Abstract
Alterations in glycosylation play an important role during intestinal cell differentiation. Here, we compared expression of mucin-type O-glycan synthases from proliferating and differentiated HT-29 and Caco-2 cells. Mucin-type O-glycan structures were analyzed at both stages by mass spectrometry. Core2 β1,6-N-acetylglucosaminyltransferase-2 (C2GnT-2) was markedly increased in differentiated HT-29 and Caco-2 cells, but the core3 structure was hardly detectable. To determine whether such differential expression of mucin-type O-glycan structures has physiological significance in intestinal cell differentiation, expression of sucrase isomaltase (SI) and dipeptidyl-peptidase IV (DPP-IV), two well known intestinal differentiation markers, was examined. Interestingly, the fully glycosylated mature form of SI was decreased in C2GnT-2 knock-out mice but not in core2 N-acetylglucosaminyltransferase-3 (C2GnT-3) nulls. In addition, expression of SI and DPP-IV was dramatically reduced in C2GnT-1–3 triple knock-out mice. These patterns were confirmed by RNAi analysis; C2GnT-2 knockdown significantly reduced cell surface expression of SI and DPP-IV in Caco-2 cells. Similarly, overexpression of the core3 structure in HT-29 cells attenuated cell surface expression of both enzymes. These findings indicate that core3 O-glycan structure regulates cell surface expression of SI and DPP-IV and that core2 O-glycan is presumably an essential mucin-type O-glycan structure found in both molecules in vivo. Finally, goblet cells in the upper part of the crypt showed impaired maturation in the core2 O-glycan-deficient mice. These studies are the first to clearly identify functional mucin-type O-glycan structures modulating cell surface expression of SI and DPP-IV during the intestinal cell differentiation.
Keywords: Carbohydrate, Carbohydrate Biosynthesis, Carbohydrate Function, Differentiation, Glycoprotein, Core2 O-Glycan, Core3 O-Glycan, Differentiation, Dipeptidyl Peptidase-IV, Sucrase Isomaltase
Introduction
Alterations in glycan structures reportedly have significant effects on the glycoprotein function and play an important role in cell differentiation and proliferation (1). Glycan structure is determined by serial activity of several glycosyltransferases, and it has been reported that expression of specific glycosyltransferases is regulated by developmental or tumorigenic processes (2).
Among the numerous glycan structures, mucin-type O-glycans function in cell adhesion (3, 4), signaling (5), and tumorigenesis (6–8). Mucin-type O-glycans are classified as four different types: core1, core2, core3, and core4 (4). Core3 O-glycan is synthesized by single glycosyltransferase, β3-N-acetylglucosaminyltransferase-6. Reduced core3 synthase3 activity has been detected in malignant tumor cells (9, 10), and we reported that increased core3 O-glycan structure negatively regulates tumorigenesis by regulating the function of α2β1 integrin in prostate cancer (8).
In contrast to core3 O-glycan, formation of the core2 O-glycan structure containing N-acetylglucosamine β1,6 branching is catalyzed by three different glycosyltransferases as follows: core2 β1,6-N-acetylglucosaminyltransferase-1–3 (C2GnT-1–3). C2GnT-1 reportedly functions to control lymphocyte rolling (11), and its expression is highly correlated with cellular malignancy (7). C2GnT-2 has been described as the major enzyme that catalyzes formation of core2- and core4-type O-glycan structures in the intestinal tract (12). However, decreased expression of C2GnT-2 was observed in colorectal cancer compared with normal mucosa (13). C2GnT activity is increased in T-cell activation (14). C2GnT-3 is expressed in activated T-cells (15), and its deletion results in reduced thyroxine levels in the circulation (12). Recently our group published that core2 O-glycan-deficient mice show abnormal mucosal barrier formation in vivo (12). These results suggest that glycosyltransferases of mucin-type O-glycans differentially regulate cell function and that core2-type O-glycans regulate expression or sorting of highly glycosylated molecules in large intestinal enterocytes.
Changes in glycosylation likely play significant roles during the course of intestinal epithelial cell differentiation. During differentiation, as proteins are transported to different cellular compartments (16, 17), their glycan structure is modified by several glycosyltransferases. The N-glycan profile and related glycoproteins have been compared in proliferating versus differentiated HT-29 cells, which are a colon cancer line (18). However, changes in the O-glycan profile during intestinal cell differentiation have not been evaluated. Sucrase isomaltase (SI) and dipeptidyl-peptidase IV (DPP-IV) are well known marker proteins of differentiation of intestinal cells, and both are expressed in the brush border of the intestinal tract (19, 20). SI and DPP-IV are type II heavily N- and O-glycosylated membrane glycoproteins, and inhibition of N- or O-glycan modification of both molecules using glycosyltransferase inhibitors promotes abnormal apical expression of both enzymes in HT-29 and Caco-2 cells (21–23).
Enzymatic and chemical inhibition reagents are often employed to evaluate the function of specific glycosylation structures. Among them is the well known O-glycan synthesis inhibitor, 1-benzyl-2-acetamido-2-deoxy-a-d-galactopyranoside (GalNAcα-bn). GalNAca-bn inhibits almost all O-glycan syntheses (24, 25), but it is not useful to analyze specific O-glycan structures. Additionally, it has been reported that, depending on cell type, GalNAcα-bn can alter intracellular trafficking and induce expression of other glycosyltransferases such as sialyltransferase and fucosyltransferase in HT-29 cells (26).
In this study, we analyzed expression levels of several mucin-type O-glycans to understand their role in intestinal cell differentiation. RT-PCR and mass spectrometry analysis of proliferating and differentiated cells showed that core2 O-glycans and its related O-glycans are altered when cells begin to differentiate. We also found that core3 type O-glycan was not detected even in the differentiated cells. SI and DPP-IV expression was decreased on the surface of C2GnT-2 RNAi-treated Caco-2 cells and in core2 O-glycan-deficient mice. Reduced cell surface expression of SI and DPP-IV was also seen in core3 synthase-expressing HT-29 cells. These results suggest that core3 O-glycans regulate SI and DPP-IV localization and that core2 O-glycan is an essential mucin-type O-glycan structure that has a potential role in apical sorting of SI and DPP-IV in vitro and their expression in vivo.
EXPERIMENTAL AND PROCEDURES
Materials
Goat anti-SI antibody and rabbit anti-CD26 (DPP-IV) antibody were obtained from Santa Cruz Biotechnology, and anti-mouse SI antibody (HBB 2/614/88) was kindly provided by Dr. Hauri (27). Rabbit anti-occludin antibody was obtained from Invitrogen. Mouse anti-β-actin antibody and α-benzyl GalNAc (GalNAc-bn) were purchased from Sigma.
Mice
C2GnT-2, C2GnT-3, and C2GnT-1–3 triple knock-out mice were reported previously (12). Mice were treated according to guidelines of the National Institutes of Health, and experiments were approved by the Animal Research Committee of the Sanford-Burnham Medical Research Institute.
Cell Culture
HT-29 and Caco-2 cells were obtained from the American Type Culture Collection and maintained in DMEM/high glucose media supplemented with 10% FCS. HT-29 cells were differentiated by reducing glucose levels through changing media to RPMI 1640, and Caco-2 cells underwent spontaneous differentiation, according to a previous report (28). During differentiation, the media were changed once a day. A core3 synthase expression vector was constructed by inserting the open reading frame of human core3 synthase cDNA into the mammalian expression vector pcDNA3.1-hygromycin. HT-29 cells were transfected with pcDNA3.1/core3 synthase or pcDNA3.1 using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instruction. Selection was performed by a 2-week incubation in medium containing 1 mg/ml hygromycin (Promega). Hygromycin-resistant colonies were isolated and maintained in 500 μg/ml hygromycin. Expression of core3 synthase was confirmed by RT-PCR and FACS analysis according to Lee et al. (8).
Metabolic Labeling and Western Blotting
Differentiated monolayers of HT-29 or Caco-2 cells in 6-well dishes were preincubated for 2 h at 37 °C in methionine/cysteine-free RPMI 1640 medium (Mediatech) containing dialyzed 10% FBS. For metabolic pulse labeling, l-[35S]methionine and l-[35S]cysteine (Promix; GE Healthcare) were added at a concentration of 200 μCi/ml to media of each culture and incubated for 1 h at 37 °C. After rinsing three times with PBS, cells were solubilized and immunoprecipitated as described below.
Cells were solubilized in lysis buffer composed of 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 1% (w/v) Triton X-100, 5 mm sodium pyrophosphate, 10 mm NaF, 1 mm sodium orthovanadate, 10 mm β-glycerophosphate, 1 mm phenylmethylsulfonyl fluoride, and a protease inhibitor mixture (Sigma). For immunoprecipitations, whole cell lysates (500 μg) were incubated with 4 μg of goat anti-SI antibody and rabbit anti-DPP-IV antibody with 20 μl of protein A-Sepharose overnight at 4 °C. For Western analysis, protein samples were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell). Membranes were incubated with mouse anti-SI antibody (1:1000) followed by HRP-conjugated anti-mouse IgG. ECL reagents (Amersham Biosciences) were used to detect signals.
Cell Surface Biotinylation
Cells were incubated with sulfosuccinimidobiotin (Pierce) (1 mg/ml) for 1 h on ice, and the reaction was stopped with 50 mm NH4Cl. After washing two times with PBS containing 1 mm MgCl, 0.1 mm CaCl2, lysates were immunoprecipitated with goat anti-SI and rabbit anti-CD26 (DPP-IV) antibody, as described above. Biotinylated proteins were visualized using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
O-Glycan Structure Analysis
Proliferating and differentiated Caco-2 cells were suspended in 0.1 m NH4HCO3, boiled for 10 min, and lyophilized. O-Glycan was purified by β-elimination methods, according to Yu et al. (29). O-Glycans were permethylated and analyzed by MALDI-MS and MS/MS on a 4700 Proteomics Analyzer (Applied Biosystem, Farmington, MA), as described previously (29, 30).
Semi-quantitative RT-PCR
Total RNA was isolated from proliferating and differentiated HT-29 and Caco-2 cells using TRIzol (Invitrogen). RT-PCR of core1 synthase, Cosmc (31), core3 synthase (β3-N-acetylglucosaminyltransferase-6) (32), C2GnT-1 (33), C2GnT-2 (34), and C2GnT-3 (35) was undertaken. cDNA was synthesized using AmpliTaq DNA polymerase (Applied Biosystems) and the following PCR primers: Cosmc, 5-cactgtgacaaagcaga-3 (5′-primer) and 5-ggttggggtgataagtca-3 (3′-primer); core1 synthase, 5-gtgggactgaaaaccaa-3 (5′-primer) and 5-agatcagagcagcaacca-3 (3′-primer); C2GnT-1, 5′-tcggtggacacctgacgactatat-3′ (5′-primer) and 5′-aggtcataccgcttcttccacctt-3′ (3′-primer); C2GnT-2, 5′-agtccagggaatctcaaagccagt-3′ (5′-primer) and 5′-tgagctctggagcaagtcttccat-3′ (3′-primer); C2GnT-3, 5′-gacatccagttctctagacctctg-3′ (5′-primer) and 5′-aaggcgaggtacttagggagtact-3′ (3′-primer); β3-N-acetylglucosaminyltransferase-6, 5′-agcactgcagcagtggttc-3′ (5′-primer) and 5′-gaggaaggtgtccgcgaag-3′ (3′-primer), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-cctggccaaggtcatccatgaca-3′ (5′-primer) and 5′-atgaggtccaccaccctgttgct-3′ (3′-primer). The PCR was carried out at 94 °C for 5 min, followed by 28 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s and by a single incubation at 72 °C for 5 min. PCR products were separated by electrophoresis on 1% agarose gels. Expression levels were normalized to GAPDH expression.
RNAi
siRNA oligonucleotides specific for human C2GnT-2 (s17678) and a control siRNA were obtained from Ambion. Transient introduction of oligonucleotide (20 nm) was achieved using DharmaFECT-4, according to the manufacturer's instructions (Thermo Scientific). After 72 h of transfection, knockdown of both SI and DPP-IV was found to be at least 75% efficient, based on semiquantitative RT-PCR.
Immunocytochemistry
Differentiated cells were fixed by incubation with PBS containing 4% paraformaldehyde for 10 min at room temperature. After washing with PBS three times for 5 min each, cells were blocked with PBS containing 5% bovine serum albumin for 1 h at room temperature and then incubated with goat anti-SI and rabbit anti-CD26 (DPP-IV) antibodies (1:100) for 1 h at room temperature. Primary antibody binding was detected with an Alexa Fluor 488-labeled antibody to goat IgG (Invitrogen).
Immunohistochemical Analysis
To detect SI or DPP-IV in mouse intestine, tissues from wild-type or C2GnT-1–3 triple knock-out mice were fixed in 0.1 m phosphate buffer containing 4% paraformaldehyde and embedded in paraffin or frozen, respectively. For SI detection, dewaxed sections were pretreated with hydroperoxide and a nonspecific staining reagent (Dako Cytomation) for 10 min at 37 °C and then incubated with 1:100 diluted goat anti-SI antibody (Santa Cruz Biotechnology) at 4 °C overnight. Sections were then incubated with 1:200 diluted horseradish peroxidase-conjugated anti goat IgG antibody. Immunoreactivity was visualized using the manufacturer's protocol for DAB substrate kit (Zymed Laboratories Inc.), and sections were counterstained with Gills' hematoxylin solution. For DPP-IV detection, sections were washed with PBS for 1 min and fixed in 0.1 m phosphate buffer containing 4% paraformaldehyde for 10 min at room temperature. After washing with PBS three times, sections were incubated with 5% goat serum in PBS for 1 h. Sections were reacted with rabbit anti-DPP-IV antibody (1:50) at 4 °C overnight and then reacted with Alexa Fluor 488 anti-rabbit IgG (Invitrogen).
Mucin Histochemistry
Hematoxylin and eosin staining was used for morphological examination. Periodic acid-Schiff reaction and Alcian blue, pH 2.5, staining were carried out for mucin histochemistry. For immunohistochemistry for MUC2 mucin, microwave irradiation in a 1.0 mm EDTA, pH 8.0, was carried out before incubation of rabbit polyclonal anti-MUC2 antibody (H-300; Santa Cruz Biotechnology). Envision+ (Dako), which is dextran polymers conjugating a large number of goat antibodies against rabbit immunoglobulins and horseradish peroxidase, was used for secondary antibody, and the peroxidase reaction was developed using a diaminobenzidine/H2O2 solution, and counterstaining was performed with hematoxylin.
RESULTS
Core2 O-Glycan Is Increased in Differentiated HT-29 and Caco-2 Cells
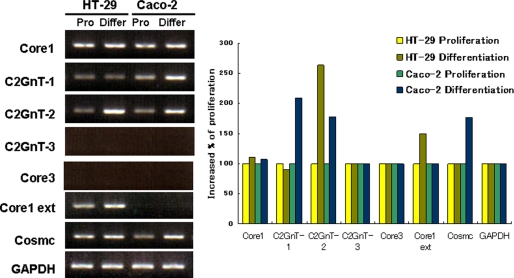
Because glycosylation patterns reportedly differ during cell differentiation, expression levels of several glycosyltransferases (Fig. 1) important for synthesis of mucin-type O-glycan structure were measured in two different intestinal cell lines HT-29 and Caco-2 by RT-PCR (Fig. 2A). In proliferating conditions, core1 synthase and its chaperone protein, Cosmc, were abundantly expressed in both HT-29 and Caco-2 cells. However, over the course of differentiation, expression of core1 synthase remained fairly unchanged. Caco-2 cells, unlike HT-29 cells, showed increased C2GnT-1 expression in differentiated conditions; however, C2GnT-2 expression was highly increased in both differentiated HT-29 and Caco-2 cells compared with proliferating conditions (Fig. 2B). Core3 synthase (β3-N-acetylglucosaminyltransferase-6) and C2GnT-3 were not detected in either line, even in differentiation conditions, and only HT-29 cells showed expression of core1 extension (Fig. 2B).
FIGURE 1.
Illustration of mucin-type O-glycan synthesis. Starting from N-acetylgalactosamine on serine or threonine residues in a polypeptide, core1 synthase transfers galactose to make the core1 structure Galβ1→ 3GalNAcα1→Ser/Thr, and core3 synthase (b3GnT-6) transfers N-acetylglucosamine to form a core3 structure GlcNAcβ1→3GalNAcα1→Ser/Thr (core3). The core1 extension enzyme β3GnT-3 transfers N-acetylglucosamine to a core1 structure to make a core1 extension structure, and core1 is converted to core2 by C2GnT-1, C2GnT-2, and C2GnT-3.
FIGURE 2.
Expression of enzymes forming mucin-type O-glycans. Total RNA was isolated from proliferating and differentiated HT-29 and Caco-2 cells. Cells at 30–40% confluency were used as proliferating (Pro) cells, and 3 days after confluency cells were judged to be differentiated (Differ). In the case of HT-29 cells, culture media were changed to RPMI 1640 when it reached confluency. After reverse transcription, levels of cDNAs encoding enzymes were semi-quantitatively estimated by PCR (left panel). mRNA levels were normalized to that of glyceraldehyde phosphate dehydrogenase (GAPDH). Expression levels were assessed by the ImageJ program, and levels of each mRNA in differentiating cells were compared with that seen in proliferating cells (right panel).
To confirm these findings, we analyzed cell surface O-glycan structures in proliferating and differentiated HT-29 and Caco-2 cells by mass spectrometry (Fig. 3). We found that core1- and core2-related O-glycan structures constitute a major population of O-glycans in both HT-29 and Caco-2 cells. Interestingly, the relative intensity of core2-based O-glycan structures (m/z 983.5, 1157.6, 1344.7, 1518.8, 1705.9, and 1800.0) gradually increased with the degree of differentiation. However, we did not observe detectable levels of core3 and core1 extended structure in either line. These results suggest that core2 O-glycans may have an important function as these cells differentiate, and their levels are regulated transcriptionally, possibly through up-regulation of the C2GnT-2 gene.
FIGURE 3.
Comparison of mucin-type O-glycans by mass spectrometric analyses of proliferating and differentiated Caco-2 cells. MALDI-MS analysis of permethylated O-glycans in positive ion mode from proliferating and differentiated Caco-2 cells. 30–40% confluent Caco-2 cells served as proliferating cells. At both 3 and 13 days after confluency, Caco-2 cells were assessed as differentiated and further differentiated cells. The m/z values of the labeled peaks correspond to monoisotopic mass, and assignment of the molecular composition is shown in the figure. Ions at m/z 983.5, 1157.6, 1344.7, 1518.8, 1705.9, and 1800.0 correspond to core3 O-glycans, Gal→GlcNAc→(Gal→)GalNAcitol, Gal→(Fuc→)GlcNAc→(Gal→)GalNAcitol, Gal→GlcNAc→(Sialic acid→Gal→)GalNAcitol, Gal→(Fuc→)GlcNAc→(sialic acid→Gal→)GalNAcitol, (sialic acid)2→Gal→GlcNAc→(Gal→)GalNAcitol, and (sialic acid)2→Gal→(Fuc→)GlcNAc→(Gal→)GalNAcitol, respectively.
Mature Form of Sucrase Isomaltase Is Decreased in C2GnT-2 Knock-out Mice
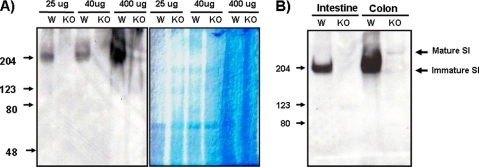
Because the core2-type O-glycan structure was dramatically increased in differentiated HT-29 and Caco-2 cells, we hypothesized that core2-type O-glycans could play an important role in the intestinal tract in vivo. To elucidate that function, we first examined expression of the differentiation marker SI. Interestingly, the highly glycosylated, mature form of SI was decreased in C2GnT-2 knock-out mouse intestine, although no difference relative to wild-type mice was detected in C2GnT-3 knock-out mice (Fig. 4). Proper maturation of SI is reportedly required to regulate apical sorting (36). Taken together, these data suggest that C2GnT-2-mediated core2 O-glycans have an important role in expression of a functional form of SI.
FIGURE 4.
Maturation of SI is attenuated in C2GnT-2-deficient mice. Expression of SI was compared from two different intestinal tissue samples from wild-type and C2GnT-2-deficient mice (A). Attenuated maturation was seen only in C2GnT-2-deficient and not in C2GnT-3-deficient mice (B). Four mg of each tissue sample was homogenized, and the resulting supernatant was used in Western blotting. Anti-goat SI antibody was used in this experiment as described under “Experimental and Procedures.”
C2GnT-2 Knockdown Promotes Decreased Cell Surface Expression of SI and DPP-IV
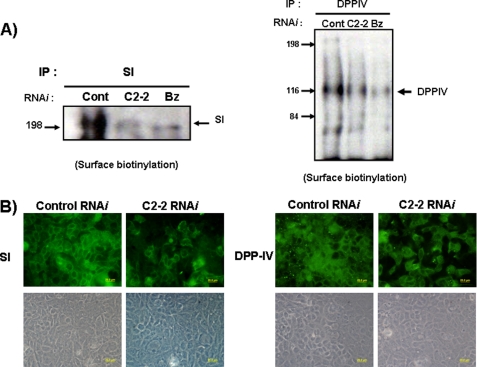
To confirm a role for C2GnT-2-mediated glycosylation in the maturation of SI and DPP-IV, we evaluated cell surface expression of SI and DPP-IV after down-regulation of C2GnT-2 expression by RNAi. Because DPP-IV reportedly exhibits a functional O-glycan structure similar to SI (26), we evaluated expression of both molecules in C2GnT-2 knockdown Caco-2 cells. After confirming that a specific RNAi sequence for C2GnT-2 efficiently inhibited C2GnT-2 expression in Caco-2 cells (Fig. 5A), SI or DPP-IV protein levels were examined by Western blotting and immunoprecipitation using metabolically labeled proteins. Because there is no high quality antibody commercially available for immunoblotting mouse DPP-IV, we were only able to compare DPP-IV molecular weight differences with and without RNAi treatment. SI and DPP-IV from GalNAc-bn-treated Caco-2 cells showed a decreased molecular weight, as reported previously (23, 37), and only DPP-IV showed significant decreases in molecular weight after RNAi treatment (Fig. 4B, right panel). However, cell surface expression of both SI and DPP-IV was dramatically decreased in C2GnT-2 RNAi-treated cells based on cell surface biotinylation and immunoprecipitation analysis (Fig. 6A). Those levels were further reduced when the most O-glycan structures were eliminated by GalNAc-bn treatment (Fig. 6A). Immunocytochemistry analysis confirmed these following findings (Fig. 6B): control RNAi-treated Caco-2 cells showed greater numbers of SI- and DPP-IV-positive cells than did C2GnT-2 knockdown cells in a nonpermeable condition. These results support the idea that C2GnT-2-mediated core2 O-glycan structure is important for cell surface SI and DPP-IV expression in intestinal Caco-2 cells.
FIGURE 5.
Targeted inhibition of C2GnT-2 by RNAi in HT-29 and Caco-2 cells. A, 20 nmol of control (Cont) RNAi and C2GnT-2-targeted (C2-2) RNAi was transfected using DharmaFECT-4, according to the manufacturer's protocols. Most C2GnT-2 expression was attenuated following transfection of C2GnT-2-targeted RNAi. B, SI expression was determined using an anti-SI antibody (HBB/214/88) after transfection of control RNAi and C2GnT-2 RNAi (left panel). In the case of DPP-IV, 35S-metabolically labeled protein was used for immunoprecipitation (IP) (right panel). GalNac-bn treatment served as a positive control to inhibit O-glycosylation of both SI and DPP-IV in Caco-2 cells.
FIGURE 6.
Cell surface expression of SI and DPP-IV is attenuated by targeted knockdown of C2GnT-2. Confluent Caco-2 cells were treated with control RNAi (Cont), C2GnT-2 RNAi (C2-2) and 4 mm GalNAc-bn (Bz) for 3 days. Surface-biotinylated Caco-2 cells (A) were immunoprecipitated (IP) using anti-goat SI (left panel) and anti-rabbit CD26 (DPP-IV) antibodies (right panel). Confluent Caco-2 cells on glass bottom plates were treated with control RNAi and C2GnT-2 RNAi. Three days later, SI and DPP-IV cell surface expression was assessed by immunofluorescence (upper panels) (B). Cell density of the area depicted is shown by phase-contrast microscopy (lower panels). Scale bar, 20 μm.
Core2 O-Glycan Is Important for Expression of SI and DPP-IV
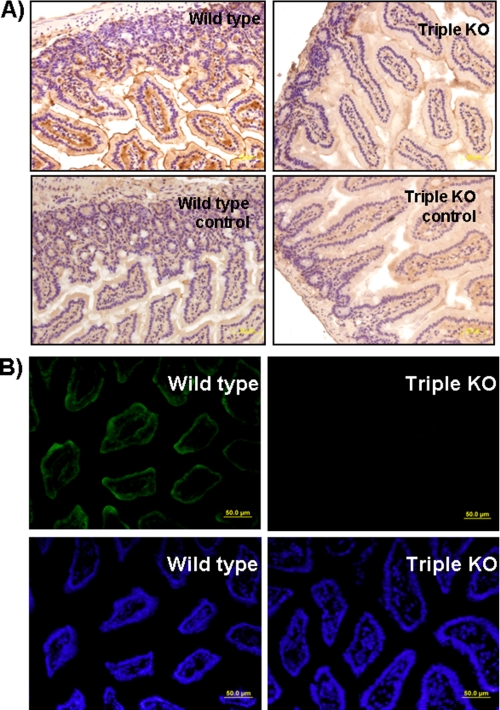
Although defective maturation of SI and reduced cell surface expression of SI and DPP-IV were seen in the C2GnT-2 single knock-out mouse intestine and in a C2GnT-2 knockdown experiment, respectively, interpretation of these findings was complicated by the fact that there are two other core2 O-glycan synthases. To eliminate the contribution of these factors, SI expression levels were determined in wild-type and in C2GnT-1–3 triple knock-out mice by Western blotting. Surprisingly, SI expression levels were dramatically reduced in triple knock-out compared with wild-type mice (Fig. 7). Immunohistochemical analysis showing weak expression of SI in C2GnT-1–3-deficient mice compared with wild-type mice confirmed these findings (Fig. 8A). DPP-IV positivity was also decreased in C2GnT-1–3 knock-out mouse intestine (Fig. 8B). These results strongly suggest that the core2 mucin-type O-glycan structure is essential for the expression of these proteins in vivo.
FIGURE 7.
SI expression is decreased in C2GnT-1–3 triple knock-out mice. The intestine and colon of wild-type and C2GnT-1–3 triple knock-out mice were homogenized, and the supernatant was isolated. A, expression of SI was determined by Western blotting with increasing loading amounts (left panel), and the same amount of protein was used in a control experiment and checked by Coomassie staining (right panel). B, intestine and colon tissues were isolated from another pair of wild-type and C2GnT-1–3 triple knock-out mice, and SI expression was similarly examined. 40 μg of cell lysate was used, and anti-goat SI antibody (1:1000) served as the first antibody. W, wild-type; KO, C2GnT-1–3 triple knock-out.
FIGURE 8.
Immunohistochemical and immunofluorescence analysis of C2GnT-1–3 triple knock-out mice. Sections from the intestine of wild-type and C2GnT-1–3 triply deficient mice were stained with goat anti-SI and rabbit anti CD26 (DPP-IV) antibody. A, paraffin sections were used for SI detection, and positive SI signals were strongly detected in wild-type mouse tissues (upper left panel). Control staining without the first antibody showed no signal (lower panel). Weak expression of SI was shown in C2GnT-1–3 triply deficient mouse intestine, compared with that of wild-type mice. B, frozen sections were used for DPP-IV detection, and Alexa Fluor-labeled anti-rabbit IgG served as second antibody (upper panels). Nuclear staining with DAPI was used as the control for DPP-IV staining (lower panels). Positive SI and DPP-IV signals were detected in the apical area of intestinal enterocytes. Scale bar, 50 μm.
Increased Core3 O-Glycan Structure Decreases Cell Surface Expression of SI and DPP-IV
Because the core3-type O-glycan structure was not detected in differentiated HT-29 and Caco-2 cells, we compared cell surface expression of SI and DPP-IV using mock-transfected (HT-29-mock) and core3 synthase-expressing HT-29 cells (HT-29-core3). After confirming expression of core3 synthase in HT-29 cells by direct and indirect methods such as RT-PCR and FACS analysis, respectively (Fig. 9), cell surface expression of SI and DPP-IV was compared in HT-29-mock and HT-29-core3 cells. Interestingly, DPP-IV surface expression was strongly decreased, and the mature form of SI was slightly decreased in HT-29-core3 cells (Fig. 10A). However, immunocytochemistry analysis showed that DPP-IV and SI positivity was decreased in HT-29-core3 cells. In addition, the staining pattern of DPP-IV and SI, which is normally apical, changed to the basolateral surface in confluent HT-29-core3 cells (Fig 10B). These data suggest that core3 O-glycans can regulate DPP-IV and SI and that increased core3 O-glycan structure may attenuate cell surface expression of both molecules.
FIGURE 9.
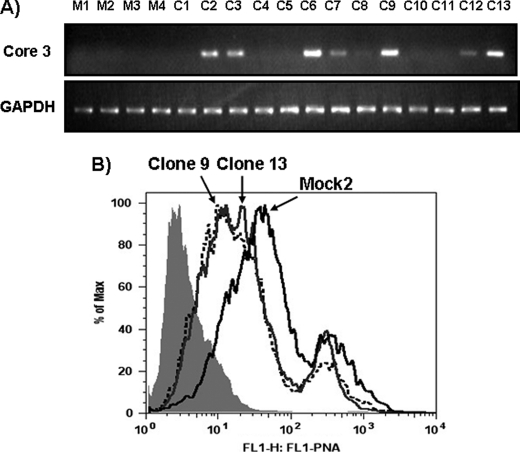
Establishment of core3 synthase-expressing HT-29 cells. A, HT-29 cells were transfected with mock and core3 synthase expression plasmids, and several hygromycin-resistant clones of each were isolated. A, RT-PCR results of core3 synthase expression of each mock- and pcDNA-core3-transfected clone. mRNA levels were normalized to that of glyceraldehyde phosphate dehydrogenase (GAPDH). B, FACS analysis for PNA (peanut agglutinin) staining after neuraminidase treatment. Two different core3-expressing HT-29 clones were compared with mock-transfected HT-29 cells. M, mock-transfected clone; C, core3-transfected clone.
FIGURE 10.
Cell surface expression of SI and DPP-IV is decreased in core3 synthase-expressing HT-29 cells compared with mock-transfected cells. A, differentiated mock- and core3 synthase-transfected HT-29 cells (mock clone 2 and 3 and core3-expressing clones 9 and 13) were biotinylated, and cell surface expression of SI (right) and DPP-IV (left) was compared after immunoprecipitation (IP) with DPP-IV and SI antibodies. B, DPP-IV and SI cell surface expression was examined by immunofluorescence. Both mock- and core3 synthase-transfected HT-29 cells were stained with goat anti-SI (upper right) or rabbit anti-CD26 (DPP-IV; upper left) antibodies in nonpermeable conditions. Cell density of the depicted areas is shown by phase-contrast microscopy (lower panels). Arrowhead and arrows indicate the apical and basolateral area, respectively. Scale bar, 20 μm.
Core 2 O-Glycan Is Important for Functional Maturation of Goblet Cells
The above findings suggest that colonic epithelial differentiation might be altered in the C2GnT-deficient mouse. Because the expressions of SI and DPP-IV were defective in the C2GnT-1–3-deficient mouse compared with wild-type mouse, we examined more closely the morphology of colonic mucosa from both wild-type and C2GnT-1–3-deficient mice. Histological examinations of the large intestines revealed that the goblet cells lining the mucosal crypt of triple knock-out mice were increased in number compared with those of wild-type mice, and the mucin vacuoles in these cells were also enlarged in size (Fig. 11). Interestingly, the mature goblet cells containing larger mucin vacuoles, which were found in the upper portion of the crypt in wild-type mice, already appeared in the crypt base of the triple knock-out mice. We also found that MUC2 mucin in the triple knock-out mice was secreted by the goblet cells largely located in lower half of the crypt, whereas MUC2 mucin in the wild-type mice was distributed throughout the mucosal crypt (Fig. 11). These results indicate that the goblet cells in the upper part of crypts of the triple knock-out mice hardly synthesize MUC2 and thus exhibit a defect in functional maturation of goblet cells. In addition, the acidity of goblet cell mucin secreted by the triple knock-out mice was reduced (Fig. 11), suggesting that sialylation and/or sulfation of O-glycans in the goblet cells were decreased in knock-out mice. Because the core2 branch is highly sialylated, the loss of core2 branch likely contributes to diminished acidity of mucin secretions from the C2GnT-1–3 triple-deficient mice.
FIGURE 11.
Histology of the large intestine of wild-type and C2GnT-1–3 triple knock-out mice. HE, PAS, and AB indicate hematoxylin and eosin, periodic acid-Schiff, and Alcian blue, pH 2.5, respectively. WT, wild-type mice; KO, C2GnT-1–3 triple knock-out mice; mm, muscularis mucosae; bar, 50 μm.
DISCUSSION
In this study, we investigated detailed O-glycan structures over the course of intestinal cell differentiation by mass spectrometry. We observed not only structural differences but also transcriptional changes in specific glycosyltransferases catalyzing mucin-type O-glycan synthesis as cells differentiated.
Among the three different glycosyltransferases responsible for synthesis of the core2 O-glycan structure, C2GnT-2 expression was most greatly increased in differentiated compared with proliferating HT-29 and Caco-2 cells, although C2GnT-1 also showed increases in differentiated HT-29 cells (Fig. 2). In support of this finding, mucin-type O-glycan structures based on core2-type O-glycans were mainly detected in differentiated Caco-2 cells, and their relative levels increased as cells differentiated (Fig. 3). We also showed that expression of the intestinal markers SI and DPP-IV was down-regulated in core2 O-glycan-deficient mice. To our knowledge, this is the first data demonstrating that O-glycan modification by a specific glycosyltransferase functionally regulates expression of SI and DPP-IV in vivo.
Core1-type O-glycan structure reportedly plays an important role in development (38) and is abundantly expressed in intestinal cells. Although increased core1 synthase levels are only marginally higher than those of C2GnT-2, total expression levels of core1 synthase in differentiated Caco-2 cells were similar to those of C2GnT-2 (Fig. 2). This could account for why an increased disialylated core1 structure (m/z 1256.7) was detected in differentiated Caco-2 cells. Using the core1-type O-glycan structure as an acceptor, the core2 O-glycan structure is synthesized by three C2GnTs (Fig. 1). Conversely, the core1 O-glycan structure remained in C2GnT-1–3 triple knock-out mice as well as on the SI and DPP-IV in the intestinal tract. In our study, incompletely glycosylated SI and reduced expression of SI and DPP-IV were shown in C2GnT knock-out mice. Taken together, these results suggest that the C2GnT-mediated O-glycan structure constitutes an essential minimum O-glycan structure important for expression of both SI and DPP-IV.
In differentiated Caco-2 cells, although the core2-based O-glycan structure was predominant, C2GnT-2 could make not only core2 but also the core4 structure. Because the core4 structure could be detected in the normal intestinal tract, it may also function in the expression of SI and DPP-IV in vivo. Detailed analysis of the glycan structure using purified SI and DPP-IV proteins should be informative in future analysis of the function of both core2 and core4 structures. The fact that there is decreased expression of MUC2 in the mucosal crypt of C2GnT-1–3 triple knock-out mice compared with wild type (Fig. 11) could be a reason for the increased permeability shown in core2 O-glycan-deficient mice in our previous study (12).
In addition, there is a possibility that defective function of absorptive cells might cause abnormal size and maturation of goblet cells in C2GnT-1–3 triple knock-out mice. It is also possible that the anomaly in the differentiation of absorptive and goblet cells is caused by impaired secretion of MUC2. These data support the functional role of core2 O-glycan in intestinal track in vivo.
Although both Caco-2 and HT-29 lines are often used to analyze enterocytic function, both are intestinal carcinoma cells. Core3 structure is reportedly down-regulated in several cancers (9, 10), possibly accounting for why core3 O-glycan was not detected in our study (Fig. 1). To understand the role of core3 mucin-type O-glycan structure in SI and DPP-IV expression, cell surface expressions of both proteins were compared in mock versus core3 synthase-transfected HT-29 cells (Fig. 10). Interestingly, increasing core3 structure hindered cell surface expression of both SI and DPP-IV, and core3-expressing HT-29 cells exhibited an expression pattern characteristic of the basolateral surface. Our group previously reported that an increased core3 structure on α2β1 integrin attenuated its cell surface expression on prostate cancer cells (8). These results suggest that core3 type O-glycan is a functional mucin-type O-glycan structure required for proper sorting of SI and DPP-IV in intestinal epithelial cells.
In polarized epithelial cells, two sorting pathways have been suggested. First, the trans-Golgi network and other components sort proteins to the surface by carrier proteins. Second, the transcytosis pathway delivers proteins to another surface via early and recycling endosomes (39). Although unknown mechanisms likely regulate both pathways, glycosylation patterns are thought to underlie these processes. In fact, it is suggested that intracellular lectin-like proteins such as mannose 6-phosphate receptor, calnexin, calreticulin, and vesicular integral membrane protein 36 play important roles in intracellular vesicular transport (40, 41). In addition, a specific glycosylation structure by single glycosyltransferase such as core fucosylation on hepatic glycoprotein is suggested to be a signal for secretion into liver bile ducts (42). O-Glycosylation of SI and DPP-IV has been proposed to regulate apical sorting by several groups, because apical SI and DPP-IV expression is disrupted by GalNAc-bn, a strong inhibitor of almost all O-glycan synthesis. In this study, cell surface expression of SI and DPP-IV was decreased by knockdown of a single glycosyltransferase, C2GnT-2. Interestingly, decreased expression of SI was detected in C2GnT-1–3 triple knock-out mice. However, only the mature form of SI was attenuated without changing overall expression levels in C2GnT-2 single deficiency mice, which show partially decreased core2 synthase activity (Fig. 4). These results, taken together, suggest that not all O-glycans, but rather C2GnT-2-mediated O-glycans, can function as signaling factors in apical sorting as well as protein expression. It is also possible that novel sorting machinery complexes include core2 O-glycan recognition molecules, an idea that requires further study.
In summary, ours is the first description of the function of a specific mucin-type core2 O-glycan structure in vivo in intestinal cells. Using C2GnT-deficient mice, C2GnT-mediated O-glycan structures, primarily the core2 O-glycan structure, were determined to be important for SI and DPP-IV expression. Our analysis should lead to further exploration of carbohydrate-mediated mechanism in intracellular trafficking.
Acknowledgments
We thank the staff of the laboratories of Drs. Minoru and Michiko Fukuda for useful discussion, and Dr. Elise Lamar for critical reading of the manuscript.
This work was supported by National Institutes of Health Grants CA33000 and P01 CA71932.
- core3 synthase
- β3-N-acetylglucosaminyltransferase-6
- C2GnT
- core2β1,6-N-acetylglucosaminyltransferase
- SI
- sucrase isomaltose
- DPP-IV
- dipeptidyl-peptidase IV.
REFERENCES
- 1.Lowe J. B., Marth J. D. (2003) Annu. Rev. Biochem. 72, 643–691 [DOI] [PubMed] [Google Scholar]
- 2.Dennis J. W., Granovsky M., Warren C. E. (1999) BioEssays 21, 412–421 [DOI] [PubMed] [Google Scholar]
- 3.Tsuiji H., Takasaki S., Sakamoto M., Irimura T., Hirohashi S. (2003) Glycobiology 13, 521–527 [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M. (2002) Biochim. Biophys. Acta 1573, 394–405 [DOI] [PubMed] [Google Scholar]
- 5.D'Alessandris C., Andreozzi F., Federici M., Cardellini M., Brunetti A., Ranalli M., Del Guerra S., Lauro D., Del Prato S., Marchetti P., Lauro R., Sesti G. (2004) FASEB J. 18, 959–961 [DOI] [PubMed] [Google Scholar]
- 6.Bao X., Kobayashi M., Hatakeyama S., Angata K., Gullberg D., Nakayama J., Fukuda M. N., Fukuda M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12109–12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatakeyama S., Kyan A., Yamamoto H., Okamoto A., Sugiyama N., Suzuki Y., Yoneyama T., Hashimoto Y., Koie T., Yamada S., Saito H., Arai Y., Fukuda M., Ohyama C. (2010) Int. J. Cancer 127, 1052–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S. H., Hatakeyama S., Yu S. Y., Bao X., Ohyama C., Khoo K. H., Fukuda M. N., Fukuda M. (2009) J. Biol. Chem. 284, 17157–17169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavasseur F., Dole K., Yang J., Matta K. L., Myerscough N., Corfield A., Paraskeva C., Brockhausen I. (1994) Eur. J. Biochem. 222, 415–424 [DOI] [PubMed] [Google Scholar]
- 10.Vavasseur F., Yang J. M., Dole K., Paulsen H., Brockhausen I. (1995) Glycobiology 5, 351–357 [DOI] [PubMed] [Google Scholar]
- 11.Mitoma J., Petryniak B., Hiraoka N., Yeh J. C., Lowe J. B., Fukuda M. (2003) J. Biol. Chem. 278, 9953–9961 [DOI] [PubMed] [Google Scholar]
- 12.Stone E. L., Ismail M. N., Lee S. H., Luu Y., Ramirez K., Haslam S. M., Ho S. B., Dell A., Fukuda M., Marth J. D. (2009) Mol. Cell. Biol. 29, 3770–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang M. C., Chen H. Y., Huang H. C., Huang J., Liang J. T., Shen T. L., Lin N. Y., Ho C. C., Cho I. M., Hsu S. M. (2006) Oncogene 25, 3267–3276 [DOI] [PubMed] [Google Scholar]
- 14.Piller F., Le Deist F., Weinberg K. I., Parkman R., Fukuda M. (1991) J. Exp. Med. 173, 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merzaban J. S., Zuccolo J., Corbel S. Y., Williams M. J., Ziltener H. J. (2005) J. Immunol. 174, 4051–4059 [DOI] [PubMed] [Google Scholar]
- 16.Hein Z., Hooper N. M., Naim H. Y. (2009) Exp. Cell Res. 315, 348–356 [DOI] [PubMed] [Google Scholar]
- 17.Scheiffele P., Peränen J., Simons K. (1995) Nature 378, 96–98 [DOI] [PubMed] [Google Scholar]
- 18.Vercoutter-Edouart A. S., Slomianny M. C., Dekeyzer-Beseme O., Haeuw J. F., Michalski J. C. (2008) Proteomics 8, 3236–3256 [DOI] [PubMed] [Google Scholar]
- 19.Semenza G., Brunner J., Wacker H. (1983) CIBA Found. Symp. 95, 92–112 [DOI] [PubMed] [Google Scholar]
- 20.Gorvel J. P., Ferrero A., Chambraud L., Rigal A., Bonicel J., Maroux S. (1991) Gastroenterology 101, 618–625 [DOI] [PubMed] [Google Scholar]
- 21.Naim H. Y., Joberty G., Alfalah M., Jacob R. (1999) J. Biol. Chem. 274, 17961–17967 [DOI] [PubMed] [Google Scholar]
- 22.Alfalah M., Jacob R., Preuss U., Zimmer K. P., Naim H., Naim H. Y. (1999) Curr. Biol. 9, 593–596 [DOI] [PubMed] [Google Scholar]
- 23.Alfalah M., Jacob R., Naim H. Y. (2002) J. Biol. Chem. 277, 10683–10690 [DOI] [PubMed] [Google Scholar]
- 24.Patsos G., Hebbe-Viton V., San Martin R., Paraskeva C., Gallagher T., Corfield A. (2005) Biochem. Soc. Trans. 33, 721–723 [DOI] [PubMed] [Google Scholar]
- 25.Patsos G., Robbe-Masselot C., Klein A., Hebbe-Viton V., Martin R. S., Masselot D., Graessmann M., Paraskeva C., Gallagher T., Corfield A. (2007) Biochem. Soc. Trans. 35, 1372–1374 [DOI] [PubMed] [Google Scholar]
- 26.Gouyer V., Leteurtre E., Delmotte P., Steelant W. F., Krzewinski-Recchi M. A., Zanetta J. P., Lesuffleur T., Trugnan G., Delannoy P., Huet G. (2001) J. Cell Sci. 114, 1455–1471 [DOI] [PubMed] [Google Scholar]
- 27.Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. (1985) J. Cell Biol. 101, 838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosentino S., Gravaghi C., Donetti E., Donida B. M., Lombardi G., Bedoni M., Fiorilli A., Tettamanti G., Ferraretto A. (2010) J. Nutr. Biochem. 21, 247–254 [DOI] [PubMed] [Google Scholar]
- 29.Yu S. Y., Wu S. W., Khoo K. H. (2006) Glycoconj. J. 23, 355–369 [DOI] [PubMed] [Google Scholar]
- 30.Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J. M., Yu S. Y., Kawashima H., Saito H., Ohtsubo K., Marth J. D., Khoo K. H., von Andrian U. H., Lowe J. B., Fukuda M. (2007) Nat. Immunol. 8, 409–418 [DOI] [PubMed] [Google Scholar]
- 31.Ju T., Cummings R. D. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai T., Inaba N., Naundorf A., Zhang Y., Gotoh M., Iwasaki H., Kudo T., Togayachi A., Ishizuka Y., Nakanishi H., Narimatsu H. (2002) J. Biol. Chem. 277, 12802–12809 [DOI] [PubMed] [Google Scholar]
- 33.Bierhuizen M. F., Fukuda M. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 9326–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machida E., Nakayama J., Amano J., Fukuda M. (2001) Cancer Res. 61, 2226–2231 [PubMed] [Google Scholar]
- 35.Schwientek T., Yeh J. C., Levery S. B., Keck B., Merkx G., van Kessel A. G., Fukuda M., Clausen H. (2000) J. Biol. Chem. 275, 11106–11113 [DOI] [PubMed] [Google Scholar]
- 36.Keiser M., Alfalah M., Pröpsting M. J., Castelletti D., Naim H. Y. (2006) J. Biol. Chem. 281, 14393–14399 [DOI] [PubMed] [Google Scholar]
- 37.Wetzel G., Heine M., Rohwedder A., Naim H. Y. (2009) Biol. Chem. 390, 545–549 [DOI] [PubMed] [Google Scholar]
- 38.Xia L., Ju T., Westmuckett A., An G., Ivanciu L., McDaniel J. M., Lupu F., Cummings R. D., McEver R. P. (2004) J. Cell Biol. 164, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huet G., Gouyer V., Delacour D., Richet C., Zanetta J. P., Delannoy P., Degand P. (2003) Biochimie 85, 323–330 [DOI] [PubMed] [Google Scholar]
- 40.Hauri H., Appenzeller C., Kuhn F., Nufer O. (2000) FEBS Lett. 476, 32–37 [DOI] [PubMed] [Google Scholar]
- 41.Yamashita K., Hara-Kuge S., Ohkura T. (1999) Biochim. Biophys. Acta 1473, 147–160 [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa T., Uozumi N., Nakano M., Mizuno-Horikawa Y., Okuyama N., Taguchi T., Gu J., Kondo A., Taniguchi N., Miyoshi E. (2006) J. Biol. Chem. 281, 29797–29806 [DOI] [PubMed] [Google Scholar]