
- S1P
- site-1 protease
- S2P
- site-2 protease
- VacA
- vacuolating cytotoxin A
- XBP
- X box binding protein.
REFERENCES
- 1.Huang J. Q., Sridhar S., Hunt R. H. (2002) Lancet 359, 14–22 [DOI] [PubMed] [Google Scholar]
- 2.Chan F. K., Sung J. J., Chung S. C., To K. F., Yung M. Y., Leung V. K., Lee Y. T., Chan C. S., Li E. K., Woo J. (1997) Lancet 350, 975–979 [DOI] [PubMed] [Google Scholar]
- 3.Murakami K., Okimoto T., Kodama M., Tanahashi J., Yasaka S., Inoue K., Uchida M., Anan J., Mizukami K., Abe T., Watada M., Fujioka T. (2009) J Gastroenterol 44, 40–43 [DOI] [PubMed] [Google Scholar]
- 4.Papatheodoridis G. V., Sougioultzis S., Archimandritis A. J. (2006) Clin. Gastroenterol Hepatol. 4, 130–142 [DOI] [PubMed] [Google Scholar]
- 5.Chan F. K., Chung S. C., Suen B. Y., Lee Y. T., Leung W. K., Leung V. K., Wu J. C., Lau J. Y., Hui Y., Lai M. S., Chan H. L., Sung J. J. (2001) N. Engl. J. Med. 344, 967–973 [DOI] [PubMed] [Google Scholar]
- 6.de Leest H. T., Steen K. S., Lems W. F., Bijlsma J. W., van de Laar M. A., Huisman A. M., Vonkeman H. E., Houben H. H., Kadir S. W., Kostense P. J., van Tulder M. W., Kuipers E. J., Boers M., Dijkmans B. A. (2007) Helicobacter 12, 477–485 [DOI] [PubMed] [Google Scholar]
- 7.Kanatani K., Ebata M., Murakami M., Okabe S. (2004) J. Physiol. Pharmacol. 55, 207–222 [PubMed] [Google Scholar]
- 8.Chang C. C., Chen S. H., Lien G. S., Lou H. Y., Hsieh C. R., Fang C. L., Pan S. (2005) World J. Gastroenterol. 11, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida N., Sugimoto N., Hirayama F., Nakamura Y., Ichikawa H., Naito Y., Yoshikawa T. (2002) Gut 50, 594–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtenberger L. M. (2001) Biochem. Pharmacol. 61, 631–637 [DOI] [PubMed] [Google Scholar]
- 11.Ligumsky M., Golanska E. M., Hansen D. G., Kauffman G. L., Jr. (1983) Gastroenterology 84, 756–761 [PubMed] [Google Scholar]
- 12.Tanaka K., Tomisato W., Hoshino T., Ishihara T., Namba T., Aburaya M., Katsu T., Suzuki K., Tsutsumi S., Mizushima T. (2005) J. Biol. Chem. 280, 31059–31067 [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi S., Gotoh T., Tomisato W., Mima S., Hoshino T., Hwang H. J., Takenaka H., Tsuchiya T., Mori M., Mizushima T. (2004) Cell Death Differ. 11, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 14.Tomisato W., Tanaka K., Katsu T., Kakuta H., Sasaki K., Tsutsumi S., Hoshino T., Aburaya M., Li D., Tsuchiya T., Suzuki K., Yokomizo K., Mizushima T. (2004) Biochem. Biophys. Res. Commun. 323, 1032–1039 [DOI] [PubMed] [Google Scholar]
- 15.Tomisato W., Tsutsumi S., Rokutan K., Tsuchiya T., Mizushima T. (2001) Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1092–G1100 [DOI] [PubMed] [Google Scholar]
- 16.Aburaya M., Tanaka K., Hoshino T., Tsutsumi S., Suzuki K., Makise M., Akagi R., Mizushima T. (2006) J. Biol. Chem. 281, 33422–33432 [DOI] [PubMed] [Google Scholar]
- 17.Tomisato W., Tsutsumi S., Hoshino T., Hwang H. J., Mio M., Tsuchiya T., Mizushima T. (2004) Biochem. Pharmacol 67, 575–585 [DOI] [PubMed] [Google Scholar]
- 18.Suemasu S., Tanaka K., Namba T., Ishihara T., Katsu T., Fujimoto M., Adachi H., Sobue G., Takeuchi K., Nakai A., Mizushima T. (2009) J. Biol. Chem. 284, 19705–19715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsumi S., Namba T., Tanaka K. I., Arai Y., Ishihara T., Aburaya M., Mima S., Hoshino T., Mizushima T. (2006) Oncogene 25, 1018–1029 [DOI] [PubMed] [Google Scholar]
- 20.Namba T., Hoshino T., Tanaka K., Tsutsumi S., Ishihara T., Mima S., Suzuki K., Ogawa S., Mizushima T. (2007) Mol Pharmacol. 71, 860–870 [DOI] [PubMed] [Google Scholar]
- 21.Ishihara T., Hoshino T., Namba T., Tanaka K., Mizushima T. (2007) Biochem. Biophys. Res. Commun. 356, 711–717 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman R. J. (2002) J. Clin. Invest. 110, 1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ron D. (2002) J. Clin. Invest. 110, 1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. (2000) Mol. Cell. Biol. 20, 6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000) Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 26.Mori K. (2003) Traffic 4, 519–528 [DOI] [PubMed] [Google Scholar]
- 27.Lou L. X., Geng B., Yu F., Zhang J., Pan C. S., Chen L., Qi Y. F., Ke Y., Wang X., Tang C. S. (2006) Life Sci. 79, 1856–1864 [DOI] [PubMed] [Google Scholar]
- 28.Covacci A., Telford J. L., Del Giudice G., Parsonnet J., Rappuoli R. (1999) Science 284, 1328–1333 [DOI] [PubMed] [Google Scholar]
- 29.Suerbaum S., Michetti P. (2002) N. Engl. J. Med. 347, 1175–1186 [DOI] [PubMed] [Google Scholar]
- 30.Hawkey C. J., Tulassay Z., Szczepanski L., van Rensburg C. J., Filipowicz-Sosnowska A., Lanas A., Wason C. M., Peacock R. A., Gillon K. R. (1998) Lancet 352, 1016–1021 [DOI] [PubMed] [Google Scholar]
- 31.Wu C. Y., Kuo K. N., Wu M. S., Chen Y. J., Wang C. B., Lin J. T. (2009) Gastroenterology 137, 1641–1648 e1–2 [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki E., Wada A., Kumatori A., Nakagawa I., Funao J., Nakayama M., Hisatsune J., Kimura M., Moss J., Hirayama T. (2006) J. Biol. Chem. 281, 11250–11259 [DOI] [PubMed] [Google Scholar]
- 33.Boquet P., Ricci V., Galmiche A., Gauthier N. C. (2003) Trends Microbiol. 11, 410–413 [DOI] [PubMed] [Google Scholar]
- 34.Mine T., Endo C., Kushima R., Kushima W., Kobayashi I., Muraoka H., Taki R., Fujita T. (2000) Aliment. Pharmacol. Ther. 14, 199–204 [DOI] [PubMed] [Google Scholar]
- 35.Kim H., Seo J. H., Kim K. H. (2003) Ann. N.Y. Acad. Sci. 1010, 90–94 [DOI] [PubMed] [Google Scholar]
- 36.Shibata W., Hirata Y., Maeda S., Ogura K., Ohmae T., Yanai A., Mitsuno Y., Yamaji Y., Okamoto M., Yoshida H., Kawabe T., Omata M. (2006) J. Pathol. 210, 306–314 [DOI] [PubMed] [Google Scholar]
- 37.Yeo M., Park H. K., Kim D. K., Cho S. W., Kim Y. S., Cho S. Y., Paik Y. K., Hahm K. B. (2004) Proteomics 4, 3335–3342 [DOI] [PubMed] [Google Scholar]
- 38.Kitao Y., Ozawa K., Miyazaki M., Tamatani M., Kobayashi T., Yanagi H., Okabe M., Ikawa M., Yamashima T., Stern D. M., Hori O., Ogawa S. (2001) J. Clin. Invest. 108, 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asahi H., Koshida K., Hori O., Ogawa S., Namiki M. (2002) BJU Int. 90, 462–466 [DOI] [PubMed] [Google Scholar]
- 40.Sawai N., Kita M., Kodama T., Tanahashi T., Yamaoka Y., Tagawa Y., Iwakura Y., Imanishi J. (1999) Infect. Immun. 67, 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 42.Nadanaka S., Yoshida H., Kano F., Murata M., Mori K. (2004) Mol. Biol. Cell 15, 2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. (1999) Mol. Biol. Cell 10, 3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Namba T., Tanaka K., Ito Y., Ishihara T., Hoshino T., Gotoh T., Endo M., Sato K., Mizushima T. (2009) Am. J. Pathol. 174, 1786–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotoh T., Oyadomari S., Mori K., Mori M. (2002) J. Biol. Chem. 277, 12343–12350 [DOI] [PubMed] [Google Scholar]
- 46.Yoshida H., Haze K., Yanagi H., Yura T., Mori K. (1998) J. Biol. Chem. 273, 33741–33749 [DOI] [PubMed] [Google Scholar]
- 47.Namba T., Homan T., Nishimura T., Mima S., Hoshino T., Mizushima T. (2009) J. Biol. Chem. 284, 4158–4167 [DOI] [PubMed] [Google Scholar]
- 48.Shirin H., Sordillo E. M., Oh S. H., Yamamoto H., Delohery T., Weinstein I. B., Moss S. F. (1999) Cancer Res. 59, 2277–2281 [PubMed] [Google Scholar]
- 49.Ashktorab H., Neapolitano M., Bomma C., Allen C., Ahmed A., Dubois A., Naab T., Smoot D. T. (2002) Microbes Infect. 4, 713–722 [DOI] [PubMed] [Google Scholar]
- 50.Hong M., Li M., Mao C., Lee A. S. (2004) J. Cell. Biochem. 92, 723–732 [DOI] [PubMed] [Google Scholar]
- 51.Ye J., Rawson R. B., Komuro R., Chen X., Davé U. P., Prywes R., Brown M. S., Goldstein J. L. (2000) Mol. Cell 6, 1355–1364 [DOI] [PubMed] [Google Scholar]
- 52.Huang T., Wan Y., Zhu Y., Fang X., Hiramatsu N., Hayakawa K., Paton A. W., Paton J. C., Kitamura M., Yao J. (2009) J. Cell. Biochem. 107, 973–983 [DOI] [PubMed] [Google Scholar]
- 53.VanSlyke J. K., Musil L. S. (2002) J. Cell Biol. 157, 381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamoto C., Sugano K., Ota S., Sakaki N., Takahashi S., Yoshida Y., Tsukui T., Osawa H., Sakurai Y., Yoshino J., Mizokami Y., Mine T., Arakawa T., Kuwayama H., Saigenji K., Yakabi K., Chiba T., Shimosegawa T., Sheehan J. E., Perez-Gutthann S., Yamaguchi T., Kaufman D. W., Sato T., Kubota K., Terano A. (2006) Eur. J. Clin. Pharmacol 62, 765–772 [DOI] [PubMed] [Google Scholar]
- 55.Molinari M., Galli C., Norais N., Telford J. L., Rappuoli R., Luzio J. P., Montecucco C. (1997) J. Biol. Chem. 272, 25339–25344 [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Wandinger-Ness A., Goldenring J. R., Cover T. L. (2004) Mol. Biol. Cell 15, 1946–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomisato W., Takahashi N., Komoto C., Rokutan K., Tsuchiya T., Mizushima T. (2000) Dig. Dis. Sci. 45, 1674–1679 [DOI] [PubMed] [Google Scholar]
- 58.Amieva M. R., Vogelmann R., Covacci A., Tompkins L. S., Nelson W. J., Falkow S. (2003) Science 300, 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]