Abstract
Toll-like receptors (TLRs) associate with adaptor molecules (MyD88, Mal/TIRAP, TRAM, and TRIF) to mediate signaling of host-microbial interaction. For instance, TLR4 utilizes the combination of both Mal/TIRAP-MyD88 (MyD88-dependent pathway) and TRAM-TRIF (MyD88-independent pathway). However, TLR5, the specific receptor for flagellin, is known to utilize only MyD88 to elicit inflammatory responses, and an involvement of other adaptor molecules has not been suggested in TLR5-dependent signaling. Here, we found that TRIF is involved in mediating TLR5-induced nuclear factor κB (NFκB) and mitogen-activated protein kinases (MAPKs), specifically JNK1/2 and ERK1/2, activation in intestinal epithelial cells. TLR5 activation by flagellin permits the physical interaction between TLR5 and TRIF in human colonic epithelial cells (NCM460), whereas TLR5 does not interact with TRAM upon flagellin stimulation. Both primary intestinal epithelial cells from TRIF-KO mice and TRIF-silenced NCM460 cells significantly reduced flagellin-induced NFκB (p105 and p65), JNK1/2, and ERK1/2 activation compared with control cells. However, p38 activation by flagellin was preserved in these TRIF-deficient cells. TRIF-KO intestinal epithelial cells exhibited substantially reduced inflammatory cytokine (keratinocyte-derived cytokine, macrophage inflammatory protein 3α, and IL-6) expression upon flagellin, whereas control cells from TRIF-WT mice showed robust cytokine expression by flagellin. Compare with TRIF-WT mice, TRIF-KO mice were resistant to in vivo intestinal inflammatory responses: flagellin-mediated exacerbation of colonic inflammation and dextran sulfate sodium-induced experimental colitis. We conclude that in addition to MyD88, TRIF mediates TLR5-dependent responses and, thereby regulates inflammatory responses elicited by flagellin/TLR5 engagement. Our findings suggest an important role of TRIF in regulating host-microbial communication via TLR5 in the gut epithelium.
Keywords: Epithelial Cell, Inflammation, Intestine, Toll-like Receptor (TLR), TRIF
Introduction
Pattern-recognition receptors mediate recognition of microbes in multicellular organisms, leading to activation of innate and adaptive immune and inflammatory responses. Toll-like receptors (TLRs)3 are the most intensively characterized member of pattern-recognition receptors and detect microbe-associated molecular patterns present in a wide range of microorganisms (1). Upon recognition of microbes, TLRs recruit a single or a combination of adaptor molecule(s) containing a Toll-interleukin 1 receptor (TIR) domain to its cytoplasmic TIR domain. At least four TIR domain-containing adaptors (MyD88, Mal/TIRAP, TRAM, and TRIF) bind to TLRs to mediate TLR-dependent signaling (2). Subsequently, these adaptor molecules associate with IL-1 receptor-associated kinases to mediate the signaling to a member of tumor necrosis factor receptor-associated factor family (e.g. TRAF6). Thereby, TLR-induced signaling leads to activation of inhibitor κB kinases and MAPKs to activate transcription factors such as NFκB, activator protein-1, and interferon-regulatory factors (IRFs), followed by inducing a pleiotropic gene expression involved in immune and inflammatory responses (2–4).
TLR4, the specific receptor for lipopolysaccharide (LPS), utilizes an axis of the Mal/TIRAP-MyD88 pathway at the plasma membrane to induce inflammatory gene expression (MyD88-dependent pathway) and is concomitantly internalized to endosome by TRAM to exploit TRIF to elicit type I interferon production involved in anti-viral activity (MyD88-independent or TRIF-dependent pathway) (2, 5). TLR2 uses an axis of the Mal/TIRAP-MyD88 pathway to induce inflammatory gene expression. TLR3 exclusively couples with TRIF to induce type I interferon production.
TLR5, specifically recognizing flagellin, is involved in promoting the pathophysiology of inflammatory bowel disease by mediating a host-microbial interaction in the gut (6, 7). In addition, TLR5 is abundantly expressed in virtually most types of epithelial cells from various mucosal organs including the gastrointestinal tract (8, 9), lung (10), or uterus (11). A subset of lamina propria dendritic cells, CD11c+CD11b+, in the small intestine are also suggested to respond to flagellin (12). Unlike TLR4 which mediates both MyD88- and TRIF-dependent signaling, so far a single adaptor molecule MyD88 is known to solely mediate TLR5-induced responses to elicit inflammatory responses (13), and to the best of our knowledge, no TRIF-dependent signaling has been suggested for TLR5-dependent signaling.
Here, we demonstrate that in addition to MyD88, TRIF adaptor molecule also mediates TLR5-induced intracellular signaling and cytokine expression. Our findings suggest that like TLR4 mostly in immune cells, TLR5 utilizes both MyD88- and TRIF-dependent signaling pathways at least in intestinal epithelial cells.
EXPERIMENTAL PROCEDURES
Materials and Mice
Human colonic epithelial cells (NCM460) were cultivated as described previously (9, 14). NCM460-MyD88-knockdown (KD) cells and NCM460-TLR5-KD cells were described in our previous publication (14). Antibodies against human TRIF, phospho-ERK1/2, phospho-p38, phospho-Akt, phospho-p105 (NFκB), phospho-p65 (NFκB), ERK1/2, Akt, and MEK1/2 were purchased from Cell Signaling Technology (Danvers, MA). MyD88 antibody (HFL-296) was from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody recognizing phospho-JNK1/2 was from Invitrogen. HA and FLAG (M2) antibodies were from Roche Applied Science and Sigma-Aldrich, respectively. Flagellin purified from Salmonella typhimurium (<0.1 endotoxin unit/μg of purified protein determined by the Limulus Amebocyte Lysate test) was purchased from Enzo Life Sciences (Farmingdale, NY) and dissolved in endotoxin-free water. Human TRAM expression construct was obtained from InvivoGen (San Diego, CA), and the FLAG epitope tag was attached to the C terminus to generate the TRAM-FLAG expression construct. THP-1 cells were maintained in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin.
MyD88-KO mice (15) were kindly provided by Dr. Shuzio Akira (University of Osaka Medical School, Japan) and back-crossed into C57BL/6 mice for more than eight generations prior to use. Lps2, a nonfunctional co-dominant allele of TRIF, was previously induced by N-ethyl-N-nitrosourea mutagenesis on the C57BL/6 background mouse (16). TRIF-KO (TRIFLps2/Lps2), C57BL/6, C3H/HeJ, and C3H/HeOuJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and maintained in the animal facilities of the Division of Laboratory Animal Medicine, UCLA, under the approval by the Institutional Animal Care and Use Committee of UCLA.
Isolation and Culture of Primary Mouse Intestinal Epithelial Cells
We isolated primary intestinal epithelial cells from mouse small intestine by modifying previously published procedures (17, 18). Small intestine was dissected out, opened longitudinally, and washed in Ca2+- and Mg2+-free Hanks' balanced salt solution containing antibiotics (amphotericin B, 25 ng/ml, and penicillin-streptomycin, 100 units/ml). Intestines were cut into small fragments and then incubated for 10 min at room temperature with 20 ml of Hanks' balanced salt solution containing collagenase XΙa (60 units/ml), dispase (0.02 mg/ml), BSA (2%), and soybean trypsin inhibitor (0.2 mg/ml). Fetal bovine serum (FBS, 10%) was added and vigorously resuspended, followed by harvesting supernatants after 60-s sedimentations in sedimentation DMEM containing sorbitol (10%), FBS (5%), and antibiotics. The epithelial fragments were centrifuged at 300 rpm for 3 min, and the pellet was resuspended in DMEM containing sorbitol (2%) and FBS (2.5%). Small sheets of intestinal epithelium (organoids) were suspended in DMEM containing FBS (10%) with antibiotics and plated into mouse fibronectin (Innovative Research, Novi, MI) (3 μg/cm2)-coated dishes. Cells were cultured in 5% CO2 at 37 °C, and organoids were attached to a plate in 1–2 days. Cells were then cultivated in medium containing equal volumes of DMEM and Ham's 12 medium (Lonza, Basel, Switzerland), and FBS (10%) with antibiotics, and the medium was replaced every other day. Proliferative and viable cells were spread out from organoids. We were able to cultivate these cells over 4 weeks in culture dishes.
Silencing the Expression of MyD88 or TRIF in NCM460 Cells
The silencing vector expressing shRNA against human MyD88 or TRIF was obtained from InvivoGen. MyD88-KD NCM460 cells were described in our previous publication (14). For TRIF-KD cells, NCM460 cells were stably transfected with human TRIF shRNA expression vector, and individual clones were determined for silenced TRIF expression by immunoblot analysis. For MyD88/TRIF-double knockdown (MyD88/TRIF-2KD) cells, MyD88-KD cells were stably transfected with human TRIF shRNA expression construct. Multiple clones of the cells were obtained, and by immunoblot assay with TRIF antibody, we confirmed that endogenous TRIF expression was successfully knocked down in these cells. The scrambled shRNA control vector was stably transfected into NCM460 cells and used as the control cells.
DSS-induced Colitis and Flagellin-mediated Exacerbation of DSS-induced Colitis
DSS is known to directly disrupt the colonic epithelium causing colitis (19). For DSS-induced colitis model, mice were fed with DSS (MP Biomedicals, Santa Ana, CA) in regular drinking water, as described previously (7, 20). For the flagellin-exacerbated DSS-induced colitis as we previously described (7), mice were fed with DSS during the entire experimental period to compromise the integrity of intestinal epithelium. Four days after DSS administration, flagellin (0.8–1.0 μg/mice) was administered daily by rectal enema for additional 5 days. The survival rate was evaluated as described previously (7, 20).
Immunofluorescence Staining
Primary mouse intestinal epithelial cells harvested from mouse small intestine were plated on a mouse fibronectin-coated chamber slide. After 2 weeks of culture, cells were washed twice with PBS and fixed in methanol-acetone (1:1) for 10 min at −20 °C. Cells were washed with PBS and blocked with 1% normal goat serum and 0.1% Triton X-100 in PBS for 1 h at room temperature. Cells were then incubated overnight at 4 °C with primary antibodies: mouse anti-pancytokeratin monoclonal antibody (1:50 dilution, Sigma-Aldrich) or mouse anti-β-catenin monoclonal antibody (1:50 dilution, Sigma-Aldrich). Cells were washed three times with PBS and incubated with FITC-conjugated goat anti-mouse secondary antibody (1:100 dilution; Jackson ImmunoResearch Laboratory) for 1 h at room temperature. Cells were then rinsed and mounted with DAPI mounting solution. Images were visualized with an Axio Imager Z1 microscope (Carl Zeiss) and captured with an AxioCam MRm digital camera and processed with Adobe Photoshop.
Cell Transfection
Human colonic epithelial cells (NCM460) were plated in 60-mm dishes (1.5 × 106 cells) and stabilized overnight. Using SuperFect transfect reagent from Qiagen (Valencia, CA), cells were transfected with the appropriate plasmid DNA. A consistent amount of total DNA was kept by adding the empty vector to each transfection. One day after transfection, cell lysates were prepared in lysis buffer. To generate stably transfected cells, cells were plated in the proper medium containing selecting antibiotics.
Measuring KC, IL-6, Macrophage Inflammatory Protein 3α, and IFN-β Production
An enzyme-linked immunosorbent assay (ELISA) was performed to measure the level of the cytokine expression using the appropriate kits from BIOSOURCE International (Camarillo, CA) and R&D Systems (Minneapolis, MN) by following the manufacturer's instructions. All assays were performed in triplicate, and data are shown as mean ± S.D.
Luciferase Reporter Assays and Immunoblot Assay
These assays were performed as described in our previous publications (9, 14).
RESULTS
TLR5 Interacts with TRIF, but Not with TRAM upon Flagellin Stimulation
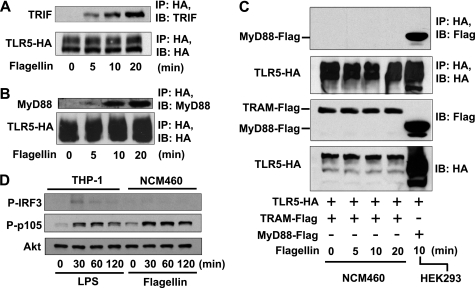
Flagellin stimulation allows TLR5 to recruit MyD88 adaptor molecule to its cytoplasmic TIR domain to mediate MyD88-dependent signaling in intestinal epithelial cells (9, 14). To test whether TRIF adaptor molecule, mediating MyD88-independent signaling of TLRs, participates in TLR5-dependent responses in intestinal epithelial cells, we first examined whether TLR5 is able to interact physically with TRIF in response to flagellin. Human colonic epithelial cells (NCM460) were stably transfected with HA-tagged TLR5 expression construct (TLR5-HA) to generate NCM460-TLR5-HA cells because a specific antibody for an immunoprecipitation of endogenous TLR5 protein is not presently available. Co-immunoprecipitation analysis between TLR5-HA and endogenous TRIF or MyD88 showed that flagellin stimulation induced an interaction between TLR5 and TRIF (Fig. 1A) as well as an interaction between TLR5 and MyD88 (Fig. 1B) in NCM460 cells.
FIGURE 1.
TLR5 interacts with TRIF and MyD88, but not with TRAM upon flagellin stimulation in human colonic epithelial (NCM460) cells. A and B, NCM460 cells transfected with TLR5-HA were stimulated with flagellin (100 ng/ml). Lysates were immunoprecipitated (IP) using HA antibody and immunoblotted (IB) using antibodies to endogenous TRIF (A) and MyD88 (B). C, NCM460 cells were stably co-transfected with TLR5-HA and TRAM-FLAG constructs. HEK293 cells were co-transfected with TLR5-HA and MyD88-FLAG constructs. After flagellin stimulation, cell extracts were immunoprecipitated using HA antibody and immunoblotted using FLAG antibody. D, THP-1 cells and NCM460 cells were stimulated with LPS (1.0 μg/ml) and flagellin, respectively. Total cell extract was used for immunoblotting to evaluate IRF3 phosphorylation (P-IRF3) and NFκB activation (P-p105). Akt blotting was measured as a loading control. All data are representative of at least three independent experiments.
In the case of TLR4, an adaptor molecule TRAM is recruited to the cytoplasmic domain of TLR4 prior to interacting with TRIF upon LPS stimulation (5). Having found that TLR5 interacts with TRIF in response to flagellin, we next examined whether flagellin stimulation induces the interaction between TLR5 and TRAM. NCM460 cells were co-transfected with TLR5-HA and TRAM-FLAG constructs, followed by flagellin stimulation for co-immunoprecipitation assay. We found that TLR5 did not recruit TRAM upon flagellin stimulation, whereas flagellin stimulation resulted in an evident interaction between TLR5 and MyD88 (Fig. 1C).
These results demonstrate that in addition to interacting with MyD88, TLR5 also recruits TRIF adaptor molecule upon flagellin stimulation. This finding suggests that TRIF physically interacts with TLR5 and thereby participates in mediating TLR5-dependent responses. Intriguingly, we could not observe the phosphorylation of IRF3 in flagellin-treated NCM460 cells, whereas LPS stimulation clearly induces IRF-3 phosphorylation in human monocytes (THP-1) (Fig. 1D). This observation implies that TRIF adaptor in the TLR5-dependent signaling pathways may not be implicated in activating IRF3 as it is elsewhere.
Silencing TRIF Expression Reduces TLR5-induced NFκB, JNK1/2, and ERK1/2 Activation in Human Colonic Epithelial Cells
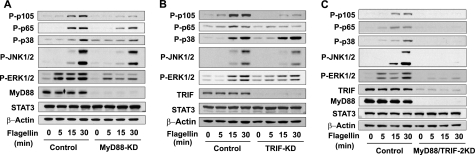
The interaction between TLR5 and TRIF upon flagellin prompted us to investigate whether TRIF is involved in mediating TLR5-dependent signaling including NFκB and MAPK activation in intestinal epithelial cells. To study this, using NCM460 cells, we generated MyD88-KD cells as previously reported (14). As expected, NFκB activation (evaluated by phosphorylation of p105 and p65) by flagellin was reduced in MyD88-KD cells, compared with control cells that were stably transfected with scrambled shRNA encoding construct (Fig. 2A). Similarly, impaired MyD88 expression resulted in reduced MAPK (p38, JNK1/2, and ERK1/2) activation in response to flagellin compared with control cells.
FIGURE 2.
Silencing TRIF expression reduces TLR5-dependent signaling in NCM460 cells. A, MyD88-KD NCM460 cells (14) and the control cells transfected with the scrambled shRNA construct were stimulated with flagellin (100 ng/ml), followed by evaluating NFκB (P-p105 and P-p65) and MAPK (p38, JNK1/2, and ERK1/2) activation. Silencing MyD88 expression was also confirmed. B, TRIF-KD NCM460 cells were generated by stably transfecting human TRIF shRNA expression construct into NCM460 cells. TLR5-induced signaling was examined in TRIF-KD cells. Silenced TRIF expression was confirmed by immunoblotting. C, MyD88/TRIF-2KD cells were generated by stably transfecting TRIF shRNA encoding construct into MyD88-KD cells. Flagellin-induced NFκB and MAPK activation was examined by immunoblot analysis. Proper loading controls for the immunoblotting were included as indicated. Data presented are representative experiments of at least three independent experiments.
To determine whether TRIF is involved in TLR5-induced responses as suggested by flagellin-induced TLR5-TRIF interaction, we generated TRIF-KD NCM460 cells (Fig. 2B). Notably, silencing TRIF expression substantially reduced flagellin-induced NFκB (p105 and p65) activation (Fig. 2B). The activation of JNK1/2 and ERK1/2 by flagellin was also reduced in TRIF-KD cells compared with control cells. However, TRIF-KD and control cells exhibited a similar extent of p38 activation in response to flagellin.
Next, we generated MyD88 and TRIF-double knockdown (MyD88/TRIF-2KD) NCM460 cells (Fig. 2C) and tested TLR5-induced signaling. We found that flagellin-stimulated NFκB activation was almost completely abolished in MyD88/TRIF-2KD cells (Fig. 2C). Furthermore, the activation of MAPKs, including JNK1/2, ERK1/2, and p38 was dramatically diminished in MyD88/TRIF-2KD cells compared with control cells. Our data show that the inhibitory levels of NFκB and MAPK activation are much greater in MyD88/TRIF-2KD cells than in MyD88-KD or TRIF-KD cells, indicating that the defect in both MyD88 and TRIF effectively hampers TLR5-dependent signaling. These results indicate a possibility that in addition to MyD88, TRIF may mediate TLR5-induced responses in human colonic epithelial cells.
TRIF Deficiency Inhibits TLR5-induced Signaling in Primary Mouse Intestinal Epithelial Cells
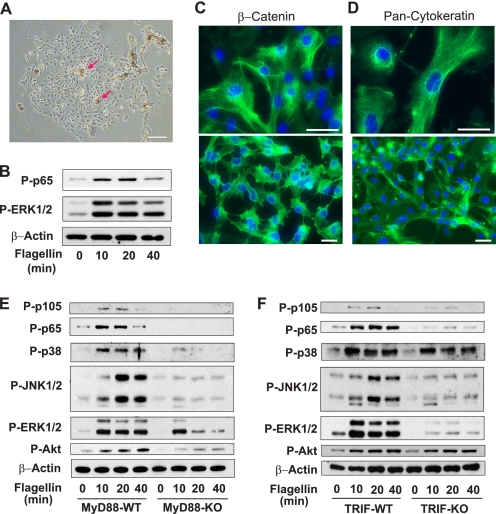
To demonstrate further the involvement of TRIF in TLR5 signaling, we isolated primary intestinal epithelial cells from TRIF-KO, MyD88-KO, and their WT control mice. Intestinal epithelial organoids (small sheets of intestinal epithelium) harvested from mouse small intestine were attached to the surface of fibronectin-coated culture dishes. We observed that proliferative and viable cells were spread out from these organoids (Fig. 3A). In about 2 weeks after plating, most of cells showed typical primary epithelial cell morphology and were developed into compact cobblestoned monolayer. In agreement with potent flagellin responses in various types of epithelial cells (9–11), these cells from mouse intestine responded to flagellin stimulation, evaluated by the phosphorylation of p65 and ERK1/2 (Fig. 3B). To confirm whether these primary cells are intestinal epithelial cells, we subsequently performed immunofluorescence staining against intestinal epithelial cell markers such as cytokeratin and β-catenin (21). We found that more than 95% of cells in coalesced and confluent or separated and sparse areas were stained with β-catenin and cytokeratin (Fig. 3, C and D). Taken together, these results indicate that these primary cells possess characteristics of intestinal epithelial cells.
FIGURE 3.
TRIF deficiency inhibits TLR5-induced signaling in primary mouse intestinal epithelial cells. A, epithelial organoids (red arrows) were harvested from small intestine of C57BL/6 mice and attached to fibronectin-coated culture surfaces. Proliferative and viable cells were spread out from these organoids in 4–7 days. A representative image was obtained in 2 weeks after plating. Scale bar, 200 μm. B, the cells from small intestine exhibited evident responses to flagellin (100 ng/ml) as shown by NFκB (P-p65) and MAPK (P-ERK1/2) activation. C and D, the cells from mouse small intestine were immunostained with monoclonal antibodies against epithelial cell markers, β-catenin or cytokeratin (21) (green). DAPI staining (blue) represents nuclei. Scale bars, 50 μm. E and F, primary intestinal epithelial cells from MyD88-KO (E), TRIF-KO (F), and WT (C57BL/6) mice were stimulated with flagellin (100 ng/ml) for the indicated time, followed by immunoblot assays. All data are representative of at least three independent experiments. β-Actin served as a loading control.
Using primary intestinal epithelial cells isolated from MyD88-KO and WT mice, we examined whether MyD88 deficiency is sufficient to block TLR5-induced signaling. Flagellin stimulation induced activation of NFκB and MAPKs in the WT cells (Fig. 3E). As expected, in MyD88-KO cells, NFκB activation by flagellin was dramatically inhibited. Surprisingly, MyD88 deficiency, however, failed to block TLR5-induced MAPK activation completely (Fig. 3E). Rather, JNK1/2 and p38 activation were still detectable in MyD88-KO cells, although the level was significantly decreased. ERK1/2 activation was still relatively robust, albeit slightly reduced in MyD88-KO cells. These results imply that MyD88 deficiency is not sufficient to block TLR5-induced signaling completely. Moreover, our findings also imply that a MyD88-independent pathway should exist to mediate TLR5-induced signaling, which circumvents the absence of MyD88 in TLR5-induced signaling pathways.
TRIF adaptor molecule mediates the signaling pathway of TLR4 and TLR3 in a MyD88-independent manner. We show that TLR5 interacts with TRIF upon flagellin stimulation (Fig. 1A), and silencing TRIF expression significantly reduces TLR5-induced responses (Fig. 2B) in human colonic epithelial cells. Based on these findings, using primary intestinal epithelial cells isolated from TRIF-KO and WT mice, we tested whether TRIF is involved in TLR5-induced signaling. Although flagellin stimulation nicely induced the activation of NFκB and MAPKs in WT cells, TRIF-KO cells exhibited dramatically reduced NFκB and MAPKs (specifically JNK1/2 and ERK1/2) activation in response to flagellin (Fig. 3F). In contrast, activation of p38 by flagellin was mostly preserved in TRIF-KO cells compared with WT cells. This result is in agreement with preserved p38 activation in TRIF-KD cells upon flagellin (Fig. 2B). Thus, TLR5-mediated p38 activation appears to be TRIF-independent and seems to be primarily governed by MyD88-dependent pathways.
Similarly, Akt phosphorylation by flagellin in TRIF-KO cells is comparable with that of WT cells, whereas it was markedly reduced in MyD88-KO cells. This result suggests that like p38 activation, Akt activation by flagellin should be primarily regulated by MyD88-dependent pathways. This finding is in agreement with our previous study demonstrating that MyD88 mediates the activation of the PI3K-Akt pathway of TLR5 (14). Taken together, these data substantiate that in addition to MyD88, TRIF mediates TLR5-induced responses in intestinal epithelial cells.
Flagellin-induced Activation of NFκB and MAPKs in the Intestinal Epithelial Cells Is Not Attributed to LPS Contamination
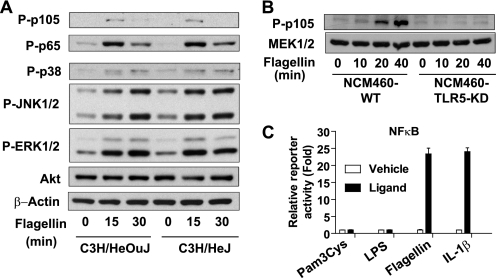
Although the above results indicate that TRIF mediates TLR5 signaling in intestinal epithelial cells, it may be possible that a potential contaminant (e.g. LPS) probably included in the flagellin preparation would have stimulated TLR5-independent responses and caused remaining MAPK activation in MyD88-KO cells (Fig. 3E). To address this concern, we performed several experiments. First, we isolated primary intestinal epithelial cells from TLR4-null mutant (C3H/HeJ) and their genetic control (C3H/HeOuJ) mice, followed by stimulating these cells with flagellin. We found that the levels of NFκB and MAPK activation by flagellin in TLR4-null cells are similar to those in WT cells (Fig. 4A). Thus, these results indicate that flagellin-induced responses in primary intestinal epithelial cells are not compounded by LPS/TLR4 engagement and exclude a possibility that potential LPS contamination in the flagellin preparation might contribute to NFκB and MAPK activation in the intestinal epithelial cells. Second, to determine whether the response activated by flagellin is specifically mediated by TLR5, we used TLR5-KD NCM460 cells, which have been previously described (13, 14). We found that flagellin-induced NFκB activation was dramatically inhibited in TLR5-KD cells, whereas it was markedly induced in WT cells (Fig. 4B). These results indicate that the activated signaling by the flagellin preparation is specifically mediated by TLR5. Third, using a NFκB reporter activity assay, we showed that NCM460 cells are potently responsive to TLR5 ligand (flagellin), but nonresponsive to TLR2 (Pam3Cys) or TLR4 (LPS) stimulation (Fig. 4C). Taken together, our data obtained from these three experiments clearly indicate that flagellin-induced responses are specifically mediated by TLR5, but not compounded by TLR4. Therefore, the remaining signaling in flagellin-treated MyD88-KO cells is not attributed to a potential contaminant such as LPS.
FIGURE 4.
Activated intracellular signaling by the flagellin preparation is not affected by the potential LPS contaminant, but rather is specifically mediated by TLR5. A, primary mouse intestinal epithelial cells from C3H/HeJ (TLR4-null) and its control C3H/HeOuJ mice were stimulated with the flagellin preparation (100 ng/ml), followed by evaluating NFκB and MAPK activation. B, TLR5-KD NCM460 and its control cells (14) were stimulated with the flagellin preparation. NFκB activation was abolished in TLR5-KD cells, whereas strong NFκB activation was observed in control cells. C, NCM460 cells are not responsive to TLR2 (Pam3Cys, 2.0 μg/ml) and TLR4-specific ligand (LPS, 2.0 μg/ml), but strongly responsive to TLR5 specific ligand (flagellin, 100 ng/ml). IL-1 receptor ligand (IL-1β, 50 ng/ml) was used as a positive control of NFκB-luciferase reporter assay in NCM460 cells. Error bars indicate ±S.D. All data are representative of at least three independent experiments.
TRIF Deficiency Inhibits Flagellin-induced Inflammatory Cytokine Expression
Having found that TRIF deficiency negatively regulates some of the TLR5-dependent intracellular signaling, we tested whether inflammatory cytokine expression induced by flagellin/TLR5 engagement is altered in the absence of TRIF expression. For this purpose, we stimulated primary intestinal epithelial cells from TRIF-KO, MyD88-KO, and WT mice with flagellin, followed by measuring TLR5-induced cytokine production. As expected, the levels of KC (mouse ortholog of human IL-8), macrophage inflammatory protein 3α, and IL-6 expression were inhibited in MyD88-KO cells compared with the levels in WT cells (Fig. 5, A–C). Notably, the cytokine expression was also dramatically suppressed in TRIF-KO cells compared with the expression level in WT cells. These results are in agreement with our findings demonstrating that NFκB and MAPK activation by flagellin is reduced in TRIF-KD and TRIF-KO cells (Figs. 2B and 3F).
FIGURE 5.
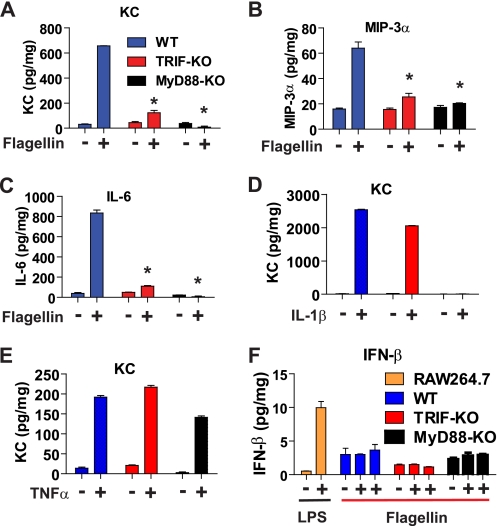
TRIF deficiency inhibits flagellin-induced cytokine expression. A–C, primary mouse intestinal epithelial cells from WT, MyD88-KO, and TRIF-KO mice were stimulated with flagellin (100 ng/ml, 8 h). KC (A), macrophage inflammatory protein 3α (MIP-3α) (B), and IL-6 (C) expression was measured by ELISA. D and E, WT, MyD88-KO, and TRIF-KO cells were stimulated with IL-1β (100 ng/ml, 8 h) (D) and TNFα (100 ng/ml, 8 h) (E), respectively. The expression of KC was measured by ELISA. F, when primary intestinal epithelial cells were stimulated with flagellin, in parallel, mouse monocytes/macrophages (RAW264.7) were stimulated with LPS (1.0 μg/ml, 8 h) to elicit IFN-β expression which was used as a positive control for IFN-β ELISA (D). Error bars indicate ±S.D. *, p < 0.01 (versus WT) as determined by one-way ANOVA followed by Bonferroni's t test.
When the primary intestinal epithelial cells are stimulated with IL-1β mediating its responses via IL-1R in a MyD88-dependent manner, IL-1β stimulation failed to induce KC cytokine expression in MyD88-KO cells, whereas it strongly induced the cytokine expression in both TRIF-KO cells and WT cells (Fig. 5D). Moreover, TNFα, utilizing non–TLR-associated signaling pathways, potently elicited the cytokine expression in the primary intestinal epithelial cells from MyD88-KO, TRIF-KO, and WT mice (Fig. 5E). These results assure that the diminished cytokine expression in flagellin-stimulated MyD88-KO and TRIF-KO cells is not due to any nonspecific effects that might allow the primary cells to be inherently less responsive to any external stimuli.
It is known that TRIF-mediated signaling leads to IFN-β expression upon TLR3 or TLR4 engagement. Thus, we tested whether flagellin/TLR5 engagement elicits IFN-β expression via TRIF in primary intestinal epithelial cells. Intriguingly, flagellin stimulation did not induce IFN-β expression in these cells, whereas LPS/TLR4 engagement strongly up-regulated IFN-β expression in mouse monocytes/macrophages (RAW264.7) (Fig. 5F). These results imply that TRIF may not be associated with IFN-β expression in TLR5-dependent responses in intestinal epithelial cells, whereas TLR3 or TLR4 utilizes TRIF to induce IFN-β expression in immune cells. Given that TRIF deficiency blocks flagellin-induced inflammatory cytokine expression, our data imply that the involvement of TRIF in TLR5-dependent responses could be associated with regulating inflammatory responses triggered by microbial recognition via TLR5.
TRIF Deficiency Confers Protective Effects in TLR5-associated Experimental Colitis
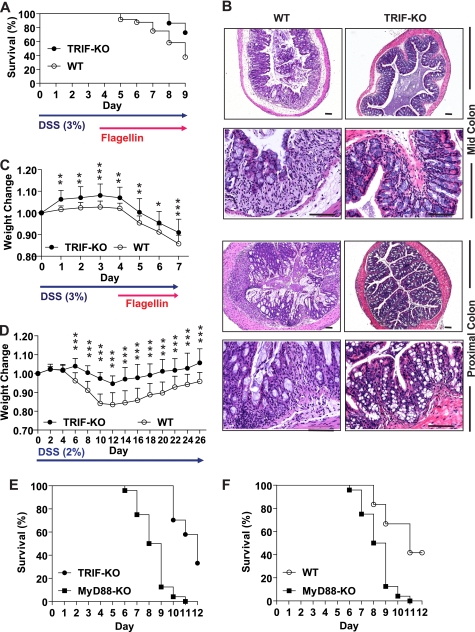
TRIF and MyD88 are key adaptor molecules to mediate MyD88-independent and MyD88-dependent signaling of TLRs, respectively. MyD88 deficiency is known to exacerbate DSS-induced experimental mouse colitis (22), whereas an effect of TRIF on intestinal inflammation still needs to be examined. Aberrant activation of TLR5 is known to associate with the development and progress of intestinal inflammation (6, 7). Indeed, we and other groups showed that activation of TLR5 by flagellin exacerbates DSS-induced colitis, suggesting a key role of TLR5-dependent signaling in the pathophysiology of colitis (7, 23). Given that TRIF appears to regulate inflammatory cytokine expression rather than IFN-β expression in flagellin-stimulated intestinal epithelial cells (Fig. 5), we tested whether TRIF deficiency alters the pathogenesis of flagellin-associated mouse colitis. To address this, TRIF-KO and their WT control mice were fed with DSS (3%) for 9 days and also treated with a flagellin enema for the last 5 days. The survival rate of TRIF-KO mice was significantly improved compared with that of WT mice (survival ratio: 72% of TRIF-KO versus 39% of WT mice at day 9) (Fig. 6A). Histological damages of colonic epithelium were more severe in WT mice than in TRIF-KO mice (Fig. 6B). We also found that TRIF-KO mice were more resistant to body weight loss than their WT control mice (weight loss: ∼9.0% of TRIF-KO versus ∼14.3% of WT mice at day 7) in this experimental setting (Fig. 6C). These results suggest that TRIF deficiency ameliorates flagellin-mediated exacerbation of DSS-induced colitis.
FIGURE 6.
TRIF deficiency confers protective effects against DSS-induced colitis and flagellin-mediated exacerbation of DSS-induced colitis. A–C, flagellin-exacerbated DSS-induced colitis was relieved in TRIF-KO mice compared with WT mice. TRIF-KO (n = 14) and WT (n = 21) mice were fed with DSS (3%) for total 9 days, and mice were treated with flagellin enema (0.8–1.0 μg/mouse/day) during the last 5 days. A, log-rank test was used to compare significant survival difference, followed by the multiple-comparison Bonferroni method (p < 0.01) B, representative H&E-stained mid- and proximal colon tissues were prepared from flagellin-exacerbated DSS-induced colitis of TRIF-KO and WT mice presented in A. Scale bars, 100 μm. C, body weight change for these mice was evaluated. D, TRIF-KO (n = 16) and WT (n = 16) mice were fed with a sublethal dose (2%) of DSS over 26 days to induce mild colitis, and weight change was measured. Data for body weight change were compared by ANOVA, followed by the multiple-comparison Bonferroni t test to assess differences between groups. E and F, MyD88-KO mice are more susceptible to DSS-induced colitis than TRIF-KO and WT mice. TRIF-KO (n = 27), MyD88-KO (n = 24), and WT (C57BL/6) (n = 12) mice were provided with DSS (4%) to induce colitis. Difference in the survival is shown by a Kaplan-Meier plot. The log-rank test was used to compare significant survival difference between MyD88-KO and TRIF-KO mice group (E) and MyD88-KO and WT mice group (F), and followed by the multiple-comparison Bonferroni method (p < 0.0001; ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Furthermore, we examined whether in the absence of flagellin enema, TRIF deficiency would alter the pathophysiology of colitis represented by body weight loss at a chronic mouse colitis model induced by low and sublethal dose of DSS (2%) feeding for 26 days. We found that TRIF-KO mice are more resistant to body weight loss than their WT control mice in this colitis model (weight loss: 5.4% of TRIF-KO mice versus 17.5% of WT mice at day 12) (Fig. 6D). Moreover, the weight loss was less evident and recovered better in TRIF-KO mice than in WT mice. These results suggest that TRIF deficiency makes the mouse tolerable to chronic colitis induced by low dose DSS feeding.
When TRIF-KO mice are compared with MyD88-KO mice in acute DSS (4%)-induced colitis, we found that TRIF-KO mice are more resistant to DSS-induced colitis than MyD88-KO mice (Fig. 6E). Given the fact that MyD88-KO mice are highly susceptible to DSS (4%)-induced colitis (Fig. 6F), TRIF and MyD88 seem to play a different role at least in DSS-induced colitis. Together, these results suggest that TRIF deficiency may provide protective effects on DSS-induced colitis as well as flagellin-mediated exacerbation of DSS-induced colitis.
In summary, our results suggest that in addition to MyD88, TRIF physically interacts with TLR5 upon flagellin stimulation and participates in mediating TLR5-induced NFκB and MAPK (specifically JNK1/2 and ERK1/2) activation, consequently regulating inflammatory cytokine expression at least in intestinal epithelial cells.
DISCUSSION
Intestinal epithelial cells are in continuous contact with enteric microbes at the front line of host-microbial interaction inside the gut. Given that TLR2 and TLR4 responses are almost negligible in many intestinal epithelial cell lines (13, 14, 24, 25) and most epithelial cell lines are strongly responsive to flagellin via TLR5 (9–11), TLR5 appears to play an important role in a communication between the intestinal epithelium and enteric microbes. Indeed, TLR5 contributes to preserving crypt stem cell proliferation in the intestine and thereby, protecting the gastrointestinal tract from radiation-induced damages (26). On the other hand, aberrant activation of TLR5 by flagellin is associated with developing intestinal inflammation (6, 7). Thus, the engagement of TLR5 in the gut seems to provide one of the inevitable means to regulate intestinal homeostasis. Regarding the molecular mechanism by which TLR5 mediates intracellular signaling, the immediate adaptor molecule MyD88 is an essential component. Therefore, blocking MyD88 expression is known to down-regulate most TLR5-induced responses (13, 14). Other than MyD88, an involvement of any other adaptor molecules such as TRIF has not been suggested in TLR5-dependent signaling.
The present results suggest that in addition to MyD88, TRIF participates in mediating TLR5-induced intracellular signaling in intestinal epithelial cells. To substantiate this notion, we present three pieces of evidence: First, flagellin stimulation induces a physical interaction between TLR5 and TRIF in a time-dependent manner (Fig. 1A). Second, impaired TRIF expression reduces TLR5-induced NFκB and MAPK activation in response to flagellin, whereas WT cells were featured with potent activation of the signaling (Figs. 2 and 3). Third, TRIF deficiency inhibited flagellin-induced cytokine expression, compared with WT cells (Fig. 5). This combined evidence strongly supports that TRIF is another adaptor molecule mediating TLR5-induced responses at least in intestinal epithelial cells.
Although MyD88- and TRIF-mediated signaling pathways of TLR5 are able to activate NFκB and MAPKs in common and thereby regulate flagellin-induced cytokine expression, they seem to play a different physiological role in the intestinal epithelium. As reported previously (22), MyD88-KO mice are highly susceptible to DSS-induced colitis (Fig. 6, E and F). In contrast, TRIF-KO mice appear to be moderately resistant to DSS-induced colitis (Fig. 6D). According to the previous study (22), MyD88 deficiency results in reduced epithelial cell proliferation in the intestine, which renders MyD88-KO mice more susceptible to DSS-induced colitis. Indeed, our recent study demonstrates that in the TLR5-induced signaling pathway, MyD88 is linked to activate the PI3K-Akt signaling pathway which governs cell survival and growth (14). Our data also showed that MyD88-KO cells have reduced Akt activation upon flagellin stimulation (Fig. 3E). By analogy, we believe that MyD88 deficiency could inhibit PI3K-Akt signaling, resulting in reduced intestinal epithelial cell survival and growth. In this context, MyD88-KO mice would be characterized with enhanced susceptibility to DSS-induced colitis. In contrast, TRIF deficiency does not alter flagellin-induced Akt activation compared with WT cells (Fig. 3F). Instead, together with reduced inflammatory cytokine expression in flagellin-treated TRIF-KO epithelial cells, TRIF-KO mice are featured with better prognosis for DSS-induced colitis than WT mice (Fig. 6D). Based on these observations, it appears that the MyD88-mediated signaling is poised to govern cell survival and proliferation, whereas the TRIF-mediated signaling could be prone to regulate inflammatory responses in intestinal epithelial cells.
Given that TRIF is involved in signaling from other TLRs that may be involved in the intestinal inflammation, in addition to TLR5, it is possible that the protective effect provided by TRIF deficiency in DSS-induced colitis would be associated with other TLRs. However, TLR5 activation by flagellin administration in DSS-treated mice worsens colitis (7, 23), suggesting the involvement of TLR5 activation in intestinal inflammatory responses. Flagellin-enhanced colitis is relieved in TRIF-KO mice compared with WT mice (Fig. 6, A–C). These considerations imply that the TRIF-mediated response in the intestine is associated with TLR5-dependent signaling.
Even though TLR5 residing in the plasma membrane is a specific receptor for flagellin, Ipaf, one of the nucleotide-binding oligomerization domain–leucine-rich repeat family proteins (called NLRs), has been also suggested to recognize flagellin in the cytosol of macrophages to activate caspase-1 (27). Thus, there may be a possibility that the remaining signaling in flagellin-treated MyD88-KO cells might be due to the flagellin recognition by Ipaf in cytosol. However, such possibility is unlikely in our experimental setting, because (i) TLR5 physically interacts with TRIF upon flagellin stimulation in a time-dependent manner in intestinal epithelial cells (Fig. 1); (ii) Ipaf does not associate with TRIF; instead it interacts with caspase-1 adaptor ASC to activate caspase-1 (27, 28); (iii) to activate cytosolic Ipaf, purified flagellin should be delivered into the cell cytosol by protein transduction system such as liposome or pore-forming protein (29, 30). In our experiments, none of the protein delivery systems was used for stimulating the cells with purified flagellin, which should activate TLR5 at the plasma membrane. Together, these results clearly indicate that TLR5 couples with TRIF to mediate the intracellular signaling generated from flagellin-TLR5 engagement.
The involvement of TRIF in TLR signaling primarily activates IRF-3 to induce IFN-β expression for antiviral defense. Concomitantly, it also leads to NFκB activation to regulate various cytokine expressions. Although our data show that TRIF participates in TLR5-induced responses, we could not see IRF-3 activation in intestinal epithelial cells by flagellin stimulation. Moreover, flagellin stimulation did not induce IFN-β expression in intestinal epithelial cells (Fig. 5F). Thus, it appears that TRIF in TLR5-induced responses may be associated with regulating NFκB and MAPK activation rather than IRF-3 activation and IFN-β expression, and this subsequently mediates TLR5-dependent inflammatory responses.
Meanwhile, our recent study showed that enhanced TRIF expression leads to caspase activation, which in turn degrades TLR5 protein and consequently inhibits TLR5-induced responses in NCM460 or HEK293 cells (31). Given that the engagement of TRIF in TLR-dependent signaling results in caspase activation which is in parallel to TLR-induced NFκB and MAPK activation pathways (32), the involvement of TRIF in flagellin/TLR5-elicited responses is probably able to provoke caspase activation which subsequently causes TLR5 protein degradation. Based on this information, in addition to the TRIF adaptor mediating NFκB and MAPK activation in TLR5-dependent signaling, it is likely that TRIF contributes to a negative feedback loop in flagellin/TLR5-induced responses by eliciting caspase-dependent TLR5 protein degradation.
In summary, our data demonstrate that TRIF is an adaptor molecule involved in TLR5-induced responses at least in the intestinal epithelial cells. Because TLR5 is an important microbial recognition molecule in the gastrointestinal tract, our findings suggest an important role of TRIF in regulating host-microbial communication via TLR5 in the gut epithelium.
Acknowledgment
We thank Dr. S. Akira (Osaka University, Japan) for MyD88-KO mice.
This work was supported, in whole or in part, by National Institutes of Health Grants DK072471 (to C. P.), DK083336 (to E. I.), and DK079015 (S. H. R.). This work was also supported by a Young Clinical Scientist Award (to S. H. R. and E. I.) from the Flight Attendant Medical Research Institute, Inc.
- TLR
- Toll-like receptor
- DSS
- dextran sulfate sodium
- IRF
- IFN-regulated factor
- KC
- keratinocyte-derived cytokine
- KD
- knockdown
- 2KD
- double knockdown
- TIR
- Toll-interleukin 1 receptor
- Mal
- MyD88 adaptor-like
- MyD88
- myeloid differentiation factor 88
- TIRAP
- TLR/IL1R-domain-containing adaptor protein
- TRAM
- TRIF-related adaptor molecule
- TRIF
- TIR domain-containing adaptor-inducing IFN-β.
REFERENCES
- 1.Palm N. W., Medzhitov R. (2009) Immunol. Rev. 227, 221–233 [DOI] [PubMed] [Google Scholar]
- 2.Kenny E. F., O'Neill L. A. (2008) Cytokine 43, 342–349 [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. (2007) Nature 449, 819–826 [DOI] [PubMed] [Google Scholar]
- 4.Kawai T., Akira S. (2008) Ann. N.Y. Acad. Sci. 1143, 1–20 [DOI] [PubMed] [Google Scholar]
- 5.Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) Nat. Immunol. 9, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodes M. J., Cong Y., Elson C. O., Mohamath R., Landers C. J., Targan S. R., Fort M., Hershberg R. M. (2004) J. Clin. Invest. 113, 1296–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee S. H., Im E., Riegler M., Kokkotou E., O'Brien M., Pothoulakis C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13610–13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gewirtz A. T., Navas T. A., Lyons S., Godowski P. J., Madara J. L. (2001) J. Immunol. 167, 1882–1885 [DOI] [PubMed] [Google Scholar]
- 9.Rhee S. H., Keates A. C., Moyer M. P., Pothoulakis C. (2004) J. Biol. Chem. 279, 25179–25188 [DOI] [PubMed] [Google Scholar]
- 10.Blohmke C. J., Victor R. E., Hirschfeld A. F., Elias I. M., Hancock D. G., Lane C. R., Davidson A. G., Wilcox P. G., Smith K. D., Overhage J., Hancock R. E., Turvey S. E. (2008) J. Immunol. 180, 7764–7773 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer T. M., Desouza K., Fahey J. V., Beagley K. W., Wira C. R. (2004) Immunology 112, 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu S., Fujimoto K., Jang M. H., Yang B. G., Jung Y. J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K. J., Akira S. (2008) Nat. Immunol. 9, 769–776 [DOI] [PubMed] [Google Scholar]
- 13.Rhee S. H., Im E., Pothoulakis C. (2008) Gastroenterology 135, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee S. H., Kim H., Moyer M. P., Pothoulakis C. (2006) J. Biol. Chem. 281, 18560–18568 [DOI] [PubMed] [Google Scholar]
- 15.Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. (1998) Immunity 9, 143–150 [DOI] [PubMed] [Google Scholar]
- 16.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S. O., Goode J., Lin P., Mann N., Mudd S., Crozat K., Sovath S., Han J., Beutler B. (2003) Nature 424, 743–748 [DOI] [PubMed] [Google Scholar]
- 17.Evans G. S., Flint N., Somers A. S., Eyden B., Potten C. S. (1992) J. Cell Sci. 101, 219–231 [DOI] [PubMed] [Google Scholar]
- 18.Macartney K. K., Baumgart D. C., Carding S. R., Brubaker J. O., Offit P. A. (2000) J. Virol. 74, 5597–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitajima S., Takuma S., Morimoto M. (1999) J. Vet. Med. Sci. 61, 67–70 [DOI] [PubMed] [Google Scholar]
- 20.Im E., Choi Y. J., Pothoulakis C., Rhee S. H. (2009) J. Nutr. 139, 1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaya T., Miyazawa K., Takakura I., Itani W., Watanabe K., Ohwada S., Kitazawa H., Rose M. T., McConochie H. R., Okano H., Yamaguchi T., Aso H. (2008) Am. J. Physiol. Gastrointest Liver Physiol 295, G273–G284 [DOI] [PubMed] [Google Scholar]
- 22.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 23.Carvalho F. A., Barnich N., Sauvanet P., Darcha C., Gelot A., Darfeuille-Michaud A. (2008) Inflamm. Bowel Dis. 14, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 24.Abreu M. T., Vora P., Faure E., Thomas L. S., Arnold E. T., Arditi M. (2001) J. Immunol. 167, 1609–1616 [DOI] [PubMed] [Google Scholar]
- 25.Melmed G., Thomas L. S., Lee N., Tesfay S. Y., Lukasek K., Michelsen K. S., Zhou Y., Hu B., Arditi M., Abreu M. T. (2003) J. Immunol. 170, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 26.Burdelya L. G., Krivokrysenko V. I., Tallant T. C., Strom E., Gleiberman A. S., Gupta D., Kurnasov O. V., Fort F. L., Osterman A. L., Didonato J. A., Feinstein E., Gudkov A. V. (2008) Science 320, 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. (2004) Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 29.Sun Y. H., Rolán H. G., Tsolis R. M. (2007) J. Biol. Chem. 282, 33897–33901 [DOI] [PubMed] [Google Scholar]
- 30.Amer A., Franchi L., Kanneganti T. D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. (2006) J. Biol. Chem. 281, 35217–35223 [DOI] [PubMed] [Google Scholar]
- 31.Choi Y. J., Im E., Pothoulakis C., Rhee S. H. (2010) J. Biol. Chem. 285, 21382–21390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebsamen M., Meylan E., Curran J., Tschopp J. (2008) Cell Death Differ 15, 1804–1811 [DOI] [PubMed] [Google Scholar]