Abstract
Elastic fibers are extracellular structures that provide stretch and recoil properties of tissues, such as lungs, arteries, and skin. Elastin is the predominant component of elastic fibers. Tropoelastin (TE), the precursor of elastin, is synthesized mainly during late fetal and early postnatal stages. The turnover of elastin in normal adult tissues is minimal. However, in several pathological conditions often associated with inflammation and oxidative stress, elastogenesis is re-initiated, but newly synthesized elastic fibers appear abnormal. We sought to determine the effects of reactive oxygen and nitrogen species (ROS/RNS) on the assembly of TE into elastic fibers. Immunoblot analyses showed that TE is oxidatively and nitrosatively modified by peroxynitrite (ONOO−) and hypochlorous acid (HOCl) and by activated monocytes and macrophages via release of ONOO− and HOCl. In an in vitro elastic fiber assembly model, oxidatively modified TE was unable to form elastic fibers. Oxidation of TE enhanced coacervation, an early step in elastic fiber assembly, but reduced cross-linking and interactions with other proteins required for elastic fiber assembly, including fibulin-4, fibulin-5, and fibrillin-2. These findings establish that ROS/RNS can modify TE and that these modifications affect the assembly of elastic fibers. Thus, we speculate that oxidative stress may contribute to the abnormal structure and function of elastic fibers in pathological conditions.
Keywords: Elastin, Extracellular Matrix, Fibrillin, Oxidative Stress, Reactive Oxygen Species (ROS), Coacervation, Desmosine, Fibulins
Introduction
Elastic fibers are complex, insoluble extracellular matrix structures that are abundant in tissues such as the arteries, skin, and lungs. They provide architectural support, as well as the stretch and recoil required for normal function of these tissues. Elastic fibers consist of two major components, elastin and microfibrils. Elastin is an amorphous component that comprises most (>90%) of the mass of the mature elastic fiber. Microfibrils are fibrillar components that are rich in acidic glycoproteins, such as fibrilins (Fbns),2 fibulins (Fblns), and microfibril-associated glycoproteins (MAGPs). Other components, such as glycosaminoglycans (GAGs), lysyl oxidase (LOX), and other elastin-binding proteins are also present in elastic fibers (1–4).
Elastogenic cells, such as fibroblasts and smooth muscle cells, secrete elastin as a soluble, precursor protein called tropoelastin (TE). Assembly of monomeric TE into elastic fibers is a multi-step process. TE monomers self-associate/coacervate into aggregates which are deposited onto pre-existing microfibrils. TE interacts with a number of different matrix proteins during the assembly process. For example, Fbln-4 and Fbln-5 facilitate TE alignment for cross-linking and deposition of cross-linked TE aggregates onto microfibrils. Microfibrillar components (MAGP-1, Fbn-1, and Fbn-2) align TE aggregates to undergo further cross-linking to form mature elastic fibers (5–9).
Elastin is mainly synthesized during late fetal and early postnatal stages and its turnover in normal adult tissues is negligible. However, in cardiovascular and pulmonary diseases, such as atherosclerosis and emphysema/chronic obstructive pulmonary disease (COPD), excessive degradation, and/or inefficient repair of elastic fibers results in compromised tissue function. New elastin synthesis occurs in these pathological conditions suggesting elastic fiber repair mechanisms are activated. However, the integrity and organization of the elastic fibers is disrupted and the network discontinuous (10–14). These data suggest that there is aberrant assembly of newly synthesized TE into elastic fibers.
Oxidative stress has been implicated in the pathogenesis of several cardiovascular and pulmonary diseases. Oxidants can be generated by external factors such as cigarette smoke, or internal factors such as inflammatory cells, and mitochondrial respiration. These systems produce various reactive oxygen and nitrogen intermediates, such as superoxide anion (O2˙̄), hydrogen peroxide (H2O2), hydroxyl radical (·OH), nitric oxide (NO), nitrite (NO2−) and peroxynitrite (ONOO−) (15–17). When free radicals exceed endogenous antioxidant capacity, they modify proteins, lipids, and nucleic acids. For example, carbonyl formation on side chains of specific amino acids (Lys, Arg, Pro, and Thr) occurs during oxidative stress, whereas 3-nitrotyrosine (N-Tyr) formation is a common modification that occurs as a result of nitration. These modifications have also been reported in many diseases, including COPD, atherosclerosis, and other cardiovascular diseases (18–30).
There is evidence that matrix proteins can be modified by reactive oxygen species (ROS) resulting in an alteration of protein structure and function. For example, oxidative modifications to collagen cause a change in the elasticity of the skin, as well as stiffer and more brittle cartilage (31, 32). However, few studies have examined the effects of oxidation of elastin (33, 34). We hypothesized that oxidants generated during oxidative stress in pathological conditions can modify newly synthesized TE and impair the assembly of TE into elastic fibers. To test this hypothesis, we investigated the effects of oxidizing agents on TE and the effects of oxidized TE on different steps of elastic fiber assembly. We found that oxidants modified TE and that oxidized TE was not assembled into elastic fibers.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
ONOO−, the anti-N-Tyr polyclonal antibody, protein A/G plus-agarose beads and the OxyBlot Protein Oxidation Detection kit were purchased from Millipore Corp. (Bedford, MA). Hypochlorous acid (HOCl), H2O2, uric acid (UA), lipoic acid (LA), Escherichia coli lipopolysaccharide (LPS), N-formyl-Met-Leu-Phe (fMLP), and protease inhibitor mixture were purchased from Sigma-Aldrich. The anti-elastin BA4 monoclonal antibody was purchased from Abcam Inc (Cambridge, MA). The anti-V5 antibody was purchased from Invitrogen (Carlsbad, CA). The TRITC-conjugated anti-mouse, FITC-conjugated anti-rabbit, and HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell Culture
CHOKI cells stably expressing a 6xHis-tagged fragment of human Fbn-2 encompassing amino acids 1–1114 (7) were maintained in Ham's F-12 supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), 2 mm l-glutamine, hygromycin B (100 μg/ml), and zeocin (100 μg/ml). CHOKI cells stably expressing V5/6xHis double-tagged full-length rat Fbln-4 or V5/6xHis double-tagged full-length rat Fbln-5 (kind gifts from Dr. H. Yanagisawa (9) were maintained in the same media. Human retinal pigmented epithelial ARPE-19 cells (American Type Tissue Culture, Rockville, MD) were maintained in DMEM supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and 2 mm l-glutamine.
Purification of Proteins
Full-length bovine TE (35) and full-length mouse MAGP1 (36) were expressed as His6 fusion proteins in M15 E. coli and purified as previously described using Ni-NTA agarose beads (Qiagen Inc, Valencia, CA). The purified proteins were dialyzed against 50 mm glacial acetic acid, lyophilized, and further purified by reverse phase high performance liquid chromatography. Fractions containing TE or MAGP1 were pooled and lyophilized. The lyophilized proteins were resuspended in sterile water and subjected to amino acid analysis and immunoblot analysis using the appropriate anti-elastin or anti-MAGP1 antibody.
The human Fbn-2 fragment, full-length rat Fbln-4, and full-length rat Fbln-5 were purified from conditioned media of transfected stable CHOKI cell lines as previously described (7, 9). Cells were maintained confluent for 1 week in serum-free SFM4-CHO medium (Hyclone, Waltham, MA), and secreted Fbn-2 fragment, Fbln-4 and Fbln-5 were purified from the conditioned medium using Ni-NTA-agarose beads following the manufacturer's protocol. The purified Fbn-2 fragment, Fbln-4, and Fbln-5 were then dialyzed against buffer containing 50 mm Tris (pH 7.5), 150 mm NaCl, and 1 mm EDTA and subjected to amino acid analysis and immunoblot analysis using an anti-Gly antibody for Fbn-2 (7) or the anti-V5 antibody for Fbln-4 and Fbln-5.
ONOO−, HOCl, and H2O2 Exposure of TE
Aliquots of ONOO− in 0.3 n NaOH were stored at −80 °C, and immediately prior to each assay, the concentration of ONOO− was determined spectrophotometrically at 302 nm (ϵM = 1,670 m−1 cm−1) and diluted in 0.01 n NaOH. Aliquots of HOCl were stored at −20 °C, and immediately prior to each assay, the concentration of HOCl was determined spectrophotometrically at 292 nm (ϵM = 1,670 m−1 cm−1) (37). An 8.8 m stock of H2O2 was stored at room temperature. HOCl and H2O2 were diluted in sterile water. TE was diluted in Tris-buffered saline (50 mm Tris, pH 7.5 and 150 mm NaCl), and ONOO−, HOCl, or H2O2 was added while vortexing. The solutions were incubated at room temperature for 5 min. The pH was monitored to make sure that each reaction was performed at neutral pH.
Detection of Oxidative and Nitrosative Protein Modifications
Oxidation of TE was detected by using the OxyBlot Protein Oxidation Detection kit (Millipore) according to the manufacturer's recommendations. Treated and untreated TE was derivatized with 2,4-dinitrophenylhydrazine (DNP), separated on an 8% SDS-polyacrylamide gel under reducing conditions, transferred to a Immobilon-P PVDF membrane (Millipore), and incubated with an anti-DNP antibody and an HRP-conjugated anti-rabbit secondary antibody. Nitration of TE was detected by separation of treated and untreated TE on an 8% SDS-polyacrylamide gel under reducing conditions, transferred onto PVDF membranes, and incubated with an anti-N-Tyr antibody, followed by HRP-conjugated anti-rabbit secondary antibody. Blots were developed using the ECL Plus Western blotting Detection System (Amersham Biosciences), and subsequent autoradiography.
Isolation of Mouse Peritoneal Macrophages and Human Monocytes
8-week-old C57BL/6 mice obtained from Taconic Farms (Germantown, NY) were housed in a pathogen-free animal facility under the veterinary care of the Department of Comparative Medicine at Washington University School of Medicine. Resident peritoneal mouse macrophages were isolated as previously described (38). Saline was injected into the peritoneal space and the lavage fluids of 3–4 mice were pooled. The predominance of macrophages in the fluid (>95%) was confirmed by Wright-stained cytospins. Experiments were performed on at least four independent cell isolations. All procedures were approved by the Washington University School of Medicine Animal Studies Committee and were performed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Human monocytes were isolated as previously described (38). Briefly, 60 ml of whole blood was obtained by venipuncture from healthy adult human volunteers and layered on a Histopaque (Sigma-Aldrich) gradient. Cells that sedimented at the plasma-histopaque interface were collected, washed several times with PBS, and the percentage of monocytes assessed by Wright-stained cytospins (38). Experiments were performed on at least four independent cell isolations. This study was reviewed and approved by the Washington University School of Medicine Human Studies Committee. Volunteers gave informed, written consent.
Activation of Monocytes and Macrophages
Macrophages and monocytes (1 × 105) were diluted in DMEM containing protease inhibitor mixture and incubated with 1 μg of TE in the presence or absence of 10 μm fMLP plus 100 μg/ml LPS for 15 min at room temperature. In some experiments, 1 mm uric acid, the ONOO−-specific scavenger, 1 mm lipoic acid, the HOCl-specific scavenger, or both were incubated with the cells prior to the addition of activators. After 15 min of incubation, the solutions were centrifuged to pellet the cells and the supernatants containing TE was analyzed for oxidative and nitrosative modifications as described above. TE was incubated with cells in the absence of activators as a negative control.
Quantitative Densitometry
Immunoblots were scanned and analysis of the optical densities was performed using the public domain NIH Image program (developed at National Institutes of Health). The data represent the mean ± S.E. from at least five independent experiments. All quantification was done on exposures in which individual bands were not yet saturating.
Immunoprecipitation-Western Analysis
Immunoprecipitation of TE was performed as mentioned elsewhere (39). Briefly, unmodified and ONOO−-modified TE were incubated with the anti-N-Tyr antibody in buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, and 1% Nonidet P-40 at 4 °C overnight with gentle agitation. Protein A/G plus-agarose beads were added to each tube and incubated for 1 h at 4 °C with gentle agitation. The immune complex was centrifuged and the supernatant containing unbound, unmodified TE was collected. The beads with bound, nitrated TE were washed three times with 50 mm Tris-HCl (pH 7.5) with 400 mm NaCl and once with 10 mm Tris-HCl (pH 6.8). SDS-PAGE sample buffer containing β-mercaptoethanol was added to both supernatant and pellet and boiled for 10 min. Samples were adjusted for equal volume, separated on a 6% SDS-polyacrylamide gel, and transferred onto Immobilon-PVDF transfer membranes (Millipore). Oxidized, immunoprecipitated and unoxidized, soluble TE were detected using an anti-elastin antibody an HRP-conjugated anti-rabbit secondary, and the ECL Plus Western blotting Detection System, and subsequent autoradiography.
In Vitro Elastic Fiber Assembly Assay
Incorporation of exogenously added TE into the microfibrils of ARPE-19 cells was performed as previously described (40). ARPE-19 cells were plated on four-well chamber culture slides and maintained at confluency for 8–10 days, after which oxidized and unoxidized TE were added at a final concentration of 0.1 mg/ml in normal growth media described above. Cells were incubated for 1 h or 16 h at 37 °C and then fixed with ice-cold methanol. TE deposition onto pre-existing microfibrils was detected by immunofluorescence microscopy using the anti-elastin BA4 monoclonal antibody, followed by TRITC-conjugated anti-mouse secondary antibody. Nitration of TE was confirmed using the rabbit anti-N-Tyr antibody, followed by a FITC-conjugated anti-rabbit secondary antibody.
Coacervation Assay
Coacervation was carried out as previously described (41). TE was exposed to ONOO− as described above and coacervation was monitored by increasing the solution temperature at a rate of 1 °C/min with constant stirring at 1000 rpm and measuring the absorbance at 440 nm using a Shimadzu UV-2401PC UV-visible recording spectrophotometer equipped with temperature controller. TE exposed to equal amount of NaOH was used as a carrier alone control.
Desmosine Analysis
Cross-linking of TE was measured by the formation of desmosine. ARPE-19 cells were plated on 100 mm dishes and maintained confluent for 8–10 days, after which oxidized and unoxidized TE were added at a final concentration of 0.1 mg/ml in normal growth media described above. Cells were incubated with the TE for 16 h at 37 °C, washed with PBS, and scraped into 1 ml of water. The samples were centrifuged, and the pellet was hydrolyzed in 6 n HCl overnight at a constant temperature of 110 °C. The desmosine content in hydrolysate was determined by radioimmunoassay (42, 43) and normalized to total protein.
Solid-Phase Binding Assay
Solid-phase binding of TE by Fbln-4, Fbln-5, Fbn-2, and MAGP1 was analyzed as previously described (7, 9). Flat-bottomed microtiter plates (Costar, NY) were coated with 1 μg/well of unmodified, ONOO−-modified, or HOCl-modified TE in 10 mm carbonate buffer (pH 9.2) at 4 °C overnight. The plates were rinsed with PBS, blocked with nonfat dry milk in PBS, and incubated with soluble ligands (Fbn-2, Fbln-4, Fbln-5, or MAGP1) in blocking buffer at 37 °C for 3 h. In the case of Fbln-4 and Fbln-5, binding was carried out in the presence of 2 mm CaCl2. The plates were then washed with PBS and incubated with primary antibody (anti-V5 antibody for Fbln-4 and Fbln-5, anti-Gly antibody for Fbn-2 (7); and anti-MAGP1 antibody for MAGP1 (36) for 2 h at room temperature, followed by HRP-conjugated secondary antibody. Binding of ligands was quantified using the ABTS Peroxidase Substrate System (KPL, Gaithersburg, MD) and measuring the absorbance at 410 nm. Equal coating of oxidized and unoxidized TE of the microtiter plate was confirmed using the BA4 anti-elastin antibody followed by HRP-conjugated anti-mouse secondary antibody (data not shown).
Statistical Analysis
All statistical analysis was performed with the SPSS 13 program. Paired Student's t test was used to analyze the relationship between unmodified and modified conditions. Data are representative of at least three independent experiments expressed as means ± S.E. A p value of less than 0.05 is considered significant.
RESULTS
TE Is a Substrate for Oxidation and Nitration by Reactive Oxygen and Nitrogen (ROS/RNS) Species
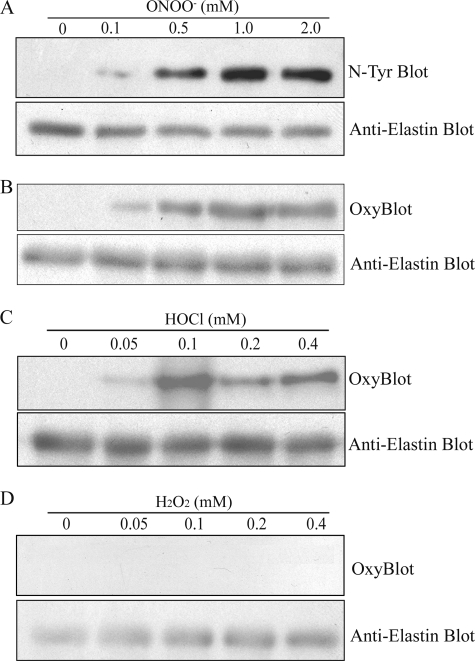
Elastin synthesis in normal adult tissues is negligible, however, in aging and several cardiovascular and pulmonary diseases, elastin synthesis is reinitiated in attempts to repair damaged elastic fibers (10–14). Because elastogenesis occurs in an oxidative environment, we examined whether TE becomes modified by exposure to ROS/RNS. Purified recombinant TE was exposed to ONOO−, HOCl, or H2O2 and modifications to TE were examined by immunoblot analysis. Concentrations of oxidants used were in a range similar to that produced by inflammatory cells (44–46). ONOO− exposure of TE induced N-Tyr (Fig. 1A) and carbonyl formation (Fig. 1B) of TE in a dose-dependent manner. Neither oxidation nor nitration of TE was detected in unmodified samples. Exposure of TE to HOCl also induced carbonyl formation (Fig. 1C), but H2O2 exposure did not (Fig. 1D). Exposure to ONOO−, HOCl, or H2O2 did not induce degradation or precipitation of TE, as shown by anti-elastin blots (Fig. 1). These data indicate that ONOO− and HOCl induces oxidative and nitrosative modifications to TE.
FIGURE 1.
TE is modified by reactive oxygen and nitrogen species. Recombinant bovine TE (1 μg) was exposed to indicated concentrations of ONOO− (A and B), HOCl (C), or H2O2 (D) and subjected to immunoblot analyses for nitrosative modifications using an anti-nitrotyrosine (N-Tyr) antibody (A) and oxidative modifications using OxyBlot protein oxidation detection kit (B–D). The same membranes were also probed with an anti-elastin antibody to control for loading. Data are representative of at least three independent experiments.
Activated Macrophages and Monocytes Oxidatively Modify TE via Release of ONOO− and HOCl
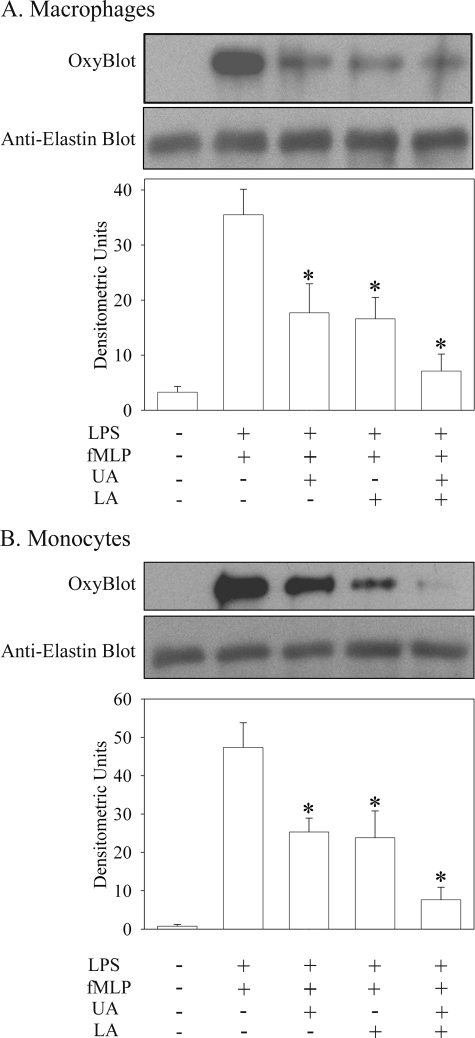
Because injury-induced elastogenesis often occurs in inflammatory conditions, we examined whether ROS/RNS generated by activated inflammatory cells could modify TE. Purified TE was incubated with resident mouse peritoneal macrophages or human monocytes in the absence or presence of LPS and fMLP, which will activate the cells. Because activated monocytes and macrophages produce proteases that could degrade TE as well as ROS/RNS in response to LPS and fMLP, the incubation was performed in the presence of protease inhibitors. Incubation of TE with monocytes (Fig. 2A) or macrophages (Fig. 2B) alone failed to induce carbonyl formation of TE. In contrast, in the presence of LPS and fMLP, activated monocytes and macrophages induced the oxidation of TE, as determined by OxyBlot analyses.
FIGURE 2.
Activated monocytes and macrophages oxidatively modify TE via release of ONOO− and HOCl. Mouse peritoneal macrophages (A) and human monocytes (B) were incubated with TE in the absence or presence of combination of LPS (100 μg/ml) and fMLP (10 μm). Activation was also performed in the presence of the ONOO−-specific scavenger UA, the HOCl-specific scavenger LA, or both scavengers. Carbonyl formation on TE was detected by OxyBlot analysis. The same membranes were also probed with an anti-elastin antibody to control for loading. The immunoblots were scanned and quantified using the NIH image program. The data represent the mean ± S.E. from at least five independent experiments. *, p < 0.05 compared with cells activated with LPS and fMLP.
To determine which ROS/RNS produced by the activated cells induced the carbonyl formation of TE, cells were activated in presence of uric acid (UA), a ONOO−-specific scavenger, LA, a HOCl-specific scavenger, or both. A significant reduction in the oxidation of TE was observed when either UA or LA was present in both macrophage and monocyte cells (∼50% decrease, p < 0.05). Oxidation of TE was further reduced when cells were activated in the presence of both UA and LA (∼80% decrease, p < 0.05). This reduction was not due to precipitation and/or degradation of TE in the presence of UA or LA, as equal amounts of TE were detected in each lane following reprobing of the blot with the anti-elastin antibody (Fig. 2). These data suggest that activated macrophages and monocytes release both ONOO− and HOCl, which in turn can oxidatively modify TE.
Oxidized TE Is Not Assembled into Elastic Fibers
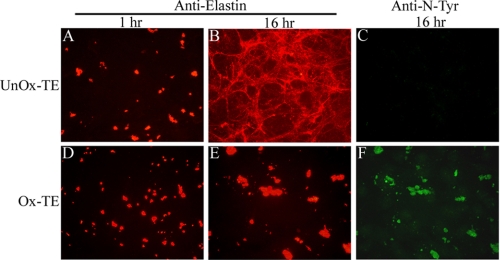
To determine whether oxidative modifications of TE alter elastic fiber assembly, we used an in vitro model system for elastic fiber assembly (40). ARPE cells secrete and organize microfibrillar components, including multiple isoforms of Fbln, Fbn, and MAGPs, into the extracellular matrix, but do not produce TE. However, when TE is provided in the culture medium, the TE is assembled with microfibrils to form elastic fibers, which can be detected by immunofluorescence using an anti-elastin antibody. Consistent with previous findings (40), deposition of unmodified TE into the microfibril-rich matrix of ARPE cells occurs first as small globules at 1 h (Fig. 3A) and then redistributes into elastin-containing microfilaments over time (Fig. 3B). Similar to unmodified TE, ONOO−-modified TE was deposited on the microfibril-rich matrix as globules at 1 h after addition to ARPE cells (Fig. 3D). In contrast to unmodified TE, however, ONOO−-modified TE organized into larger globules (Fig. 3E) after 16 h of incubation. Incubation of unmodified or ONOO−-modified TE in the absence of cells resulted in a fairly uniform distribution of TE with few small particulates (data not shown). These data suggest that the formation of larger globules of ONOO−-modified TE was cell-mediated and not merely due to enhanced precipitation of oxidized TE. Staining with anti-N-Tyr antibody was performed to confirm the modification induced by ONOO− (Fig. 3F), which is absent in unmodified sample (Fig. 3C). Similar results were obtained when TE was modified with HOCl (supplemental Fig. S1). These data suggest that oxidative modifications of TE prevent its assembly into elastic fibers.
FIGURE 3.
Oxidative modification of TE prevents elastic fiber assembly. ARPE cells were incubated with 100 μg/ml unmodified (A–C) and ONOO−-modified (D–F) TE for 1 h (A, D) or 16 h (B, C, E, F). Incorporation of TE in the pre-existing microfibrillar matrix was detected by immunofluorescence using an anti-elastin antibody. N-Tyr modification of TE induced by ONOO− exposure was confirmed by immunostaining using the anti-N-Tyr antibody (C, F). Images (×40) are representative of at least five independent experiments.
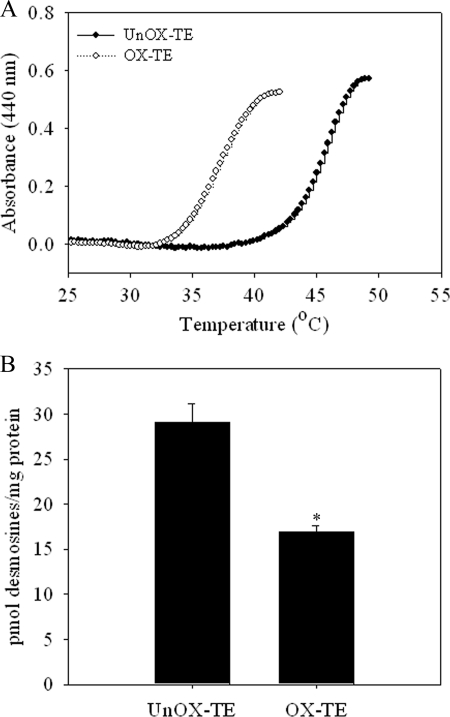
Oxidation of TE Decreased the Optimal Coacervation Temperature
During elastic fiber assembly, TE monomers undergo self-association through interactions between hydrophobic domains of TE to form aggregates through a process called coacervation. These aggregates are then deposited on pre-formed microfibrils to form elastic fibers. Because oxidized TE formed large aggregates and did not form elastic fibers (Fig. 3), we examined whether oxidative modifications of TE alter the process of coacervation. Similar to previous studies (47) unoxidized TE coacervated at ∼42 °C (Fig. 4A). However, oxidation of TE induced by ONOO− exposure reduces the coacervation temperature by almost 10 °C (Fig. 4A). These data suggest that oxidation of TE increased the propensity for self-aggregation.
FIGURE 4.
Oxidative modification of TE promotes coacervation, but decreases cross-linking. A, oxidized and unoxidized TE was dissolved in 50 mm Tris, 150 mm NaCl, and kinetics of coacervation was monitored at 400 nm. B, ARPE cells were maintained at confluency for 8–10 days and then incubated with 100 μg/ml unmodified or ONOO−-modified TE for 16 h. Cross-linking of TE was determined by desmosine analysis. Desmosine was quantified by radioimmunoassay and total protein was determined by amino acid analysis. Results are displayed as the mean ratio of pmol of desmosines/mg protein of at least five independent experiments ± S.E. *, p < 0.05 compared with unmodified TE.
Oxidative Modifications of TE Inhibit Cross-linking
Elastic fiber assembly involves cross-linking of soluble TE monomers into insoluble functional polymers. To determine whether oxidative modifications affect cross-linking of Lys residues of TE, we used the in vitro model system for elastic fiber assembly and measured desmosine formation, a marker of cross-linking. ARPE cells incubated with ONOO−-modified TE showed significantly decreased desmosine/total protein ratio (∼45% reduction, p < 0.05) compared with unmodified TE (Fig. 4B). Supporting the immunofluorescence data (Fig. 3), these results suggest that oxidized TE has decreased ability to assemble into microfilaments.
Oxidation of TE Inhibits Its Association with Fbln-4 and Fbln-5
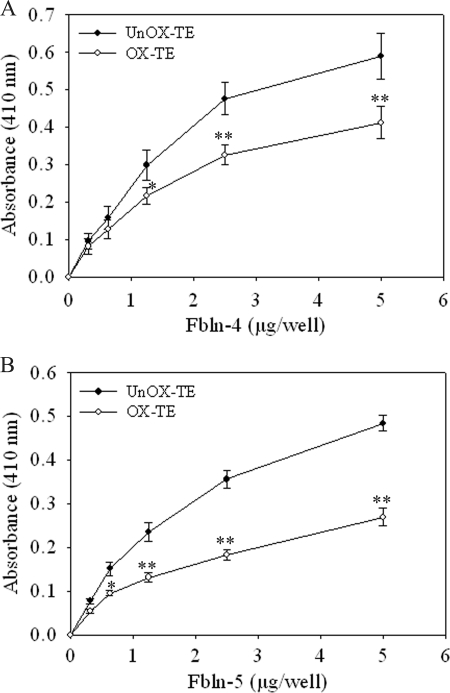
Fbln-4 and Fbln-5 interact with TE and facilitate cross-linking of TE monomers to form aggregates and deposition of aggregates onto microfibrils. To determine whether oxidative modifications of TE alter its interactions with Fbln-4 and Fbln-5, we examined the binding of Fbln-4 and Fbln-5 with unmodified and ONOO−-modified TE using a solid phase binding assay. ONOO−-modified and unmodified TE bound equally to the microtiter plates as determined using an anti-elastin antibody (data not shown). Fbln-4 bound to unmodified TE in a dose-dependent manner. However, Fbln-4 showed a significant reduction (∼35% decrease, p < 0.005) in binding to ONOO−-modified TE as compared with that of unmodified TE at 5 μg/well (Fig. 5A). Fbln-5 showed a significant reduction (∼50% decrease, p < 0.005) in binding to ONOO−-modified TE as compared with that of unmodified TE at 5 μg/well (Fig. 5B). The TE was 65% oxidized (supplemental Fig. S2), which likely explains the incomplete reduction of binding. Higher concentrations of ONOO− resulted in complete inhibition of binding (data not shown). Similar results were obtained when TE was modified with HOCl (Fbln-4, ∼35% reduction; Fbln-5, 50% reduction) (data not shown). There was minimal binding of Fbln-4 and Fbln-5 to wells coated with nonfat milk alone. These data suggest that oxidation of TE prevents elastic fiber assembly, in part by altering interactions with Fbln-4 and Fbln-5.
FIGURE 5.
Oxidative modification of TE reduced binding to Fbln-4 and Fbln-5. A 96-well non-tissue culture plates coated with 1 μg/well of unmodified (UnOx-TE; closed circle) or ONOO−-modified (Ox-TE; open circle) were incubated with indicated concentrations of V5-tagged Fbln-4 (A) or Fbln-5 (B) at 37 °C for 3 h. Following washing, bound Fbln-4 or Fbln-5 was detected using an anti-V5 antibody, HRP-conjugated secondary antibody, and the ABTS Peroxidase Substrate System, and measuring the absorbance at 410 nm. Data represent the mean of at least three independent experiments done in duplicate ± S.E. *, p < 0.05; **, p < 0.005 compared with unmodified TE.
Oxidation of TE Inhibits Binding with Fbn-2 but Not with MAGP1
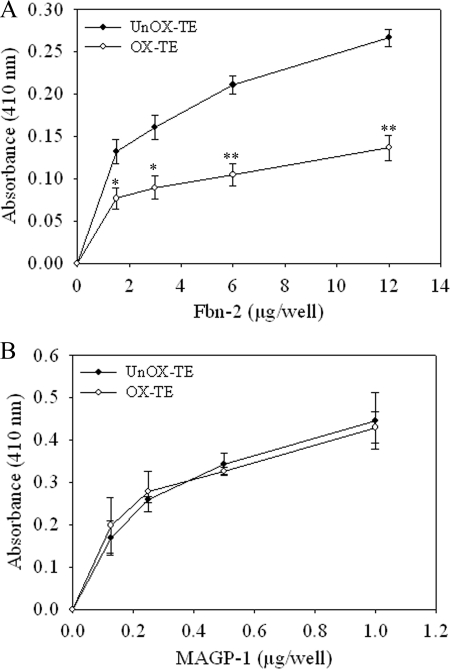
Microfibrils are composed of Fbns, MAGPs, and several other components. Others and we have shown that TE directly interacts with N-terminal of Fbn-2 and full-length MAGP1 (7, 48) and that these interactions play a role in elastic fiber assembly. To further decipher the mechanism of inhibition of elastic fiber assembly by oxidation of TE, we examined whether oxidative modifications of TE alter its interaction with Fbn-2 and MAGP1 using a solid phase binding assay. Fbn-2 bound to unmodified TE in a dose-dependent manner. However, Fbn-2 showed a significant reduction (∼55% decrease, p < 0.005) in binding to ONOO−-modified TE as compared with that of unmodified TE at 12 μg/well (Fig. 6A). Similar results were obtained when TE was modified with HOCl, but to a lesser degree (∼25% reduction) (data not shown). In contrast, MAGP1 bound equally to unmodified and ONOO−-modified (Fig. 6B) or HOCl-modified TE (data not shown). There was minimal binding of Fbn-2 or MAGP1 to wells coated with nonfat milk alone. These data suggest that oxidation of TE alters its interaction with critical components of microfibrils, and thus prevents elastic fiber assembly.
FIGURE 6.
Oxidative modification of TE reduced binding to Fbn-2 but not to MAGP1. A 96-well non-tissue culture plates coated with 1 μg/well of unmodified (UnOx-TE, closed circle) or ONOO−-modified (Ox-TE, open circle) were incubated with indicated concentrations of Fbn-2 fragment (A) or MAGP1 (B) at 37 °C for 3 h. Following washing, bound Fbn-2 or MAGP1 was detected using an anti-Gly antibody for Fbn-2 or anti-MAGP1 antibody, HRP-conjugated secondary antibody, and the ABTS Peroxidase Substrate System, and measuring the absorbance at 410 nm. Data represent the mean of at least three independent experiments done in duplicate ± S.E. *, p < 0.05; **, p < 0.005 compared with unmodified TE.
DISCUSSION
Many studies have focused on elastic fiber destruction as the critical process leading to inflammatory and degenerative changes in several cardiovascular and pulmonary diseases. Our studies suggest that oxidative modification of TE also plays a role in the development of these diseases by preventing elastic fiber assembly and repair. We found that TE is modified by ONOO− and HOCl released by activated macrophages and monocytes, and that modified TE was unable to incorporate into pre-existing microfibrils and thus cannot assemble into elastic fibers. In these studies, we detected N-Tyr and carbonyl formation on Lys, Arg, Pro, or Thr (Figs. 1 and 2). However we cannot rule out the possibility that modifications to other amino acid residues could occur during oxidative stress. TE contains several amino acids that are potentially susceptible to oxidative modification, including Tyr, Lys, Arg, Pro, Thr, Cys, Met, Val, Leu, and Phe residues (1). Thus, modifications to any of these residues could alter the function of TE and contribute to the aberrant elastic fiber assembly seen in several pathologic conditions. Additional studies using mass spectrometry are needed to determine the amino acid residues that are modified by ROS/RNS, and determine which of these modifications impact elastic fiber assembly.
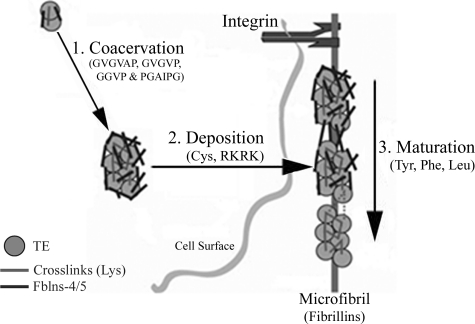
Elastic fiber assembly is a multistep process (Fig. 7) in which TE monomers are secreted from elastogenic cells and self-assemble/coacervate to form microaggregates that interact with Fbln-4 and Fbln-5 (step 1). Fbln-4 and Fbln-5 help facilitate the cross-linking of TE monomers to form appropriately sized, larger aggregates and mediate the deposition of the TE aggregates onto pre-existing microfibrils (step 2). Through interactions with microfibril components, the TE aggregates become aligned with precise positioning so that cross-linking domains are juxtaposed. Finally, LOX facilitates cross-linking and the generation of mature elastic fibers (step 3) (8, 49, 50). At each of these steps, critical amino acids in TE have been identified, many of which could be susceptible to oxidative modifications and thus could affect elastic fiber assembly at various steps.
FIGURE 7.
Model of elastic fiber assembly. Step 1, TE is transported to assembly sites on the plasma membrane where it self assembled via coacervation into aggregates that are cross-linked by a LOX. Hydrophobic domains of TE containing Pro residue assist in the process of coacervation. Interaction with Fbln-4 and/or -5 may facilitate cross-linking or possibly help limit the size of the TE aggregates. Step 2, the aggregates are then transferred to extracellular microfibrils, which interact with the cell through integrins. Fbln-4 and/or -5 assist the transfer of elastin aggregates on to the microfibril while the C-terminal domain of TE that contains Cys and terminates into Lys residues direct its association with microfibrils. Step 3, elastin aggregates on the microfibril align and are further cross-linked by LOX to form the mature elastic fiber. Tyr, Phe, and Leu residues contribute in the alignment of TE aggregates on microfibril.
Oxidative Modifications to TE Promote Coacervation
An initial step in elastic fiber assembly is coacervation, which takes place through the interaction between the hydrophobic domains of TE, such as repetitive sequences of GVGVP, GGVP, GVGVAP, and PGAIPG (Fig. 7, step 1). In addition the process of coacervation is highly influenced by the overall charge state of the TE molecule (2, 51–54). For example, the interaction of negatively charged molecules such as GAGs with positively charged Lys residues of TE molecules has been shown to promote coacervation at lower temperatures by interfering with the charge interactions (2, 25, 50, 55, 56). We speculate that oxidation neutralizes the Lys residues of TE, allows the hydrophobic domains to more easily interact and thus promote coacervation at lower temperature.
It has been postulated that Fbln-4 and/or Fbln-5 interactions with TE regulate the size of the TE aggregates and play a role in the deposition of aggregates onto microfibrils by forming a ternary complex with TE and Fbns in the microfibrils (8, 57, 58). Oxidation of TE reduced the interactions with Fbln-4 and Fbln-5 (Fig. 5) and resulted in the formation of larger aggregates (Fig. 3E). Thus, by blocking the interactions between TE and Fblns-4/-5, oxidative modifications to TE could block elastic fiber assembly by promoting uncontrolled coacervation of TE monomers and/or by disruption of the ternary complex required for deposition of TE onto the microfibrils.
Oxidative Modifications to TE Could Inhibit Deposition onto Elastic Fibers
Another potential mechanism by which oxidative modifications to TE could alter elastic fiber assembly is that modifications to the C-terminal domain of TE could affect deposition of TE aggregates onto microfibrils (Fig. 7, step 2). The C-terminal domain of TE is highly conserved across species. Removal of the C-terminal domain reduces the ability of TE to assemble into elastic fibers (4). This region contains two Cys residues that form a disulfide bond. Disruption of the disulfide bond reduces the ability of TE to assemble into elastic fibers (4, 59). In addition, the C-terminal region contains a positively charged RKRK sequence, also shown to play a role in elastic fiber assembly. Thus, oxidation of the Cys, Lys, and/or Arg residues in the C-terminal domain could potentially be another mechanism by which oxidation of TE prevents elastic fiber assembly.
Oxidative Modifications to TE Inhibits Cross-linking
During cross-linking of TE, four Lys residues in KA (Lys-Ala) cross-linking domains are aligned to be in close proximity for processing by LOX to form tetrafunctional cross-links of desmosine or isodesmosine (60). Hydrophobic amino acid residues (Tyr, Phe, Ile, or Leu) next to the Lys residues play a critical role in maintaining the optimal microenvironment for proper cross-linking (8, 47–52) (Fig. 7, step 3). We found that oxidative modification of TE inhibits desmosine formation (Fig. 4B). There are several possible explanations of how oxidation of TE resulted in decreased cross-linking: first, oxidative modifications to the Lys residues of TE could render the Lys residues incapable of cross-linking; second, juxtaposed Tyr or Phe residues could become modified, resulting in failure of alignment of the cross-linking domains for cross-linking; and third, oxidation of TE could inhibit cross-linking by interfering with the formation of the ternary complex with Fblns-4 (Fig. 5) and Fbns (Fig. 6A), and thus prevent elastic fiber assembly.
Potential Consequences of Oxidative Stress on Elastic Fiber Assembly
Evidence of abnormal assembly of newly synthesized elastin is present in several cardiovascular and pulmonary diseases, suggesting a lack of efficient assembly/repair of elastic fibers where oxidative stress is elevated (10–12). Our results show that oxidative modification of TE alters elastic fiber assembly in an in vitro elastic fiber assembly model. When unmodified TE is added in this system, it becomes incorporated into the pre-existing matrix deposited by the ARPE cells. In contrast, when ONOO−-modified TE is added, the oxidized TE remains as large aggregates and does not assemble into microfilaments. This scenario may occur in vivo in settings of high oxidative stress and increased synthesis of TE, as seen in several cardiovascular and pulmonary diseases. Just as likely to occur in vivo are oxidative modifications to the pre-existing elastic fibers, which too could impact elastic fiber repair. Using our in vitro model system, we found that when the pre-existing ARPE matrix was exposed to ONOO− prior to the addition of unoxidized, “newly synthesized” TE, the unmodified TE was unable to incorporate into the oxidized matrix (data not shown). This observation suggests that in addition to modifying TE, oxidants generated during pathological condition can also modify other proteins involved in elastic fiber assembly, such as LOX, Fblns, Fbns, and might contribute in the production of abnormal elastic fibers.
In summary, this study was designed to investigate the effects of oxidative stress on the assembly of TE into elastic fibers. We found that TE is susceptible to oxidative modifications by ONOO− and HOCl released from activated monocytes and macrophages, and that oxidized TE cannot assemble into elastic fibers, at least in part by inhibiting critical interactions with microfibrillar components, including Fbln-4/-5 and Fbn-2, required for elastic fiber formation. We believe that these studies provide new insights into mechanisms by which abnormal elastic fibers are generated, which in turn play a role in the development of various cardiovascular and pulmonary diseases.
Acknowledgments
We thank Dr. Robert M. Senior (Washington University, St. Louis) for discussion and critical reading of the manuscript, Dr. Hiromi Yanagisawa (University of Texas Southwestern Medical Center, Dallas) for kindly providing us CHO cells expressing Fbln-4 and Fbln-5, and Dr. Sadis Matalon (University of Albama, Birmingham) for guidance in the use of peroxynitrite.
This work was supported, in whole or in part, by National Institutes of Health Grant HL29594, the Alpha-1 Foundation, and the Alan A. and Edith L. Wolff Charitable Trust/Barnes-Jewish Hospital Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- Fbn
- fibrillin
- TE
- tropoelastin
- ROS
- reactive oxygen species
- RNS
- reactive nitrogen species
- ONOO−
- peroxynitrite
- HOCl
- hypochlorous acid
- N-Tyr
- 3-nitrotyrosine
- COPD
- chronic obstructive pulmonary disease
- Fbln
- fibulin
- MAGPs
- microfibril-associated glycoproteins
- LOX
- lysyl oxidase
- LPS
- lipopolysaccharides
- fMLP
- N-formyl-Met-Leu-Phe
- UA
- uric acid
- LA
- lipoic acid.
REFERENCES
- 1.Mecham R. P. (2008) Methods 45, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrhovski B., Weiss A. S. (1998) Eur. J. Biochem. 258, 1–18 [DOI] [PubMed] [Google Scholar]
- 3.Wagenseil J. E., Mecham R. P. (2009) Physiol. Rev. 89, 957–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise S. G., Weiss A. S. (2009) Int. J. Biochem. Cell. Biol. 41, 494–497 [DOI] [PubMed] [Google Scholar]
- 5.Cirulis J. T., Bellingham C. M., Davis E. C., Hubmacher D., Reinhardt D. P., Mecham R. P., Keeley F. W. (2008) Biochemistry 47, 12601–12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trask B. C., Broekelmann T., Ritty T. M., Trask T. M., Tisdale C., Mecham R. P. (2001) Biochemistry 40, 4372–4380 [DOI] [PubMed] [Google Scholar]
- 7.Trask T. M., Trask B. C., Ritty T. M., Abrams W. R., Rosenbloom J., Mecham R. P. (2000) J. Biol. Chem. 275, 24400–24406 [DOI] [PubMed] [Google Scholar]
- 8.Wagenseil J. E., Mecham R. P. (2007) Birth Defects Res. C Embryo Today. 81, 229–240 [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa H., Davis E. C., Starcher B. C., Ouchi T., Yanagisawa M., Richardson J. A., Olson E. N. (2002) Nature 415, 168–171 [DOI] [PubMed] [Google Scholar]
- 10.Deslee G., Woods J. C., Moore C. M., Liu L., Conradi S. H., Milne M., Gierada D. S., Pierce J., Patterson A., Lewit R. A., Battaile J. T., Holtzman M. J., Hogg J. C., Pierce R. A. (2009) Eur. Respir. J. 34, 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce R. A., Albertine K. H., Starcher B. C., Bohnsack J. F., Carlton D. P., Bland R. D. (1997) Am. J. Physiol. 272, L452–L460 [DOI] [PubMed] [Google Scholar]
- 12.Krettek A., Sukhova G. K., Libby P. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 582–587 [DOI] [PubMed] [Google Scholar]
- 13.Maeda I., Kishita S., Yamamoto Y., Arima K., Ideta K., Meng J., Sakata N., Okamoto K. (2007) J. Biochem. 142, 627–631 [DOI] [PubMed] [Google Scholar]
- 14.Rongioletti F., Rebora A. (1995) Dermatology 191, 19–24 [DOI] [PubMed] [Google Scholar]
- 15.Fearon I. M., Faux S. P. (2009) J. Mol. Cell. Cardiol. 47, 372–381 [DOI] [PubMed] [Google Scholar]
- 16.Madamanchi N. R., Vendrov A., Runge M. S. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 29–38 [DOI] [PubMed] [Google Scholar]
- 17.Singh U., Jialal I. (2006) Pathophysiology 13, 129–142 [DOI] [PubMed] [Google Scholar]
- 18.Osoata G. O., Hanazawa T., Brindicci C., Ito M., Barnes P. J., Kharitonov S., Ito K. (2009) Chest 135, 1513–1520 [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa H., Shiraishi S., Hirata K., Yoshikawa J. (2003) Thorax 58, 106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gole M. D., Souza J. M., Choi I., Hertkorn C., Malcolm S., Foust R. F., 3rd, Finkel B., Lanken P. N., Ischiropoulos H. (2000) Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L961–L967 [DOI] [PubMed] [Google Scholar]
- 21.Morton L. W., Puddey I. B., Croft K. D. (2003) Biochem. J. 370, 339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi Y., Matsuno S., Kagota S., Haginaka J., Kunitomo M. (2004) Atherosclerosis 172, 259–265 [DOI] [PubMed] [Google Scholar]
- 23.Petruzzelli S., Puntoni R., Mimotti P., Pulerà N., Baliva F., Fornai E., Giuntini C. (1997) Am. J. Respir Crit. Care. Med. 156, 1902–1907 [DOI] [PubMed] [Google Scholar]
- 24.Deslee G., Adair-Kirk T. L., Betsuyaku T., Woods J. C., Moore C. H., Gierada D. S., Conradi S. H., Atkinson J. J., Toennies H. M., Battaile J. T., Kobayashi D. K., Patterson G. A., Holtzman M. J., Pierce R. A. (2010) Am. J. Respir. Cell. Mol. Biol. 43, 576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deslee G., Woods J. C., Moore C., Conradi S. H., Gierada D. S., Atkinson J. J., Battaile J. T., Liu L., Patterson G. A., Adair-Kirk T. L., Holtzman M. J., Pierce R. A. (2009) Chest 135, 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreadis A. A., Hazen S. L., Comhair S. A., Erzurum S. C. (2003) Free Radic Biol. Med. 35, 213–225 [DOI] [PubMed] [Google Scholar]
- 27.Lenz A. G., Jorens P. G., Meyer B., De Backer W., Van Overveld F., Bossaert L., Maier K. L. (1999) Eur. Respir J. 13, 169–174 [DOI] [PubMed] [Google Scholar]
- 28.Wu W., Samoszuk M. K., Comhair S. A., Thomassen M. J., Farver C. F., Dweik R. A., Kavuru M. S., Erzurum S. C., Hazen S. L. (2000) J. Clin. Invest. 105, 1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R., Cantley L. G., Kerjaschki D., Neilson E. G. (2000) J. Biol. Chem. 275, 20027–20032 [DOI] [PubMed] [Google Scholar]
- 30.Lamb N. J., Gutteridge J. M., Baker C., Evans T. W., Quinlan G. J. (1999) Crit. Care. Med. 27, 1738–1744 [DOI] [PubMed] [Google Scholar]
- 31.Verzijl N., DeGroot J., Oldehinkel E., Bank R. A., Thorpe S. R., Baynes J. W., Bayliss M. T., Bijlsma J. W., Lafeber F. P., Tekoppele J. M. (2000) Biochem. J. 350, 381–387 [PMC free article] [PubMed] [Google Scholar]
- 32.Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W., Lafeber F. P., Baynes J. W., TeKoppele J. M. (2000) J. Biol. Chem. 275, 39027–39031 [DOI] [PubMed] [Google Scholar]
- 33.Clark R. A., Szot S., Williams M. A., Kagan H. M. (1986) Biochem. Biophys. Res. Commun. 135, 451–457 [DOI] [PubMed] [Google Scholar]
- 34.Laurent P., Janoff A., Kagan H. M. (1983) Chest 83, 63S-65S [DOI] [PubMed] [Google Scholar]
- 35.Broekelmann T. J., Kozel B. A., Ishibashi H., Werneck C. C., Keeley F. W., Zhang L., Mecham R. P. (2005) J. Biol. Chem. 280, 40939–40947 [DOI] [PubMed] [Google Scholar]
- 36.Werneck C. C., Vicente C. P., Weinberg J. S., Shifren A., Pierce R. A., Broekelmann T. J., Tollefsen D. M., Mecham R. P. (2008) Blood 111, 4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris J. C., Ping-Sheng L., Zhai H. X., Shen T. Y., Mensa-Wilmot K. (1996) J. Biol. Chem. 271, 15468–15477 [DOI] [PubMed] [Google Scholar]
- 38.Adair-Kirk T. L., Atkinson J. J., Kelley D. G., Arch R. H., Miner J. H., Senior R. M. (2005) J. Immunol. 174, 1621–1629 [DOI] [PubMed] [Google Scholar]
- 39.Davis E. C., Broekelmann T. J., Ozawa Y., Mecham R. P. (1998) J. Cell. Biol. 140, 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozel B. A., Ciliberto C. H., Mecham R. P. (2004) Matrix Biol. 23, 23–34 [DOI] [PubMed] [Google Scholar]
- 41.Miao M., Cirulis J. T., Lee S., Keeley F. W. (2005) Biochemistry 44, 14367–14375 [DOI] [PubMed] [Google Scholar]
- 42.Starcher B. C., Mecham R. P. (1981) Connect Tissue Res. 8, 255–258 [DOI] [PubMed] [Google Scholar]
- 43.Wachi H., Sato F., Murata H., Nakazawa J., Starcher B. C., Seyama Y. (2005) Clin. Biochem. 38, 643–653 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y. R., Deterding L. J., Sturgeon B. E., Tomer K. B., Mason R. P. (2002) J. Biol. Chem. 277, 29781–29791 [DOI] [PubMed] [Google Scholar]
- 45.Dröge W. (2002) Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 46.Weiss S. J. (1989) N. Engl. J. Med. 320, 365–376 [DOI] [PubMed] [Google Scholar]
- 47.Wachi H., Nonaka R., Sato F., Shibata-Sato K., Ishida M., Iketani S., Maeda I., Okamoto K., Urban Z., Onoue S., Seyama Y. (2008) J. Biochem. 143, 633–639 [DOI] [PubMed] [Google Scholar]
- 48.Jensen S. A., Reinhardt D. P., Gibson M. A., Weiss A. S. (2001) J. Biol. Chem. 276, 39661–39666 [DOI] [PubMed] [Google Scholar]
- 49.Franzblau C., Foster J. A., Faris B. (1977) Adv. Exp. Med. Biol. 79, 313–327 [DOI] [PubMed] [Google Scholar]
- 50.Narayanan A. S., Page R. C., Kuzan F. (1977) Adv. Exp. Med. Biol. 79, 491–508 [DOI] [PubMed] [Google Scholar]
- 51.Lin S. Y., Hsieh T. F., Wei Y. S. (2005) Peptides 26, 543–549 [DOI] [PubMed] [Google Scholar]
- 52.Wise S. G., Mithieux S. M., Raftery M. J., Weiss A. S. (2005) J. Struct Biol. 149, 273–281 [DOI] [PubMed] [Google Scholar]
- 53.Starcher B. C., Urry D. W. (1973) Biochem. Biophys. Res. Commun. 53, 210–216 [DOI] [PubMed] [Google Scholar]
- 54.Toonkool P., Jensen S. A., Maxwell A. L., Weiss A. S. (2001) J. Biol. Chem. 276, 44575–44580 [DOI] [PubMed] [Google Scholar]
- 55.Wu W. J., Vrhovski B., Weiss A. S. (1999) J. Biol. Chem. 274, 21719–21724 [DOI] [PubMed] [Google Scholar]
- 56.Tu Y., Weiss A. S. (2008) Biomacromolecules 9, 1739–1744 [DOI] [PubMed] [Google Scholar]
- 57.Giltay R., Timpl R., Kostka G. (1999) Matrix Biol. 18, 469–480 [DOI] [PubMed] [Google Scholar]
- 58.Choudhury R., McGovern A., Ridley C., Cain S. A., Baldwin A., Wang M. C., Guo C., Mironov A., Jr., Drymoussi Z., Trump D., Shuttleworth A., Baldock C., Kielty C. M. (2009) J. Biol. Chem. 284, 24553–24567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kozel B. A., Wachi H., Davis E. C., Mecham R. P. (2003) J. Biol. Chem. 278, 18491–18498 [DOI] [PubMed] [Google Scholar]
- 60.Brown-Augsburger P., Tisdale C., Broekelmann T., Sloan C., Mecham R. P. (1995) J. Biol. Chem. 270, 17778–17783 [DOI] [PubMed] [Google Scholar]