Abstract
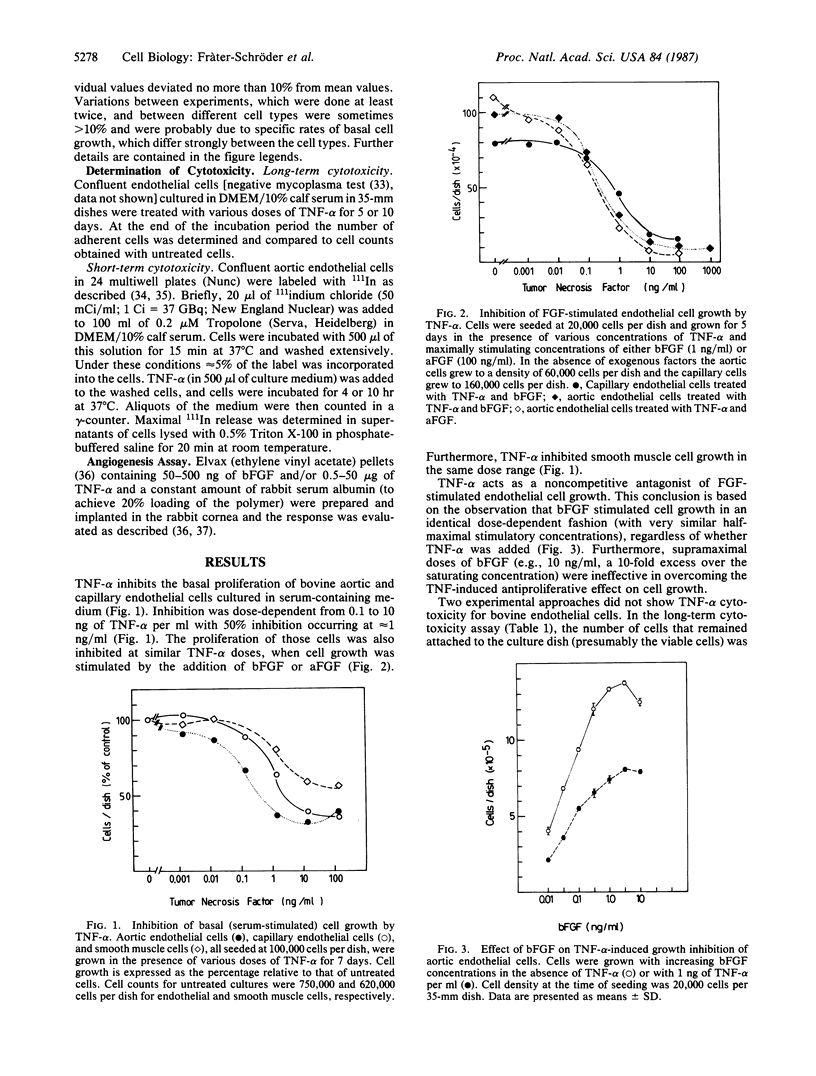
Tumor necrosis factor type alpha (TNF-alpha) inhibits endothelial cell proliferation in vitro. Basal cell growth (in the absence of exogenously added growth factor) and fibroblast growth factor (FGF)-stimulated cell proliferation are inhibited in a dose-dependent manner from 0.1 to 10 ng/ml with half-maximal inhibition occurring at 0.5-1.0 ng of TNF-alpha per ml. Bovine aortic and brain capillary endothelial and smooth muscle cells are similarly affected. TNF-alpha is a noncompetitive antagonist of FGF-stimulated cell proliferation. Its action on endothelial cells is reversible and noncytotoxic. Surprisingly, TNF-alpha does not seem to inhibit endothelial cell proliferation in vivo. In the rabbit cornea, even a high dose of TNF-alpha (10 micrograms) does not suppress angiogenesis induced by basic FGF. On the contrary, in this model system TNF-alpha stimulates neovascularization. The inflammatory response that is seen in the cornea after TNF-alpha implantation suggests that the angiogenic properties of this agent may be a consequence of leukocyte infiltration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Baird A., Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun. 1986 Jul 16;138(1):476–482. doi: 10.1016/0006-291x(86)90305-0. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bertoni G., Keist R., Groscurth P., Wyler R., Nicolet J., Peterhans E. A chemiluminescent assay for mycoplasmas in cell cultures. J Immunol Methods. 1985 Apr 8;78(1):123–133. doi: 10.1016/0022-1759(85)90335-7. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Baird A., Esch F., Ling N., Gospodarowicz D. Isolation and partial molecular characterization of pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5364–5368. doi: 10.1073/pnas.81.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Esch F., Baird A., Gospodarowicz D. Acidic fibroblast growth factor (FGF) from bovine brain: amino-terminal sequence and comparison with basic FGF. EMBO J. 1985 Aug;4(8):1951–1956. doi: 10.1002/j.1460-2075.1985.tb03876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danpure H. J., Osman S., Brady F. The labelling of blood cells in plasma with 111In-tropolonate. Br J Radiol. 1982 Mar;55(651):247–249. doi: 10.1259/0007-1285-55-651-247. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr J., Moser R., Leppert D., Groscurth P. Antiadhesive properties of biological surfaces are protective against stimulated granulocytes. J Clin Invest. 1985 Aug;76(2):535–542. doi: 10.1172/JCI112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fràter-Schröder M., Müller G., Birchmeier W., Böhlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986 May 29;137(1):295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Heimark R. L., Twardzik D. R., Schwartz S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986 Sep 5;233(4768):1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Heyns A. D., Eldor A., Vlodavsky I., Kaiser N., Fridman R., Panet A. The antiproliferative effect of interferon and the mitogenic activity of growth factors are independent cell cycle events. Studies with vascular smooth muscle cells and endothelial cells. Exp Cell Res. 1985 Dec;161(2):297–306. doi: 10.1016/0014-4827(85)90087-4. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cancer. Cell growth control mechanisms. Nature. 1986 Jul 3;322(6074):14–16. doi: 10.1038/322014a0. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Necrosin: purification and properties of a cytotoxin derived from a murine macrophage-like cell line. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7932–7936. doi: 10.1073/pnas.81.24.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu Y., Watanabe N., Sone H., Neda H., Yamauchi N., Urushizaki I. Mechanism of the cytotoxic effect of tumor necrosis factor. Jpn J Cancer Res. 1985 Dec;76(12):1193–1197. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Risau W. Developing brain produces an angiogenesis factor. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3855–3859. doi: 10.1073/pnas.83.11.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Goto T., Haranaka K., Satomi N., Nariuchi H., Mano-Hirano Y., Sawasaki Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986 Jun;76(6):1113–1121. [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Niitsu Y., Sone H., Neda H., Yamauchi N., Urushizaki I. Inhibitory effect of tumor necrosis serum on the metastasis of B-16 mouse melanoma cells. Jpn J Cancer Res. 1985 Oct;76(10):989–994. [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]