Abstract
This study investigated the role of leptin receptor (Lepr) signaling in determining the bone mechanosensitivity and also evaluated whether differences in the Lepr signaling may contribute to the differential osteogenic response of the C57BL/6J (B6) and C3H/HeJ (C3H) pair of mouse strains to mechanical stimuli. This study shows that a loading strain of ∼2,500 μϵ, which was insufficient to produce a bone formation response in B6 mice, significantly increased bone formation parameters in leptin-deficient ob−/ob− mice and that a loading strain of ∼3,000 μϵ also yielded greater osteogenic responses in Lepr-deficient db−/db− mice than in wild-type littermates. In vitro, a 30-min steady shear stress increased [3H]thymidine incorporation and Erk1/2 phosphorylation in ob−/ob− osteoblasts and db−/db− osteoblasts much greater than those in corresponding wild-type osteoblasts. The siRNA-mediated suppression of Lepr expression in B6 osteoblasts enhanced (but in osteoblasts of C3H (the mouse strain with poor bone mechanosensitivity) restored) their anabolic responses to shear stress. The Lepr signaling (leptin-induced Jak2/Stat3 phosphorylation) in C3H osteoblasts was higher than that in B6 osteoblasts. One of the three single nucleotide polymorphisms in the C3H Lepr coding region yielded an I359V substitution near the leptin binding region, suggesting that genetic variation of Lepr may contribute to a dysfunctional Lepr signaling in C3H osteoblasts. In conclusion, Lepr signaling is a negative modulator of bone mechanosensitivity. Genetic variations in Lepr, which result in a dysfunctional Lepr signaling in C3H mice, may contribute to the poor osteogenic response to loading in C3H mice.
Keywords: Bone, Gene Knock-out, Mouse Genetics, Receptors, Signal Transduction, Transgenic, Leptin Receptor, Mechanosensitivity, Mechanotransduction, Osteoblasts
Introduction
Genetics play a key role in determining the bone response to mechanical loading. Accordingly, C57BL/6J (B6)2 inbred strain mice respond to in vivo loading with an increase in bone formation, but C3H/HeJ (C3H) inbred strain mice show no such response (1–4). Quantitative trait locus mapping studies of the C3H/B6 pair of mouse strains revealed that the bone mechanosensitivity is regulated by genes located in multiple quantitative trait loci on a number of chromosomes (5, 6) and that significant interactions exist among these quantitative trait loci (6). Thus, the genetic component contributing to the differential bone response to loading in the B6/C3H pair of mouse strains is complex and involves multiple genes. Studies of the identity of and interactions among these mechanosensitivity-modulating genes would yield not only information about mechanical stimulation of bone formation but also insights into the pathophysiology of various bone-wasting diseases (e.g. osteoporosis).
Our previous studies, using primary osteoblasts of the C3H/B6 pair of mouse strains as an in vitro model system and fluid shear stress as an in vitro surrogate of mechanical strain (7), have disclosed two pieces of relevant information: 1) some of the genetic components determining bone mechanosensitivity in the C3H/B6 pair of mouse strains are intrinsic to osteoblasts, and 2) some of the “mechanosensitivity” genes contributing to the good and poor bone formation response in B6 and C3H mice, respectively, are upstream from four anabolic pathways (the Wnt (wingless- and Int-related protein), IGF-I, estrogen receptor (ER), and bone morphogenetic protein (BMP)/transforming growth factor β (TGFβ) pathways).
Our search for candidate mechanosensitivity genes focused initially on mouse chromosome 4, because functional genetic studies indicated that at least one region in mouse chromosome 4 harbors mechanosensitivity-modulating genes. Specifically, the B6.C3H-4T congenic mouse strain, which is genetically identical to B6 except that it carried a segment of C3H chromosome 4 (between 40 and 80 centimorgans), was more responsive to mechanical stimulation in periosteal bone formation than B6 mice (8). An N-ethylnitrosourea mutagenesis study in B6 mice identified a mutant exhibiting reduced bone mechanosensitivity, and the mutation was mapped to chromosome 4 (9). Because the chromosome 4 congenic locus is currently the only clearly defined chromosomal region that has demonstrated functional ability to modulate mechanosensitivity (8), we focused on candidate genes in this region.
The possibility that at least some of the mechanosensitivity genes in the B6/C3H pair of mouse strains are upstream from the four aforementioned anabolic pathways (7) led to our decision to narrow our initial search to candidate genes that may function as upstream signaling genes. It has been reported that the mechanosensitivity of the rat skeleton was markedly reduced after a long period of sustained loading (10). Loss of responsiveness after sustained activation is a hallmark characteristic of desensitization of receptor-mediated events. Thus, we decided to focus on receptor or receptor-associated genes. Although there are more than 50 receptor or receptor-associated genes located in this chromosome 4 congenic locus, we were interested in the Lepr (leptin receptor) gene for the following reasons: 1) it is located in this chromosomal 4 congenic region (8); 2) because Lepr is the cell surface receptor for leptin, Lepr is considered an upstream gene in the context of the leptin signaling pathway; 3) leptin deficiency had contrasting effects on weight-bearing (long bones) versus less weight-bearing (vertebrae) bones in mice (11); and 4) a preliminary genome-wide screen study in rats suggested that Lepr was associated with bone architecture and strength (12), both of which are key determining factors of mechanical responsiveness. The osteogenic effects of leptin can be stimulatory or inhibitory, depending on whether the route of administration is peripheral (13) or central (14). Because of this unique dual action on bone formation, it has been suggested that the leptin/Lepr signaling has a critical role in the “Mechanostat” theory of mechanical regulation of bone mass (15).
The objectives of the present study were 2-fold. The first objective was to test the hypothesis that Lepr and/or its signaling plays a modulating role in bone mechanosensitivity. Our in vivo approach to test this hypothesis was to compare the bone formation response of leptin-deficient ob−/ob− mice and also that of Lepr-deficient db−/db− mice to a 2-week four-point bending loading regimen with that of the background strain, B6 mice, or wild-type (WT) littermates, respectively. Our in vitro approach, which used primary osteoblasts as an in vitro model system and fluid shear stress as an in vitro surrogate of mechanical loading, was to compare the anabolic response of ob−/ob− and db−/db− osteoblasts to fluid shear with that of osteoblasts of corresponding WT littermates to demonstrate modulating functions of the leptin/Lepr signaling in bone cell mechanosensitivity. The second objective was to determine if a dysfunctional leptin/Lepr signaling in C3H osteoblasts may in part contribute to the contrasting osteogenic response to fluid shear stress between B6 and C3H osteoblasts. Leptin-deficient ob−/ob− mice were included in these experiments, because 1) we have previously shown that ob−/ob− mice lacked the sex-related differences in the greater periosteal expansion (16), which suggests a dysfunctional periosteal bone formation response to loading, and 2) rescue experiments with direct pretreatment with leptin protein can be performed in cells of ob−/ob− mice to confirm the functional role of leptin (or Lepr signaling) in mechanosensitivity in vitro. Such straightforward rescue experiments would not be feasible with cells of Lepr-deficient db−/db− mice.
EXPERIMENTAL PROCEDURES
Materials
Tissue culture plasticware was obtained from Falcon (Oxnard, CA). Dulbecco's modified Eagle's medium (DMEM) was from Mediatech, Inc. (Herndon, VA). Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT). [3H]Thymidine (48 Ci/mmol) was a product of Research Products International (Mount Prospect, IL). Anti-phospho-Erk1/2 (pErk1/2), anti-pan-Erk1/2, anti-actin, anti-Lepr, anti-Jak2, and anti-Stat3 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), Upstate Biotechnology, Inc. (Lake Placid, NY), or BD Transduction Laboratories (La Jolla, CA). Other chemicals were from Fisher or Sigma.
Animals
Heterozygous male and female leptin-deficient (ob+/ob−) breeder mice (B6.v-Lepob/J) as well as heterozygous male and female Lepr-deficient (db+/db−) mice (B6.BKS-Leprdb) were obtained from Jackson Laboratories (Bar Harbor, ME). A colony of each knock-out mouse strain was generated and maintained at the J. L. Pettis Memorial Veterans Affairs Medical Center. The homozygous leptin-deficient genotype in ob−/ob− mice was confirmed by RT-PCR as described previously (17), and the homozygous Lepr-deficient db−/db− genotype was also confirmed by a PCR-based end point analysis genotyping assay recommended by Jackson Laboratories. Our previous investigation of the bone phenotype of ob−/ob− mice revealed that young adult male ob−/ob− mice lacked the sex-related differences in the greater periosteal expansion and that the loss of sex-related bone size differences in young adult male ob−/ob− mice appeared to be due to a defective androgen signaling (16). To avoid potential sex-related effects, this study used only 10-week-old female leptin-deficient ob−/ob− mice and 12-week-old female db−/db− mice as well as corresponding age-matched WT littermates. Age-matched female B6 mice (also from Jackson Laboratories) were included in this study as the background strain control for ob−/ob− mice.
In Vivo Mechanical Loading Model
Our rationale for choosing the 2-week four-point bending regimen as the in vivo loading model and model itself have been described in detail previously (7). The loading was applied for 6 days/week with 1 day of rest for 2 weeks. Forty-eight h after the final loading, bone parameters of the loaded and contralateral unloaded tibiae (internal controls) were determined with peripheral quantitative computed tomography (pQCT). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the J. L. Pettis Memorial Veterans Affairs Medical Center.
Strain Gauge Measurements
The amount of mechanical strain on the loaded region of the tibia produced by each indicated load was measured by the strain gauge technique using a P-3500 portable strain indicator and a strain gauge of a specific range (EP-XX-015DJ-120) (4). Briefly, the ends of the strain gauge circuits were soldered to copper wire and glued on the medial side of the exposed tibia, 2.09 mm from the tibia-fibular junction, to provide a consistent position on the 4-mm loading zone. The copper wires were connected to the indicator, and the amounts of strain produced by the loads on the loading zone were recorded. The strain gauge data from four mice from each mouse strain were averaged for each test load.
Bone Parameter Measurements
Bone parameters (total bone mineral content, total tissue area, periosteal circumference, endosteal circumference, cortical area, cortical thickness, cortical content, material bone mineral density, and volumetric bone mineral density) were determined by pQCT (Stratec XCT 960M, Norland Medical Systems, Ft. Atkinson, WI) with the analysis threshold settings of 730–730 for cortical bone and 180–730 for cancellous bone.
Cell Cultures
Osteoblasts, isolated from calvarias of 8-week-old B6 and C3H mice by collagenase digestion as described previously (7, 18), were maintained in DMEM supplemented with 10% FBS. Osteoblasts were also isolated from calvarias of 10-week-old female ob−/ob− mice, 12-week-old female db−/db− mice, and corresponding age-matched WT littermates. Cell passage, up to passage 7, had no significant effects on the responsiveness of primary B6 osteoblasts to fluid shear stress with respect to [3H]thymidine incorporation, alkaline phosphatase activity, and Erk1/2 phosphorylation (data not shown). Thus, cells of passages 3–6 were used in this study.
Shear Stress Experiments
Fifty thousand cells were plated on each glass slide. At ∼80% confluence, the cells were serum-deprived for 24 h and subjected to a steady fluid shear stress of 20 dynes/cm2 for 30 min in the Cytodyne flow chamber as described previously (19). This dosage of shear stress is considered within the physiologically relevant range of laminar shear stress produced by the circulation (20). Replicate glass slides of cells were placed in parallel flow chambers but without the shear stress as static controls for comparison.
[3H]Thymidine Incorporation Assay
Cell proliferation was assessed by [3H]thymidine incorporation during the final 6 h of the 24 h after exposure to shear stress (7, 19).
Western Immunoblot Assays
The relative cellular pErk1/2 level was determined with the antibody against pErk1/2 and normalized against corresponding the total Erk1/2 level (determined with the anti-panErk1/2 antibody) as described previously (7, 19).
For measurements of leptin-induced activation of Jak2 (Janus kinase 2)/Stat3 (signal transducers and activators of transcription 3) signaling, primary osteoblasts isolated from B6, ob−/ob−, or C3H mice were treated with 100 ng/ml leptin for 24 h and were then subjected to the 30-min steady shear stress. Ten minutes after the shear stress, the stressed and static control cells were lysed in radioimmunoprecipitation assay buffer, and the relative levels of tyrosine-phosphorylated Jak2 (Tyr(P)-Jak2), total Jak2, Tyr(P)-Stat3, and total Stat3 were analyzed by Western immunoblots using respective specific antibodies.
Lepr Co-immunoprecipitation Assay
Ten min after the shear stress, the stressed and static control cells from three slides each were pooled and lysed in radioimmunoprecipitation assay buffer, and cell extract protein (0.5 μg) of the stressed and static control B6 osteoblasts was immunoprecipitated with the anti-Lepr antibody. The immune complex was resolved on 10% SDS-PAGE, and the co-immunoprecipitated Jak2 was identified with an anti-Jak2 antibody. The stripped blot was reblotted against an anti-Stat3 antibody to identify the co-immunoprecipitated Stat3.
Real-time RT-PCR Analyses
Real-time PCR was carried out with the SYBR Green method on the MJ Research DNA Engine Opticon® 2 system (MJ Research, Waltham, MA). Briefly, total RNA was extracted with a Qiagen mini-RNA kit (Qiagen, Valencia, CA). The purified total RNA was used as template for synthesizing cDNA by reverse transcription using random hexamer primers and Superscript II reverse transcriptase (Invitrogen). An aliquot of the cDNA was subjected to real-time PCR amplification using gene-specific primer sets listed in supplemental Table S1. An aliquot (25 μl) of reaction mixture (consisting of 2× (12.5 μl) QuantiTect SYBR Green PCR master mix, which contained the Hot Start Taq polymerase (Qiagen), 0.5 μm primers, and 1–5 μg of cDNA template) was used in each assay. The PCR amplification condition consisted of an initial 10-min hot start at 95 °C, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing, extension for 30 s at an appropriate temperature (50–72 °C) (see supplemental Table S1), and a final step of melting curve analysis from 60 to 95 °C. The data were analyzed using Opticon® Monitor software version 2.0. Data normalization was performed against β-actin mRNA, and the normalized values were used to calculate relative -fold change by the threshold cycle (ΔCT) method.
Leptin Protein Enzyme Immunometric Assay (EIA)
Secretion of leptin protein from primary B6 and C3H osteoblasts were determined by measuring the amounts of leptin protein in 24-h conditioned medium (CM). Briefly, 24-h CM (7 ml each) of replicate cultures (four slides each) of primary B6 and C3H osteoblasts without (i.e. basal) or with the 30-min shear stress were collected separately, lyophilized, and resuspended in 0.7 ml of deionized water. The amount of leptin protein in each resuspended CM was determined with a commercial mouse leptin EIA (mouse Leptin TiterZyme® EIA, catalog no. 900-019; Assay Designs, Ann Arbor, MI) and reported as pg of leptin/mg of cellular protein/24 h.
Lepr siRNA Experiments
A set of three siRNAs specific for mouse Lepr (i.e. Lepr siRNA1 (target sequence: CCC GAG CAA ATT AGA AAC AAA), Lepr siRNA2 (target sequence: ATC GAT GTC AAT ATC AAT ATA), and Lepr siRNA3 (target sequence: TTG AAG CTA AAT TTA ATT CAA)) and a non-silencing control siRNA without any homology to known mammalian genes were designed and synthesized by Qiagen. For the siRNA experiment, primary B6 or C3H osteoblasts were seeded at 60,000 cells in a 24-well plate for 24 h. The cells were transfected with the test siRNAs using the HiPerFect Transcription reagent (Qiagen). Briefly, 3 μl of HiPerFect transcription reagent was added to 100 μl of DMEM containing 75 ng of Lepr (or control) siRNA duplex. The reaction mixture was incubated for 10 min at room temperature and was added to each cell culture well containing 500 μl of fresh DMEM and 10% FBS. After 16 h of incubation at 37 °C, the medium was replaced by fresh DMEM containing FBS. The effectiveness of Lepr suppression after an additional 24–48 h of incubation at 37 °C was assessed by Western immunoblot using an anti-Lepr antibody. The protein loading was normalized against the cellular actin level using a specific anti-actin antibody.
Cloning of Full-length Lepr cDNA from B6 and C3H Osteoblasts
The full-length cDNA of the signal-transducing long form of Lepr (LRb) was cloned from both C3H and B6 osteoblasts by an RT-PCR-based cloning approach. Briefly, total RNA was purified from C3H and B6 osteoblasts with the RNeasy kit (Qiagen). Two μg of total RNA of C3H or B6 osteoblasts was reverse transcribed into first-strand cDNA using oligo(dT) primer with the RNase H-reverse transcriptase (Superscript II, Invitrogen) in the presence of the deoxynucleotides. The full-length Lepr cDNA was produced by PCR using the following primer set 5′-AAA AGG ATC CAG ATG ATG TGT CAG AAA TTC-3′ (forward) and 5′-AAA AAA GCT TTC AAA GAG TGT CCG TTC TCT-3′ (reverse). To facilitate cloning, BamHI and HindIII restriction sites (underlined), respectively, were added to the forward and reverse primer. The PCR amplification was performed with native Pfu DNA polymerase (Stratagene, La Jolla, CA) with the following conditions: a 1-min hot start at 95 °C, 35 cycles of 45-s denaturation at 95 °C, 45-s annealing at 63 °C, 1-min extension at 72 °C, and a final 10-min extension at 72 °C. The 2,615-bp Lepr PCR product of C3H and B6 osteoblasts was gel-purified, digested with BamHI and HindIII restriction enzymes, and cloned into pcDNA3.1 at these two restriction sites. The entire coding regions of the B6 and C3H Lepr genes were each determined by sequencing both strands of cDNA.
Statistical Analysis
Statistical significance was determined with a two-tailed Student's t test, and the difference was significant at p < 0.05.
RESULTS
Bone Formation Response to Mechanical Loading in Tibias of Female Leptin-deficient ob−/ob− and Lepr-deficient db−/db− Mice
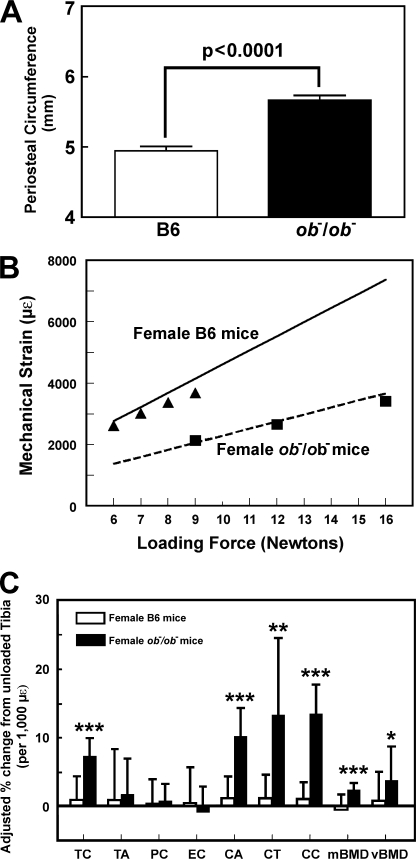
To test whether leptin/Lepr signaling has modulating functions on bone mechanosensitivity, we first compared the osteogenic response to a 2-week four-point bending loading regimen on tibias of young adult female leptin-deficient ob−/ob− mice with that on tibias of age-matched female B6 (background genetic strain control) mice. The size (periosteal circumference) of tibia of young adult female ob−/ob− mice was significantly bigger (by ∼14%) than that of age-matched female B6 tibia (Fig. 1A). Because the bone formation response is determined by mechanical strain sensed by the bone rather than the loading force per se, and because the amount of strain generated by a given load is largely determined by the size of bone, we measured, with a strain gauge, the actual strain sensed by the loaded tibias of young adult female ob−/ob− mice as opposed to age-matched female B6 tibia at various loads (Fig. 1B). The determined strain sensed by the bones compared well with the calculated strain (based on respective bone size) at various loading forces for the two mouse strains. The tibia of B6 mice with a smaller bone size had indeed experienced a much higher strain at any given load than tibias of ob−/ob− mice. Accordingly, we adjusted the loading force for each mouse strain to ensure that similar mechanical strain was applied to tibia of each mouse strain. Our past studies in B6 mice used 9 N, which showed a substantial bone formation response (4–6, 8, 9). If a 9-N load were to be used in B6 mice, a loading force of ∼16 N would be needed for ob−/ob− mice for similar mechanical strain. However, because the magnitude of bone formation response differences between the two strains at higher loads may not be as large, and because the lower strain is physiologically more relevant than the higher strain, we decided to use a 9-N load (∼2,100 μϵ) for young adult female ob−/ob− mice but only a 6-N load (∼2,500 μϵ) for young adult female B6 mice.
FIGURE 1.
The periosteal circumference of 10-week-old female B6 and ob−/ob− mice (A), the relationship between loading force (in Newtons) and mechanical strain sensed by the bone (in μϵ) in female B6 and ob−/ob− mice (B), and the mechanical strain-adjusted bone responses in the tibia (by pQCT) of female ob−/ob− mice (filled bars) after a 2-week four-point bending loading compared with those in the tibia of female B6 mice (open bars) (C). In B, the solid line represents the calculated strain for each loading force in female B6 mice, and the dotted line is the calculated strain for each loading force for female ob−/ob− mice. The filled circles represent the actual strain sensed by the bone of female B6 mice measured with a strain gauge, whereas filled squares represent the actual strain sensed by the bone of female ob−/ob− mice. In C, the bending exercise was performed in six ob−/ob− mice and twelve B6 mice. The results are shown as relative percentage change from the unloaded tibias of individual mice per 1,000 μϵ (means ± S.E. (error bars)). TC, total bone mineral content; TA, total area; PC, periosteal circumference; EC, endosteal circumference; CA, cortical area; CT, cortical thickness; CC, cortical content; mBMD, material bone mineral density; vBMD, volumetric bone mineral density. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with B6 mice.
To adjust for the small difference in the actual mechanical strain sensed by the B6 tibia as opposed to the ob−/ob− tibia, the pQCT bone parameters were reported as the percentage change from corresponding unloaded tibia per 1,000 μϵ (Fig. 1C). Consistent with the previous finding that a 6-N load is insufficient to produce a bone formation response in young adult B6 mice (4), the mechanical strain of ∼2,500 μϵ (6 N of force) had no significant effect on any of the pQCT parameters in young adult female B6 mice. In contrast, this mechanical strain significantly increased total bone mineral content, cortical area, cortical content, cortical thickness, and material and volumetric bone mineral densities at the loading site in ob−/ob− mice, indicating that ob−/ob− mice had an enhanced mechanosensitivity compared with B6 mice.
Anabolic Response of ob−/ob− Osteoblasts to Fluid Shear Stress
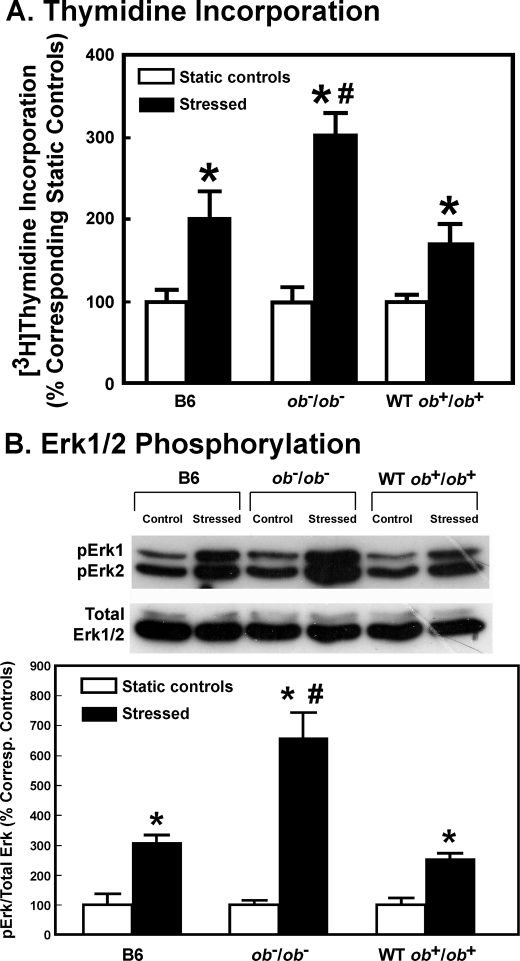
To determine whether leptin deficiency would also lead to an enhanced anabolic response to mechanical stimuli in osteoblasts in vitro, we assessed the effects of a 30-min steady fluid shear of 20 dynes/cm2 on [3H]thymidine incorporation and Erk1/2 phosphorylation in osteoblasts of young adult female ob−/ob− mice as opposed to those in osteoblasts of age-matched female B6 mice and WT littermates (ob+/ob+). Fig. 2 confirms that the shear stress stimulated [3H]thymidine incorporation and Erk1/2 phosphorylation in B6 osteoblasts. However, the same stress produced significantly greater increases in [3H]thymidine incorporation and Erk1/2 phosphorylation in ob−/ob− osteoblasts than in B6 or WT osteoblasts.
FIGURE 2.
Effects of fluid shear on [3H]thymidine incorporation (A) and Erk1/2 phosphorylation (B) of osteoblasts of young adult female B6 mice, wild-type littermates of ob−/ob− mice (WT ob+/ob+), or leptin-deficient (ob−/ob−) mice. In A, results are shown as the percentage of respective static cells (mean ± S.D. (error bars), n = 4). *, p < 0.05 versus static control; #, p < 0.05 versus the B6 and WT cells. In B, the top shows a representative blot of pErk1 and pErk2 (using an anti-pErk1/2 antibody) and total Erk1/2 (identified by an anti-pan-Erk antibody). The bottom summarizes the relative amounts of pErk1/2 normalized against total Erk, and results are shown as a percentage of respective static control (mean ± S.D., n = 4). *, p < 0.05, compared with static control; #, p < 0.05, compared with B6 osteoblasts or WT osteoblasts.
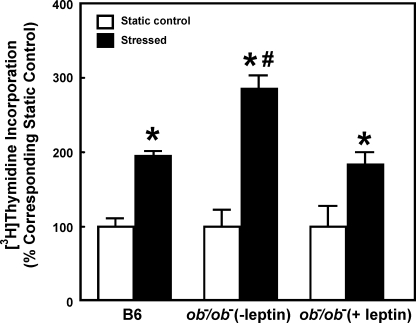
If the enhanced mitogenic response of ob−/ob− osteoblasts to fluid shear was due to a deficiency in leptin expression, pretreatment with an effective dose of leptin should block the enhanced response in ob−/ob− osteoblasts. Consistent with this speculation, the mitogenic response to shear stress in ob−/ob− osteoblasts was reduced markedly by a 2-h pretreatment with 100 ng/ml leptin, and in fact it was returned to a level that was no longer different from that seen in B6 osteoblasts (Fig. 3).
FIGURE 3.
Leptin pretreatment of ob−/ob− osteoblasts abolished their enhanced mitogenic response to the fluid shear in vitro. Osteoblasts of ob−/ob− mice were pretreated for 2 h with or without 100 ng/ml leptin. Immediately after the pretreatment, these ob−/ob− osteoblasts along with B6 osteoblasts were subjected to the 30-min fluid shear stress of 20 dynes/cm2. [3H]Thymidine incorporation was determined as described (5). Results are shown as a percentage of corresponding static controls (mean ± S.D. (error bars), n = 6). *, p < 0.05, compared with static control; #, p < 0.05, compared with B6 osteoblasts.
Effects of Leptin Deficiency on the Fluid Shear-induced Up-regulation of Genes Associated with Four Anabolic Pathways in Mouse Osteoblasts
Our previous studies revealed that fluid shear stress differentially up-regulated in B6, but not C3H, osteoblasts the expression of a number of genes associated with four anabolic pathways (the Wnt, IGF-I, ER, and BMP/TGFβ pathways) (7). To test whether the enhanced mitogenic response in leptin-deficient ob−/ob− osteoblasts is accompanied by an enhanced response in up-regulation of genes associated with these four anabolic pathways, relative expression levels of several genes associated with the IGF-I (Igf1r and c-fos), ER (Era and Ncoa1), BMP (Dlx1), and Wnt (Ctnnb1, Wnt1, and Wnt3a) pathways 4 h after the fluid shear were measured by real-time RT-PCR (supplemental Table S2). Although the shear stress significantly up-regulated the expression of each test gene in B6 and ob−/ob− osteoblasts, the up-regulation was significantly and consistently greater in ob−/ob− osteoblasts than in B6 osteoblasts. The shear stress-induced up-regulation of expression of these test genes in B6 osteoblasts as well as in ob−/ob− osteoblasts was blocked by the overnight leptin pretreatment (Table 1), indicating that the leptin signaling is probably acting upstream to these four anabolic pathways.
TABLE 1.
Effect of an overnight pretreatment with 100 ng/ml leptin on the shear stress-induced expression of genes of the IGF-I, BMP/TGFβ, ER, and Wnt signaling pathways in B6 osteoblasts and ob−/ob− osteoblasts by real-time PCR 4 h after the fluid shear (n = 6 for each)
| Gene | B6 osteoblasts |
ob−/ob− osteoblasts |
||
|---|---|---|---|---|
| Stressed/Static control (no leptin, -fold changes, mean ± S.D.) | Stressed/Static control (with leptin, -fold changes, mean ± S.D.) | Stressed/Static control (no leptin, -fold changes, mean ± S.D.) | Stressed/Static control (with leptin, -fold changes, mean ± S.D.) | |
| -fold | -fold | -fold | -fold | |
| Era | 2.40 ± 0.75a | 1.39 ± 0.25b | 3.31 ± 0.20a | 1.18 ± 0.24b |
| Igf1r | 3.30 ± 0.36a | 1.55 ± 0.29b | 4.49 ± 0.71a | 1.78 ± 0.38b |
| Dlx1 | 1.68 ± 0.48a | 0.75 ± 0.10b | 3.23 ± 0.65a | 1.12 ± 0.21b |
| Ncoa1 | 2.89 ± 0.11a | 1.34 ± 0.60b | 3.74 ± 0.88a | 0.98 ± 0.17b |
| c-fos | 3.06 ± 0.68a | 1.25 ± 0.22b | 2.56 ± 0.29a | 1.32 ± 0.08b |
| Ctnnb1 | 2.62 ± 0.54a | 0.90 ± 0.30b | 3.80 ± 0.62a | 1.08 ± 0.28b |
| Wnt1 | 2.71 ± 0.74a | 0.93 ± 0.09b | 3.65 ± 0.52a | 1.68 ± 0.34b |
| Wnt3a | 2.56 ± 0.75a | 1.66 ± 0.25b | 3.00 ± 0.62a | 1.52 ± 0.18b |
a p < 0.05, compared with the corresponding static control.
b p < 0.05, compared with those without the leptin pretreatment.
Enhanced Osteogenic Response to Mechanical Loading in Tibias of Female Lepr-deficient db−/db− Mice
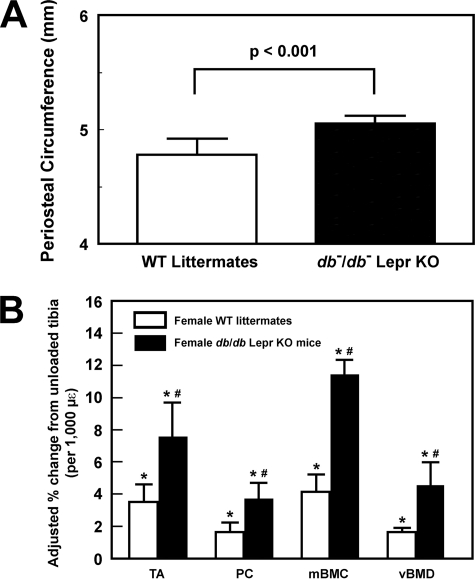
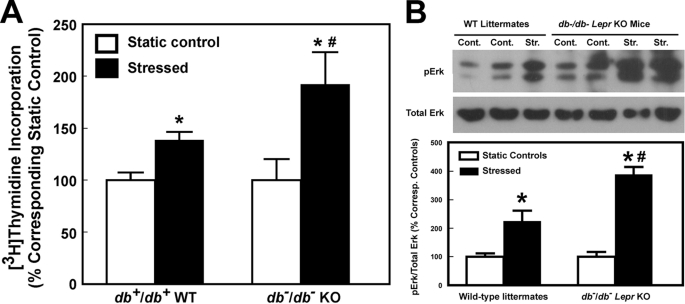
We next sought to confirm that enhanced mechanosensitivity can also be seen in Lepr-deficient db−/db− mice by comparing the periosteal bone formation response to a 2-week four-point bending loading regimen on tibia of young adult female Lepr-deficient db−/db− mice with that on tibia of age-matched female WT littermates. Like ob−/ob− mice, the periosteal circumference of tibia of 12-week-old female db−/db− mice was also bigger (by 5.6%, p < 0.001) than that of age-matched female WT littermates (Fig. 4A). Thus, we used a loading force of 9 N on db−/db− tibias (which produced a strain of ∼3,100 μϵ) and a loading force of 6.5–7.5 N on tibias of WT littermates (which yielded a strain of ∼3,000 μϵ) to ensure that a similar mechanical strain was applied to the tibia of both mouse strains. Although this mechanical strain produced significant bone formation responses (e.g. bone mineral density and volumetric bone mineral density) in both db−/db− mice and WT littermates (Fig. 4B), the response in db−/db− tibias was significantly (p < 0.01 for each) greater than that in tibias of WT littermates, confirming that mice deficient in Lepr expression indeed exhibited an enhanced bone formation response to loading.
FIGURE 4.
The periosteal circumference of 12-week-old female B6 and db−/db− mice (A) and the mechanical strain-adjusted bone responses in the tibia (by pQCT) of female db−/db− mice (filled bars) after a 2-week four-point bending loading compared with those in the tibia of female B6 mice (open bars) with a 9-N load and a 6.5–7.5-N load, respectively (B). The 9-N load on the tibia of db−/db− mice yielded an average mechanical strain of ∼3,100 μϵ, whereas the 6.5–7.5-N load on the tibia of WT littermates produced an average strain of ∼3,000 μϵ. The bending exercise was performed in six 12-week-old female db−/db− mice and six age- and sex-matched WT littermates. The results are shown as relative percentage change from the unloaded tibia of individual mice per 1,000 μϵ (means ± S.E. (error bars)). TA, total area; PC, periosteal circumference; mBMD, bone mineral density; vBMD, volumetric bone mineral density. *, p < 0.05, compared with respective unloaded contralateral tibia; #, p < 0.05, compared with WT littermates.
Enhanced Anabolic Response of db−/db− Osteoblasts to Fluid Shear Stress
Fig. 5 confirms that although the 30-min steady fluid shear at 20 dynes/cm2 enhanced [3H]thymidine incorporation and Erk1/2 phosphorylation in both db−/db− osteoblasts and WT osteoblasts, the increases were also significantly (p < 0.05) larger in db−/db− osteoblasts than in WT osteoblasts. Thus, osteoblasts of Lepr-deficient mice also have an enhanced anabolic response to mechanical stimulation.
FIGURE 5.
Effects of fluid shear on [3H]thymidine incorporation (A) and Erk1/2 phosphorylation (B) of osteoblasts of 12-week-old female Lepr-deficient db−/db− mice and of osteoblasts of age- and sex-matched littermates. In A, results are shown as the percentage of respective static cells (mean ± S.D., n = 6). *, p < 0.05 versus static control; #, p < 0.05 versus the WT littermates. In B, the top shows a representative blot of pErk1 and pErk2 (using an anti-pErk1/2 antibody) and total Erk1/2 (identified by an anti-pan-Erk antibody). The bottom summarizes the relative amounts of pErk1/2 normalized against total Erk, and results are shown as a percentage of respective static control (mean ± S.D. (error bars), n = 4–6). *, p < 0.05, compared with static control; #, p < 0.05, compared with osteoblasts of WT littermates.
Functional Role of Lepr in the Anabolic Response to the Fluid Shear Stress in Mouse Osteoblasts
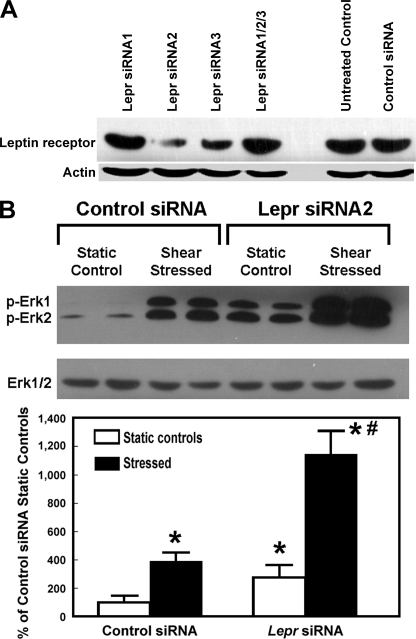
To confirm a functional role of Lepr in determining the anabolic response to mechanical stimuli in B6 osteoblasts, the effects of siRNA-mediated knockdown of Lepr expression on the mitogenic action of fluid shear stress in B6 osteoblasts were investigated. The effects of three Lepr siRNAs alone or in combination on Lepr expression in B6 were examined. The treatment with Lepr siRNA2 reduced cellular Lepr protein level by >70% (Fig. 6A). Down-regulation of Lepr expression in B6 osteoblasts by Lepr siRNA2 enhanced both basal (i.e. static control) and fluid shear-induced Erk1/2 phosphorylation much further in B6 osteoblasts (Fig. 6B). These findings together indicate that leptin/Lepr signaling has a negative modulating role in the anabolic response to mechanical stimuli in these mouse osteoblasts.
FIGURE 6.
siRNA-mediated suppression of Lepr expression enhanced basal and shear stress-induced Erk1/2 phosphorylation in B6 osteoblasts in vitro. A, the effects of the 24-h treatment of a set of Lepr siRNAs (siRNA1 to -3) alone or in combination (at a total concentration of 5 nm) on the cellular Lepr protein level in B6 osteoblasts. Cellular Lepr protein was identified by Western blots and normalized against actin. B, the effects of suppression of Lepr expression by Lepr siRNA2 on pErk1/2 levels. Total Erk1/2 was measured with an anti-pan-Erk antibody. The relative amounts of pErk1/2 were normalized against total Erk, and results are shown as a percentage of the control siRNA-treated static control cells (mean ± S.D. (error bars), n = 4–6). *, p < 0.05, compared with control siRNA-treated static control cells; #, p < 0.05, compared with stressed control siRNA-treated cells.
Role of Lepr in the Lack of an Anabolic Response in C3H Osteoblasts to Fluid Shear Stress
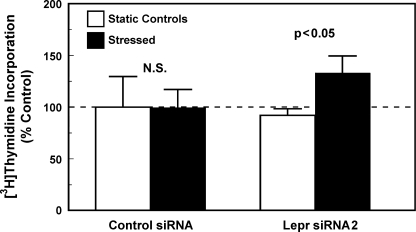
We next tested whether the lack of a significant anabolic effect in C3H osteoblasts to shear stress is related to dysfunctional Lepr or Lepr signaling. Accordingly, if the lack of a mitogenic response to fluid shear stress in C3H osteoblasts (7) is due to a dysfunctional Lepr, suppression of expression or activity of Lepr in C3H osteoblasts should restore the responsiveness of these cells to shear stress with an increase in cell proliferation. Consistent with this prediction, the siRNA-mediated suppression of Lepr expression in C3H osteoblasts restored their mitogenic responsiveness to fluid shear stress (Fig. 7). Thus, the lack of an anabolic response to shear stress in C3H osteoblasts could be due to a dysfunctional Lepr.
FIGURE 7.
Suppression of Lepr expression in C3H osteoblasts by a 24-h treatment with 5 nmLepr siRNA2 restored their mitogenic response to fluid shear stress. Primary osteoblasts isolated from 10-week-old female C3H mice were treated for 24 h with 5 nm Lepr siRNA2 (right bars) or the control siRNA (left bars). After the respective siRNA treatment, the cells were placed in the flow chamber with (dark bars) or without (open bars) subjection to a 30-min shear stress of 20 dynes/cm2. Cell proliferation was measured by [3H]thymidine incorporation during the final 6 h of a 24-h incubation. Results are shown as mean ± S.E. (error bars) (n = 4). N.S., not significant.
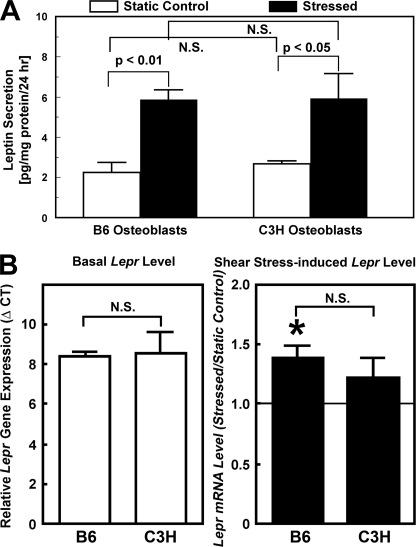
Basal and Shear Stress-induced Secretion of Leptin and Lepr Gene Expression Levels in C3H and B6 Osteoblasts
Human osteoblasts have been shown to produce and secrete leptin protein (21). To investigate the possibility that the good and poor anabolic response to shear stress of B6 and C3H osteoblasts, respectively, might be due to secretion of different amounts of leptin protein by B6 and C3H osteoblasts, we measured the amounts of leptin protein in the 24-h CM of B6 and C3H osteoblasts with or without the 30-min fluid shear (Fig. 8A). The amount of leptin in CM of each static control culture was low but detectable (∼2 pg/mg of cellular protein/24 h). The shear stress increased the CM leptin concentration by ∼3-fold in either osteoblast culture. However, there was no significant difference in basal or shear stress-induced leptin secretion levels between the B6 and C3 osteoblasts, indicating that the differential anabolic response to shear stress between B6 and C3H osteoblasts was probably not due to differences in leptin secretion.
FIGURE 8.
Comparison of basal and shear stress-induced secretion levels of leptin into CM by B6 and C3H osteoblasts in vitro (A) and basal and shear stress-induced Lepr mRNA expression levels in B6 and C3H osteoblasts (B). In A, the 24-h CM of B6 (left) or C3H osteoblasts (right) with or without the 30-min fluid shear were concentrated 7-fold, and the amounts of leptin in each concentrated CM were assayed with the mouse leptin EIA kit (Assay Designs, Inc.) and normalized against cellular protein content. Results are shown as mean ± S.D. (error bars) (n = 4 each). In B, the relative Lepr mRNA expression levels were determined by real-time RT-PCR. Left, the mRNA levels were assessed by the ΔCT method, and basal Lepr mRNA expression was reported as ΔCT compared with β-actin. Right, cells were subjected to a 30-min steady shear stress of 20 dynes/cm2. The -fold change in Lepr mRNA levels in response to the shear stress was determined by ΔΔCT, and the results were shown as ratio of the stressed cells/static control cells (mean ± S.D., n = 3 each). *, p < 0.05, compared with static control cells. N.S., not significant.
We next investigated whether the differential anabolic response was due to differences in Lepr expression levels between B6 and C3H osteoblasts by measuring the relative mRNA levels of the signal-transducing (long) form of Lepr in primary osteoblasts of these two mouse strains by real-time RT-PCR (Fig. 8B). There was no significant difference in basal Lepr mRNA levels (reported as ΔCT) (left). Although the shear stress increased the Lepr mRNA levels slightly by ∼40% in B6 osteoblasts and ∼25% in C3H osteoblasts (right), the difference between the two was not statistically significant. Thus, the difference in anabolic response to shear stress between the two mouse osteoblasts was also unlikely to be due to different expression levels of the functional form of Lepr.
Comparison of Leptin-dependent Activation of Jak2/Stat3 Signaling in C3H and B6 Osteoblasts
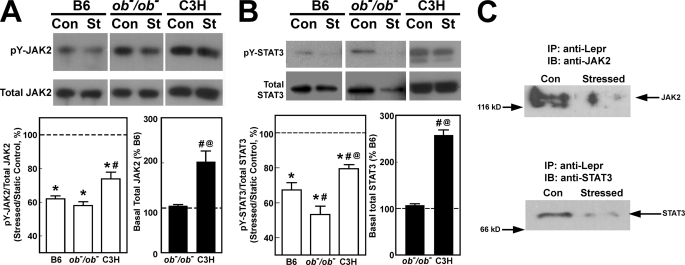
We next tested the alternative possibility that the different anabolic response to fluid shear stress of B6 and C3H osteoblasts might be due to different abilities of Lepr to trigger its signaling activity in the two mouse osteoblasts. Lepr is a member of the class I cytokine receptor family and uses the Jak2/Stat3 pathway as its primary signaling mechanism (22–24). Thus, we measured the relative ratios of Tyr(P)-Jak2/total Jak2 and Tyr(P)-Stat3/total Stat3, as indices of activation of the Lepr signaling, without (basal) or with the fluid shear in C3H and B6 osteoblasts. Osteoblasts of ob−/ob− mice were included for comparison. To ensure full activation of the Lepr signaling, the cells were pretreated with 100 ng/ml leptin for 24 h prior to the shear stress. The basal leptin-dependent increases in Tyr(P)-Jak2 (Fig. 9A) and in Tyr(P)-Stat3 (Fig. 9B) levels were 2-fold higher in C3H osteoblasts than those in B6 and ob−/ob− osteoblasts, suggesting that C3H osteoblasts might have a functionally more active Lepr signaling. The shear stress markedly reduced the relative levels of Tyr(P)-Jak2 (Fig. 9A) and Tyr(P)-Stat3 (Fig. 9B) in B6 and ob−/ob− as well as C3H osteoblasts. However, the shear stress-related reduction in the levels of these Tyr(P)-proteins was also comparatively smaller in C3H osteoblasts than in B6 osteoblasts.
FIGURE 9.
Effects of fluid shear stress on leptin-mediated induced phosphorylation of JAK2 and STAT3 (A) and on the amounts of co-immunoprecipitated Jak2 and Stat3 with Lepr in B6 osteoblasts (B). In A, primary osteoblasts isolated from B6, ob−/ob−, and C3H mice were each treated with 100 ng/ml leptin with or without the 30-min steady fluid shear stress of 20 dynes/cm2. Ten min after the shear stress, the stressed cells (St) and the corresponding static control cells (Con) were lysed in radioimmune precipitation buffer, and the relative levels of Tyr(P)-Jak2 and total Jak2 as well as those of Tyr(P)-Stat3 and total Stat3 were analyzed by Western blot. In B, immediately after the 30-min shear stress, cell extracts of the stressed and static control B6 osteoblasts were immunoprecipitated (IP) with anti-Lepr and blotted (IB) against anti-Jak2 (left) or anti-Stat3 (right).
It is interesting that the total Jak2 and Stat3 levels were 2–3-fold higher in C3H osteoblasts than in B6 or ob−/ob− osteoblasts. Thus, it may be argued that although fluid shear suppressed the leptin-mediated Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in C3H osteoblasts, the remaining Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in C3H osteoblasts were still severalfold higher than basal Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in B6 and ob−/ob− osteoblasts, simply because of the much higher basal total levels of these proteins in C3H osteoblasts.
Other members of the class I cytokine receptor family could also activate the Jak2/Stat3 signaling. To ensure that the fluid shear-mediated reduction in the Jak2/Stat3 activation was related to the Lepr signaling, we performed a co-immunoprecipitation experiment to assess the relative amounts of Lepr-associated Jak2/Stat3 in B6 osteoblasts (Fig. 9C). The fluid shear reduced markedly the amounts of Lepr-bound Jak2 and Stat3, a finding consistent with the interpretation that the reduced Jak2/Stat3 activation was in large part related to the shear stress-mediated reduction in the Lepr signaling.
Single Nucleotide Polymorphisms (SNPs) in Lepr Coding Region
A large number of SNPs have been identified in the coding and noncoding regions of human LEPR (25), and the three nonsynonymous SNPs (i.e. K109R, Q223R, and K665N SNPs) in human LEPR are associated strongly with adiposity, body composition, bone mineral density, fracture risk, and leptin response (26, 27). Because SNPs in noncoding regions are usually involved in regulation of gene expression but not gene functions, and because Fig. 8B indicates that the Lepr gene expression in C3H and B6 osteoblasts was not different, we focused on SNPs in the coding region. Comparison of the coding region of the Lepr cDNA sequence of C3H and B6 osteoblasts revealed three single nucleotide differences (Fig. 10). These differences were not PCR-introduced mutations because the same variations existed in some of the Lepr coding region sequences in the PubMed data base. Thus, there are three SNPs in the coding sequence of Lepr between the B6 and C3H mouse strains: 1) G → A SNP at nucleotide 594; 2) T → C SNP at 1041, and 3) A → G SNP at 1075. Two of the SNPs are located at the wobble base and are silent (i.e. synonymous), but the A → G SNP at 1075 is nonsynonymous and results in the I359V substitution.
FIGURE 10.
Existence of three SNPs in Lepr open reading frame between B6 and C3H osteoblasts. The capital, underlined letters in the nucleotide sequences indicate the location of SNPs. Single-letter symbols are used to represent amino acid sequence. The shaded and italicized letters show the location of the Ile → Val substitution in the amino acid sequence.
DISCUSSION
Mechanical loading is a key physiological regulatory mechanism for periosteal bone formation and is essential for the maintenance of skeletal architectural integrity and bone strength. It is now clear that determination of the overall osteogenic response to mechanical loading has a large genetic component. However, despite several genetic linkage and association studies that have identified a number of genetic loci harboring mechanosensitivity-modulating genes on various chromosomes (1–10, 28), little information about the identity of these mechanosensitivity genes is available. In this study, we provide several lines of strong in vivo and in vitro evidence that Lepr or its signaling may function as a negative modulator of bone mechanosensitivity in mice. Accordingly, we showed that young adult female leptin-deficient ob−/ob− mice exhibited an enhanced bone formation response to loading compared with young adult female B6 control mice in vivo and that the in vitro anabolic response of osteoblasts of ob−/ob− mice to a steady fluid shear stress was consistently and significantly greater than that in osteoblasts derived from WT littermates or B6 control mice. More importantly, we demonstrate that loading on tibias of young adult Lepr-deficient db−/db− mice produced significantly greater periosteal bone formation response than that on tibias of age-matched WT littermates and that osteoblasts derived from db−/db− mice also exhibited enhanced anabolic response to the fluid shear in vitro. These findings together lead us to conclude that the Lepr or its signaling has a negative modulating role in the osteogenic response to mechanical stimulation. The facts that shear stress reduced the leptin-dependent, Lepr-mediated phosphorylation of Jak2 and Stat3 along with the amounts of total Jak2 and Stat3 in ob−/ob− and also B6 osteoblasts further allow us to postulate that mechanical stimulation of the osteoblast activity and bone formation is in part mediated through suppression of the Lepr signaling in osteoblasts.
It is a general belief that osteocytes are the primary sensory cells of loading-induced fluid shear within the bone that transmit biological signals to osteoblasts at the bone surfaces (29, 30), because osteocytes are well situated within the bone to detect the fluid shear stress (29), have the ability to communicate with other bone cells through an extensive network of cellular processes connected at gap junctions (31), and respond to mechanical stimuli (32, 33). However, because primary osteocytes are difficult to isolate, we did not determine the effects of shear stress on the anabolic response of osteocytes of ob−/ob− and db−/db− mice in this study. On the other hand, there is evidence that osteoblasts also sensed and responded to mechanical signals and that the gene expression response and/or mechanotransduction mechanism in osteocytes and osteoblasts appear mostly similar (34). Therefore, we speculate that fluid shear stress would produce enhanced osteogenic responses in osteocytes of ob−/ob− and db−/db− mice in response to fluid shear stress similar to those seen in osteoblasts of these mice, such that Lepr or its signaling would play a similar negative modulating role in the overall mechanosensitivity of osteocytes.
The molecular mechanism by which Lepr (or Lepr signaling) acts to negatively modulate the anabolic response of osteoblasts to mechanical stimulation remains to be determined. However, because the fluid shear stress-induced up-regulation of expression of genes of the four anabolic signal transduction pathways (i.e. Wnt, IGF-I, ER, and BMP/TGFβ) was significantly enhanced in leptin-deficient ob−/ob− osteoblasts compared with B6 mice, and because this enhanced expression of these genes of the four anabolic pathways was abolished by the leptin pretreatment, we conclude that leptin (or its signaling) acts upstream to these four anabolic pathways to negatively modulate the anabolic response to mechanical stimulation in these osteoblasts. Consistent with the possibility that Lepr signaling is upstream from at least some of these anabolic pathways is the finding that ablation of ERα (which is essential for mechanotransduction (35)) blocked the leptin-mediated up-regulation of proopiomelanocortin in hypothalamus (36). In addition, mice deficient in ERα (36) or IGF-I (37) exhibited elevated serum leptin levels. This is consistent with a feedback up-regulation of leptin expression in ERα or IGF-I KO mice.
The mechanism by which leptin/Lepr signaling acts to negatively regulate these four anabolic pathways remains to be determined. In this regard, the activated Lepr recruited a number of signaling proteins, including SHP2 and PTP1B, through its phosphorylated tyrosine residues (22–24). There is circumstantial evidence that the Lepr signaling interacts and regulates other key signaling pathways, including the integrin, IGF-I, ER, BMP/TGFβ, and Wnt signaling pathways, through SHP2 or PTP1B (38–40). It is conceivable that the Lepr signaling may negatively modulate the anabolic response to mechanical stimulation in part by suppressing these four anabolic pathways through cross-talk involving signaling proteins, such as SHP2 and PTP1B, in osteoblasts.
Our findings that the leptin/Lepr signaling functions as a negative modulator of bone mechanosensitivity in mouse osteoblasts may have physiological implications in humans, in that it may play a role in the only recently recognized strong intraregulatory relationship between fat and bone (41). Thus, it is not surprising that leptin or Lepr signaling (the key negative regulatory hormone for body fat (42)) also plays a negative regulatory role in bone responsiveness to mechanical loading. More importantly, the findings that 1) the increase in obesity in postmenopausal women is associated with an increase in the circulating leptin level (43), 2) estrogen replacement in postmenopausal women decreases serum leptin levels (44), and 3) the PvuII and XbaI polymorphisms of ERα significantly influence the association between the Q223R polymorphism of human LEPR and peak bone mineral density (45) raise the interesting possibility that the suppressive action of leptin or Lepr signaling on bone mechanosensitivity might also have a role in the reduction in bone formation in postmenopausal osteoporotic patients. Our future studies will address these very interesting possibilities.
A major objective of this study is to evaluate whether genetic variations in Lepr may contribute in part to the contrasting osteogenic response to mechanical stimulation between the C3H and B6 pair of inbred mouse strains (1–4, 7). In this respect, this study, in addition to presenting compelling evidence that the Lepr signaling functions as a negative modulator in bone mechanosensitivity, also offers strong evidence to support our contention that there are significant differences in Lepr or its signaling between B6 and C3H osteoblasts and that these differences may in part contribute to the good and poor osteogenic response to mechanical loading in B6 and C3H mice, respectively (1–4, 7). Accordingly, we showed that down-regulation of Lepr expression by siRNA in C3H osteoblasts restored the anabolic response to the shear stress stimulation. It is also interesting to note that the basal levels of Jak2 and Stat3 in C3H were 2–3-fold higher than those in B6 and ob−/ob− osteoblasts. The leptin-induced Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in C3H osteoblasts were also 2–3-fold greater than those in B6 and ob−/ob− osteoblasts, suggesting that the basal Lepr signaling was significantly greater in C3H osteoblasts than in B6 osteoblasts. Because there were no apparent differences in the basal expression levels of Lepr mRNA or basal secretion rates of leptin protein between C3H and B6 osteoblasts in vitro, we conclude that the basal Lepr signaling activity in C3H osteoblasts is “hyperactive” compared with that in B6 osteoblasts. The reason for elevated basal levels of Jak2 and Stat3 in C3H osteoblasts is unclear. One possible explanation is that the elevated basal levels of Jak2 and Stat3 in C3H osteoblasts could be a result of the physiological feedback up-regulation of their biosynthesis of Jak2 and Stat3 in response to a “hyperactive” Lepr or Lepr signaling. We cannot, however, rule out the possibility that other genetic differences existing between B6 and C3H inbred strains of mice might be responsible for the elevated basal expression levels of Jak2 and Stat3 in C3H osteoblasts. Moreover, the increases in Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in C3H osteoblasts could merely be the consequence of the high basal Jak2 and Stat3 levels in these cells. Future work is needed to determine the mechanistic reasons for the elevated Jak2/Stat3 and Tyr(P)-Jak2/Tyr(P)-Stat3 in C3H osteoblasts compared with B6 osteoblasts.
Regardless of the reason, the higher basal Jak/Stat levels and the greater Lepr signaling in C3H osteoblasts could provide a potential explanation for the lack of an anabolic response to mechanical stimulation in these osteoblasts. Accordingly, although the shear stress also reduced the leptin-mediated Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in C3H osteoblasts, the resulting Tyr(P)-Jak2 and Tyr(P)-Stat3 levels in the stressed C3H osteoblasts were still high compared with those in B6 osteoblasts. Thus, it is conceivable that the shear stress produced by physiologically relevant levels of loading might not be sufficient to suppress the Lepr signaling in C3H mice to a level that would be low enough to allow for an anabolic response in C3H mice. Congruent with this possibility and with our contention that a significantly higher loading stress would be needed to reduce the Lepr signaling in C3H osteoblasts to levels that allow for an anabolic response are past findings that higher than physiological levels of mechanical strain are able to elicit an osteogenic response in C3H mice (46).
This study also uncovered an important piece of information that might be relevant to the potential mechanism leading to the “hyperactive” Lepr signaling in C3H osteoblasts. We found that three SNPs existed in the coding region of the Lepr gene between C3H and B6 mice. One of these SNPs is a nonsynonymous SNP that results in an I359V substitution in the Lepr protein in C3H osteoblasts. This amino acid residue is located within the Ig-like C2-type domain of the extracellular region of the protein (47). This hydrophobic, Ig-like C2-type domain is believed to be involved in the leptin binding (47). Because the leptin binding domain has been mapped to residues 323–640 (48), this Ile359 residue may be involved in the leptin binding and/or the conformational change needed for activation of the Lepr signaling. In this regard, there is strong evidence that activation of Lepr signaling was induced by ligand-dependent or -independent receptor dimerization (49, 50) or conformational change (50, 51). There is evidence that fluid shear induces conformational change in membrane receptors (52) and in membrane-associated proteins (53). Thus, it is possible that fluid shear stress alters dimerization and/or conformation of Lepr, which could then affect ligand binding affinity and/or its efficiency to activate intracellular signals. Thus, although the physiological significance of this substitution is unclear, we postulate that this I359V substitution in C3H Lepr may increase leptin binding affinity and/or may favor the conformation that would yield a more functionally active (i.e. “hyperactive”) Lepr in C3H osteoblasts. However, we cannot completely ignore the other two “silent” SNPs because it has recently been reported that a silent SNP in the MDR1 gene altered the substrate specificity of its gene product, P-glycoprotein (54). This silent SNP in MDR1 altered the kinetics of protein translation, affecting the timing of co-translational folding and insertion of the gene product into the membrane, and thereby changed the structure of the effectors and substrate binding sites.
In summary, we have demonstrated that the Lepr signaling in mouse osteoblasts functions as a negative modulator of the anabolic response to mechanical stimulation in vivo and in vitro. We also presented strong in vitro evidence supporting the possibility that mechanical stimuli, such as fluid shear stress, exert anabolic effects in part through suppression of the Lepr signaling in mouse osteoblasts. Finally, this study offers strong circumstantial evidence that the good and poor osteogenic response of B6 and C3H mice, respectively, to mechanical stimulation were in part due to a dysfunctional Lepr signaling in C3H mice, which is the result of a key genetic variation in the coding region of the Lepr between the B6 and C3H mice.
This work was supported in part by United States Army Medical Research Acquisition Activity Assistance Award DAMD17-01-1-0744). This work was also supported in part by a Merit Review provided by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. All work was performed in facilities provided by the Department of Veterans Affairs. Preliminary results were presented in abstract form (55).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- B6
- C57BL/6J inbred strain of mice
- BMP
- bone morphogenetic protein
- C3H
- C3H/HeJ inbred strain of mice
- CM
- conditioned medium
- EIA
- enzyme immunometric assay
- ER
- estrogen receptor
- pErk1/2
- phosphorylated Erk1/2
- pQCT
- peripheral quantitative computed tomography
- N
- newtons.
REFERENCES
- 1.Akhter M. P., Cullen D. M., Pedersen E. A., Kimmel D. B., Recker R. R. (1998) Calcif. Tissue Int. 63, 442–449 [DOI] [PubMed] [Google Scholar]
- 2.Kodama Y., Umemura Y., Nagasawa S., Beamer W. G., Donahue L. R., Rosen C. R., Baylink D. J., Farley J. R. (2000) Calcif. Tissue Int. 66, 298–306 [DOI] [PubMed] [Google Scholar]
- 3.Robling A. G., Turner C. H. (2002) Bone 31, 562–569 [DOI] [PubMed] [Google Scholar]
- 4.Kesavan C., Mohan S., Oberholtzer S., Wergedal J. E., Baylink D. J. (2005) J. Appl. Physiol. 99, 1951–1957 [DOI] [PubMed] [Google Scholar]
- 5.Kesavan C., Mohan S., Srivastava A. K., Kapoor S., Wergedal J. E., Yu H., Baylink D. J. (2006) Bone 39, 634–643 [DOI] [PubMed] [Google Scholar]
- 6.Kesavan C., Baylink D. J., Kapoor S., Mohan S. (2007) Bone 41, 223–230 [DOI] [PubMed] [Google Scholar]
- 7.Lau K. H., Kapur S., Kesavan C., Baylink D. J. (2006) J. Biol. Chem. 281, 9576–9588 [DOI] [PubMed] [Google Scholar]
- 8.Robling A. G., Li J., Shultz K. L., Beamer W. G., Turner C. H. (2003) FASEB J. 17, 324–326 [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A. K., Kapur S., Mohan S., Yu H., Kapur S., Wergedal J., Baylink D. J. (2005) J. Bone Miner. Res. 20, 1041–1050 [DOI] [PubMed] [Google Scholar]
- 10.Saxon L. K., Robling A. G., Alam I., Turner C. H. (2005) Bone 36, 454–464 [DOI] [PubMed] [Google Scholar]
- 11.Hamrick M. W., Pennington C., Newton D., Xie D., Isales C. (2004) Bone 34, 376–383 [DOI] [PubMed] [Google Scholar]
- 12.Turner C. H., Alam I., Sun O., Li J., Fuchs R. K., Edenberg H. J., Koller D. L., Foroud T., Econs M. J. (2005) J. Bone Miner. Res. 20, Suppl. 1, S28, abstract 1108 [Google Scholar]
- 13.Thomas T. (2004) Curr. Opin. Pharmacol. 4, 295–300 [DOI] [PubMed] [Google Scholar]
- 14.Ducy P., Amling M., Takeda S., Priemel M., Schilling A. F., Beil F. T., Shen J., Vinson C., Rueger J. M., Karsenty G. (2000) Cell 100, 197–207 [DOI] [PubMed] [Google Scholar]
- 15.Gordeladze J. O., Reseland J. E. (2003) J. Cell Biochem. 88, 706–712 [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Rundle C. H., Wergedal J. E., Srivastava A. K., Mohan S., Lau K. H. (2007) Calcif. Tissue Int. 80, 374–382 [DOI] [PubMed] [Google Scholar]
- 17.Namae M., Mori Y., Yasuda K., Kadowaki T., Kanazawa Y., Komeda K. (1998) Lab. Anim. Sci. 48, 103–104 [PubMed] [Google Scholar]
- 18.Sheng M. H., Lau K. H., Beamer W. G., Baylink D. J., Wergedal J. E. (2004) Bone 35, 711–719 [DOI] [PubMed] [Google Scholar]
- 19.Kapur S., Baylink D. J., Lau K. H. (2003) Bone 32, 241–251 [DOI] [PubMed] [Google Scholar]
- 20.Hillsley M. V., Frangos J. A. (1994) Biotechnol. Bioeng. 43, 573–581 [DOI] [PubMed] [Google Scholar]
- 21.Reseland J. E., Syversen U., Bakke I., Qvigstad G., Eide L. G., Hjertner O., Gordeladze J. O., Drevon C. A. (2001) J. Bone Miner. Res. 16, 1426–1433 [DOI] [PubMed] [Google Scholar]
- 22.Frühbeck G. (2006) Biochem. J. 393, 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartaglia L. A. (1997) J. Biol. Chem. 272, 6093–6096 [DOI] [PubMed] [Google Scholar]
- 24.Ghilardi N., Skoda R. C. (1997) Mol. Endocrinol. 11, 393–399 [DOI] [PubMed] [Google Scholar]
- 25.Thompson D. B., Ravussin E., Bennett P. H., Bogardus C. (1997) Hum. Mol. Genet. 6, 675–679 [DOI] [PubMed] [Google Scholar]
- 26.de Luis Roman D., de la Fuente R. A., Sagrado M. G., Izaola O., Vicente R. C. (2006) Arch. Med. Res. 37, 854–859 [DOI] [PubMed] [Google Scholar]
- 27.Fairbrother U. L., Tankó L. B., Walley A. J., Christiansen C., Froguel P., Blakemore A. I. (2007) J. Bone Miner. Res. 22, 544–550 [DOI] [PubMed] [Google Scholar]
- 28.Robling A. G., Warden S. J., Shultz K. L., Beamer W. G., Turner C. H. (2007) J. Bone Miner. Res. 22, 984–991 [DOI] [PubMed] [Google Scholar]
- 29.Aarden E. M., Burger E. H., Nijweide P. J. (1994) J. Cell Biochem. 55, 287–299 [DOI] [PubMed] [Google Scholar]
- 30.Lanyon L. E. (1993) Calcif. Tissue Int. 53, Suppl. 1, S102–S106; discussion S106–S107 [DOI] [PubMed] [Google Scholar]
- 31.Jones S. J., Gray C., Sakamaki H., Arora M., Boyde A., Gourdie R., Green C. (1993) Anat. Embryol. 187, 343–352 [DOI] [PubMed] [Google Scholar]
- 32.Dodds R. A., Ali N., Pead M. J., Lanyon L. E. (1993) J. Bone Miner. Res. 8, 261–267 [DOI] [PubMed] [Google Scholar]
- 33.Cheng B., Zhao S., Luo J., Sprague E., Bonewald L. F., Jiang J. X. (2001) J. Bone Miner. Res. 16, 249–259 [DOI] [PubMed] [Google Scholar]
- 34.Iqbal J., Zaidi M. (2005) Biochem. Biophys. Res. Commun. 328, 751–755 [DOI] [PubMed] [Google Scholar]
- 35.Jessop H. L., Suswillo R. F., Rawlinson S. C., Zaman G., Lee K., Das-Gupta V., Pitsillides A. A., Lanyon L. E. (2004) J. Bone Miner. Res. 19, 938–946 [DOI] [PubMed] [Google Scholar]
- 36.Hirosawa M., Minata M., Harada K. H., Hitomi T., Krust A., Koizumi A. (2008) Biochem. Biophys. Res. Commun. 371, 320–323 [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Moreno C., Pichel J. G., Chesnokova V., De Pablo F. (2004) FEBS Lett. 557, 64–68 [DOI] [PubMed] [Google Scholar]
- 38.Lund I. K., Hansen J. A., Andersen H. S., Møller N. P., Billestrup N. (2005) J. Mol. Endocrinol. 34, 339–351 [DOI] [PubMed] [Google Scholar]
- 39.Oh E. S., Gu H., Saxton T. M., Timms J. F., Hausdorff S., Frevert E. U., Kahn B. B., Pawson T., Neel B. G., Thomas S. M. (1999) Mol. Cell Biol. 19, 3205–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias-Salgado E. G., Haj F., Dubois C., Moran B., Kasirer-Friede A., Furie B. C., Furie B., Neel B. G., Shattil S. J. (2005) J. Cell Biol. 170, 837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid I. R. (2008) Osteoporos. Int. 19, 595–606 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994) Nature 372, 425–432 [DOI] [PubMed] [Google Scholar]
- 43.Hong S. C., Yoo S. W., Cho G. J., Kim T., Hur J. Y., Park Y. K., Lee K. W., Kim S. H. (2007) Menopause 14, 835–840 [DOI] [PubMed] [Google Scholar]
- 44.Lambrinoudaki I. V., Christodoulakos G. E., Economou E. V., Vlachou S. A., Panoulis C. P., Alexandrou A. P., Kouskouni E. E., Creatsas G. C. (2008) Maturitas 59, 62–71 [DOI] [PubMed] [Google Scholar]
- 45.Koh J. M., Kim D. J., Hong J. S., Park J. Y., Lee K. U., Kim S. Y., Kim G. S. (2002) Eur. J. Endocrinol. 147, 777–783 [DOI] [PubMed] [Google Scholar]
- 46.Pedersen E. A., Akhter M. P., Cullen D. M., Kimmel D. B., Recker R. R. (1999) Calcif. Tissue Int. 65, 41–46 [DOI] [PubMed] [Google Scholar]
- 47.Niv-Spector L., Gonen-Berger D., Gourdou I., Biener E., Gussakovsky E. E., Benomar Y., Ramanujan K. V., Taouis M., Herman B., Callebaut I., Djiane J., Gertler A. (2005) Biochem. J. 391, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong T. M., Huang R. R., Tota M. R., Mao C., Smith T., Varnerin J., Karpitskiy V. V., Krause J. E., Van der Ploeg L. H. (1998) Mol. Pharmacol. 53, 234–240 [DOI] [PubMed] [Google Scholar]
- 49.Devos R., Guisez Y., Van der Heyden J., White D. W., Kalai M., Fountoulakis M., Plaetinck G. (1997) J. Biol. Chem. 272, 18304–18310 [DOI] [PubMed] [Google Scholar]
- 50.Biener E., Charlier M., Ramanujan V. K., Daniel N., Eisenberg A., Bjørbaek C., Herman B., Gertler A., Djiane J. (2005) Biol. Cell 97, 905–919 [DOI] [PubMed] [Google Scholar]
- 51.Couturier C., Jockers R. (2003) J. Biol. Chem. 278, 26604–26611 [DOI] [PubMed] [Google Scholar]
- 52.Chachisvilis M., Zhang Y. L., Frangos J. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15463–15468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming I., Bauersachs J., Fisslthaler B., Busse R. (1998) Circ. Res. 82, 686–695 [DOI] [PubMed] [Google Scholar]
- 54.Kimchi-Sarfaty C., Oh J. M., Kim I. W., Sauna Z. E., Calcagno A. M., Ambudkar S. V., Gottesman M. M. (2007) Science 315, 525–528 [DOI] [PubMed] [Google Scholar]
- 55.Lau K. H., Kapur S., Amoui M., Wang X., Kesavan C., Mohan S., Baylink D. J. (2007) J. Bone Miner. Res. 22, Suppl. 1, p. S23, abstract 1223 [Google Scholar]