Abstract
Biomaterials have been shown to differentially support dendritic cell (DC) maturation, a prerequisite for an adjuvant effect. Treatment of DCs with poly(D,L-lactic-co-glycolic acid). (PLGA) films resulted in DC maturation but agarose films did not. In these studies, the biomaterial adjuvant effect was attenuated by material selection (PLGA or agarose scaffolds) or local delivery of an anti-inflammatory/immunosuppressive glucocorticoid, dexamethasone (DX), from PLGA scaffolds. Porous scaffolds (SCs) of PLGA or agarose were produced to deliver equivalent amounts of model antigen, ovalbumin (OVA). Alternatively, PLGA SCs with incorporated OVA were produced with or without DX. These SCs were implanted individually, subcutaneously, and dorsally in C57BL/6 mice. Blood was collected from mice at specific times over a 12-week duration for measurement of antibody production against OVA. Scaffolds were explanted at 12 weeks for histological examination of foreign body response. Scaffolds of PLGA, but not of agarose, were found to elicit higher antibody production against co-delivered OVA, than negative controls. Short term delivery of DX from PLGA SCs delivering OVA temporarily delayed onset of anti-OVA antibody production. More sustained release of DX at an effective dose and with an appropriate time course is expected to extend the effect of DX on the biomaterial adjuvant effect. The immunomodulatory ability of biomaterials to affect the immune response to co-delivered antigen is demonstrated wherein this immunomodulatory ability correlates with the observed in vitro differential effects of biomaterials on DC maturation.
Introduction
For functional tissue engineered devices, critical issues are maintenance of the cell phenotype, complex organ development with multiple cell types and 3-dimensionality, biomaterial scaffold design addressing mechanical, bio/chemical, and remodeling properties, and integration into living systems including host acceptance. While much consideration has been given issues such as cell source, induction and maintenance of cell differentiation, and development of novel scaffold materials, certain issues have received less attention, particularly how scaffold material selection can potentiate immune responses. Hence, scaffold material selection is a focus herein to improve host acceptance of tissue engineered devices.
Upon implantation, tissue engineered devices elicit an innate immune response towards the biomaterial scaffold, known as the foreign-body response[1-3]; these devices also may elicit an adaptive immune response towards incorporated allo- or xenogeneic cells or their shed antigens[4]. Adaptive immune responses are potentiated by adjuvants in the antigen delivery system. These principles of engaging innate and adaptive immune responses towards foreign antigens are utilized in the pharmaceutical formulations for vaccine delivery using polymers, such as chitosan[5-9] and PLGA[5, 6, 10-13]; whereas, it would be detrimental to tissue engineered device function to engage adaptive immune response to shed foreign antigens and induction of tolerance would be preferred.
Foreign antigens are presented to T cells (conductors of the adaptive immune response: cell mediated and humoral immunity) by antigen presenting cells (APCs): macrophages, B-lymphocytes and DCs. Of these cell types, DCs are the most potent APCs due to their unique ability to stimulate naïve T cells and are critical in linking innate and adaptive immunity[14, 15]. While DCs are immature, they reside in tissues and act as sentinels: detecting self and foreign antigens[16]. During the innate immune response, immature DCs recognize foreign components (particularly microbial- or viral-derived pathogen associated molecular patterns, PAMPs) through pattern recognition receptors (PRRs). Due to the signaling of PRRs, this recognition process results in DC maturation – heralded by stable, high levels of major histocompatibility complex (MHC) expression and necessary co-stimulatory molecule expression – that enables efficient antigen presentation and T cell stimulatory ability by DCs. In addition to the recognition of PAMPs, this recognition process by PRRs is also triggered in the presence of ‘danger signals’; endogenous indicators of tissue damage and cell stress[15, 17, 18]. Activators of PRR signaling and DC maturation are known as adjuvants. Mature DCs migrate to lymph nodes and stimulate T-cell activation for an antigen-specific response.
Since DCs are key in linking the innate and adaptive immune responses, we investigated modulation of in vitro immunological functionality of DCs by treatment with different biomaterials and the in vivo adaptive immune response to foreign antigens delivered with biomaterials. Specifically, DC treatment with PLGA or chitosan films induced DC maturation, while treatment with agarose or alginate films did not induce DC maturation[19, 20]. Interestingly, hyaluronic acid films inhibited DC maturation[19, 20]. The induction of DC maturation by treatment with PLGA fims in vitro[21] established a potential mechanism for the observed adjuvant effect of PLGA SCs. Specifically, PLGA SCs were shown to enhance the antigen-specific humoral immune response[22] and clonal T cell proliferation[23] to co-delivered model antigen. Furthermore, PLGA scaffolds, with associated tissue damage due to implantation, supported a high and sustained humoral immune response to co-delivered OVA, while OVA delivered from minimally-invasively injected PLGA microparticles induced a moderate and transient humoral immune response to co-delivered OVA[24]. These studies have demonstrated the potential for biomaterial scaffolds to act as adjuvants in enhancing the adaptive immune response to co-delivered antigen, presumably due to a material effect on DC maturation. Furthermore, tissue damage associated with the implantation of a construct may prime the site for the biomaterial adjuvant effect due to the generation of endogenous ‘danger signals’.
Since TE devices are intended to integrate well with the surrounding environment, strategies to minimize or avoid adjuvant effects warranted investigation. In the studies presented herein, we hypothesized the translation of the differential biomaterial effect on DC phenotype observed in vitro to the immunomodulation of the humoral immune response (adjuvant effect or not) to co-delivered antigen in vivo. For this purpose, equivalent amounts of a model antigen, OVA, were delivered from PLGA or agarose scaffolds due to their opposing effects of these materials on DC maturation in vitro[19, 20]. After implantation of porous SCs of PLGA or agarose delivering OVA, the humoral response was assessed with respect to extent of OVA-specific antibody production. In this way, a material-based means of influencing the extent of a biomaterial adjuvant effect was demonstrated.
Another approach to modulate a known biomaterial adjuvant effect was to locally deliver an anti-inflammatory/immunosuppressive glucocorticoid, DX. Not only does DX have known anti-inflammatory effects, it also has a noted effect of suppressing DC functional maturation[25-28], inducing a tolerogenic state in DCs prior to maturation[25, 27, 29], and after maturation, inducing DC-mediated humoral immunity through T-cell helper 2 response[25, 27]. For this purpose, SCs were prepared from PLGA, a biomaterial adjuvant[24], to deliver OVA with or without incorporation of DX. After SC implantation in mice, the resulting humoral immune response was determined by measuring OVA-specific antibody production. The delivery of DX had the potential to reduce the adjuvant effect of the PLGA either through a minimization of the inflammatory response or through a more specific effect on DC functional status.
Materials and Methods
Preparation of Polymer SCs
Preparation of Agarose SCs
Agarose SCs were prepared using by inverted colloidal crystal templating method using polystyrene (PS) beads as the leachable component based on a previously reported technique[30, 31], with some modifications, as described in the Supplemental Methods section. The agarose solution was prepared from SeaPlaque agarose (Lonza, Rockland, ME) as 3% (w/v) in distilled, deionized water (ddH2O) and the PS beads were 100 μm in diameter [30 ml with 2.1% (w/v) solids content (Duke Scientific, Palo Alto, CA)]. Agarose SCs (0.8 cm in diameter) were washed with 70% ethanol and sterile ddH2O and UV sterilized prior to implantation. The endotoxin content of agarose SCs was measured by Limulus Amebocyte Assay (Lonza, Walkersville, MD) according to manufacturer’s instructions, with SCs in the presence of the reagents until the addition of the stop reagent.
Preparation of PLGA SC
The PLGA SCs were prepared using the solvent casting, particulate leaching method[24] with minor modifications, as described in the Supplemental Methods section. The polymer solution was 75:25 PLGA (intrinsic viscosity = 0.55 – 0.75 dL/g, DURECT Corporation, Cupertino, CA) dissolved in dichloromethane (DCM) at 83.3 mg/ml. For preparing PLGA SC with co-delivered OVA, OVA was added to the PLGA solution at 166 mg/ml. For SCs containing DX, DX (Sigma) of was dissolved in PLGA solution at 10 mg/ml. The respective PLGA solutions were poured over NaCl particles (90-120 μm particle size; 0.3 g NaCl/mL PLGA solution), DCM evaporated and the salt leached out into water over several days. Dried PLGA SCs (0.8 cm in diameter) were cut, stored and endotoxin content determined as described for agarose SC.
In Vitro Characterization of Polymer SCs
Final OVA contents and OVA encapsulation efficiencies for agarose or PLGA SCs were determined. OVA concentrations in scaffold digest samples were determined using a custom-designed ELISA technique as previously described with modifications [32]. Dexamethasone content of scaffold digest samples was determined using HPLC as previously described [33]. Controlled release kinetics of DX from PLGA SCs was determined by placing the scaffolds in a sink of Dulbecco’s PBS (D-PBS, pH 7.4) at 37°C with samples of release buffer taken over 90 days and analyzed by HPLC. Details of these methods are found in the Supplemental Methods section.
Humoral Immune Response Assay
Animals
Animal care and treatment were in compliance with the Institution Animal Care and Use Committee at Emory University (Protocol #161-2006). Male C57BL/6 mice (8 weeks old; Jackson Labs, Bar Harbor, ME) were housed 6 mice per cage and allowed to acclimate for 1 week prior to receiving experimental treatments.
Co-Delivery of OVA with Polymer SC in Mice and Analysis of Humoral and Tissue Response
Mouse treatment groups are summarized in Tables 1 and 2 including amounts of OVA mass delivered (Tables 1 and 2), amounts of DX delivered (Table 2), amounts of polymer delivered and endotoxin contents (Tables 1 and 2). Isofluorane was used for induction and maintenance of anesthesia. For examining the effect of material selection, C57BL/6 mice received a dorsal subcutaneous implantation of a PLGA or agarose SC (2 mm thickness, 8 mm diameter) with or without OVA. For assessing the impact of anti-inflammatory drug delivery, C57BL/6 mice received a dorsal subcutaneous implantation of a PLGA SC with or without OVA each also with or without DX. Immunization with PBS with OVA served as negative control and immunization with OVA in PBS in a 1:1 dilution with Complete Freund’s Adjuvant (CFA) (Sigma) served as the positive control. Three weeks after primary immunization, all mice received an injected PBS boost solution containing OVA at the same amount as the initial delivery except for those in the positive control CFA groups which received the same amount of OVA in PBS emulsified at 1:1 in Incomplete Freund’s Adjuvant (IFA) (Sigma). Blood samples were collected from mice by retro-orbital bleeding at 2, 3 (before boost), 4, 8, and 12 weeks after primary immunization. Blood was allowed to clot at 4°C overnight and serum recovered by centrifugation (twice at 2300g, 10 min). Serum was stored at - 20°C until analysis of anti-OVA total IgG antibodies and isotypes (IgG1, IgG2a) by using an ELISA technique[22, 24], described in the Supplemental Methods section. After 12 weeks of implantation, SCs and associated tissues were excised and processed for histological and image analysis as described in the Supplemental Methods section.
Table 1.
For each treatment group, amount of OVA delivered, OVA encapsulation efficiency, OVA:polymer ratio, amount of polymer mass delivered and endotoxin contents are presented. Data are shown as mean ± SD (n = 3-4 SCs); NA=not applicable.
| Treatment Groups (7 mice per treatment group) | ||||||
|---|---|---|---|---|---|---|
| PLGA SC | PLGA OVA SC | Agarose SC | Agarose OVA SC |
PBS | CFA | |
| OVA delivered (μg) |
NA | 29.3 ± 14.7 | NA | 18.1 ± 5.2 | 23.7 | 23.7 |
| Percent OVA encapsulation efficiency |
NA | 0.17 ± 0.06% | NA | 54.5 ± 20.5% | NA | NA |
| OVA to polymer ratio (mg/mg) |
NA | 0.003 ± 0.001 | NA | 0.019 ± 0.006 | NA | NA |
| Polymer mass delivered (mg) |
10.6 ± 0.1 | 8.7 ± 1.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | NA | NA |
| Endotoxin (EU/ml) | <0.1 | 0.15 ± 0.02 | 0.22 ± 0.04 | 0.28 ± 0.04 | NA | NA |
Table 2.
For each treatment group, amount of OVA delivered, OVA encapsulation efficiency, OVA:polymer ratio, amount of DX delivered, DX encapsulation efficiency, DX:polymer ratio, amount of polymer mass delivered and endotoxin contents are presented. Data are shown as mean ± SD (n=3-8 SCs); NA=not applicable.
| Treatment Groups (8 mice per treatment group) | ||||||
|---|---|---|---|---|---|---|
| PLGA SC | PLGA OVA SC | PLGA DX SC | PLGA DX OVA SC |
PBS | CFA | |
| OVA delivered (μg) |
NA | 33.9 ± 9.1 | NA | 35.0 ± 22.7 | 34.4 | 34.4 |
| Percent OVA encapsulation efficiency |
NA | 0.14 ± 0.02% | NA | 0.14 ± 0.04% | NA | NA |
| OVA to polymer ratio (mg/mg) |
NA | 0.003 ± 0.001 | NA | 0.003 ± 0.001 | NA | NA |
| DX delivered (μg) | NA | NA | 5.3 ± 1.4 | 15.0 ± 8.7 | NA | NA |
| Percent DX encapsulation efficiency |
NA | NA | 13 ± 3% | 31 ± 7% | NA | NA |
| DX to polymer ratio (mg/mg) |
NA | NA | 0.0006 ± 0.0001 |
0.0014 ± 0.0003 |
NA | NA |
| Polymer mass delivered (mg) |
11.9 ± 3.9 | 11.3 ± 2.5 | 11.3 ± 2.1 | 10.6 ± 2.0 | NA | NA |
| Endotoxin (EU/ml) | 0.12 ± 0.02 | >1.0 | <0.1 | >1.0 | NA | NA |
Data Analysis
Four parameter logistical fit curves for ELISA were produced using GraphPad Prism 4.03 software (GraphPad Software, Inc., LaJolla, CA). Statistical differences between negative control and treatment groups was determined using one-way ANOVA at each time point with Dunnett’s post-tests using Minitab 13 software (Minitab, Inc., State College, PA). Statistical significance was noted when p<0.05.
Results
OVA and DX content of implanted agarose SCs and PLGA SCs
In Tables 1 and 2, mouse treatment groups are summarized including mass of OVA delivered (Tables 1 and 2), mass of DX delivered (Table 2), mass of polymer delivered and endotoxin contents (Tables 1 and 2). The data characterizing the delivery vehicles in Table 1 is for the experiment to compare the adjuvant effect of PLGA SC versus agarose SC. The data characterizing the delivery vehicles in Table 2 is for the experiment to assess the effect of DX delivery to mitigate the adjuvant effect of PLGA. Positive and negative control groups received OVA at equivalent amounts as for the scaffold treatment groups (Table 1 and 2). For the experiment to compare the adjuvant effect of PLGA SC versus agarose SCs, the OVA contents for the respective scaffolds were similar and hence suitable for in vivo comparison (Table 1). The scaffolds were measured for endotoxin content and found to have low levels (<0.5 EU/ml) (Table 1 and 2).
Histological examination of implanted agarose or PLGA SC from C57BL/6 mice
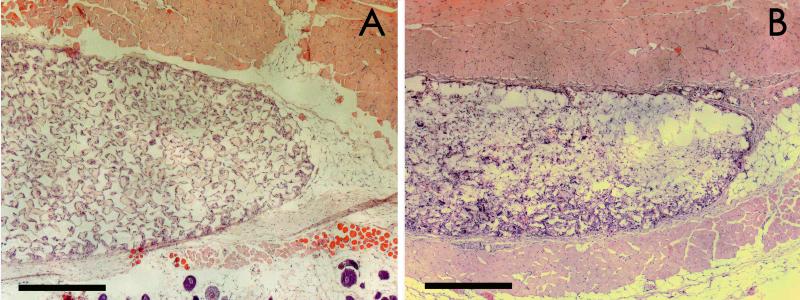
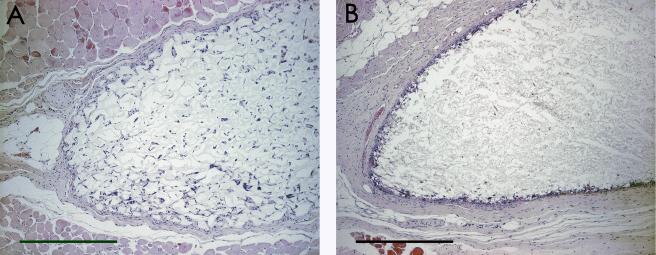
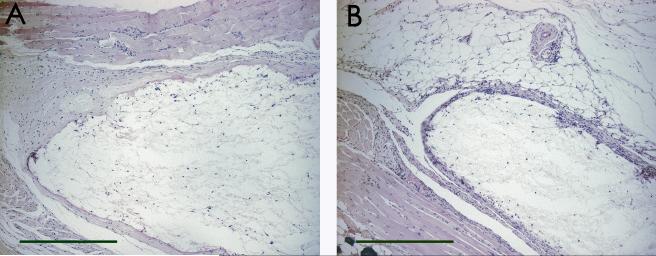
Histological analysis of the tissue reaction to agarose SCs (Figure 1a) or PLGA SCs (Figure 1b) demonstrated a moderate tissue reaction with cellular infiltration into both scaffold materials. More uniform cellular infiltration was observed for the agarose SC, prepared by a sphere templating method, than with the PLGA SC, prepared by a salt-leaching method (Figure 1 and 2). Compared to the respective scaffold tissue reactions without OVA, incorporation of OVA into the agarose SCs did not significantly affect the extent of the tissue reaction or extent of cellular infiltration (Figure 2a), while less cellular infiltration and thicker tissue reactions at the periphery of the scaffolds were observed for PLGA SCs with incorporated OVA (Figure 2b). Incorporation of DX in the PLGA SC resulted in a diminished tissue reaction surrounding the scaffold implant (Figure 3a). However, incorporation of DX into PLGA SCs also containing OVA did not demonstrate the benefit of a diminished tissue reaction due to DX delivery (Figure 3b), since the tissue reaction was similar to that elicited by PLGA SCs with OVA (Figure 2b).
Figure 1.
Histological images of subcutaneous tissue sections from C57BL/6 mice after 12 weeks of polymer SC implantation. Tissue sections were stained with H&E. Panels display both SC materials: A) agarose SC and B) PLGA SC. Scale bars are equal to 500 μm.
Figure 2.
Histological images of subcutaneous tissue sections from C57BL/6 mice after 12 weeks of polymer SC implantation with OVA incorporation. Tissue sections were stained with H&E. Panels display both SC materials: A) agarose OVA SC and B) PLGA OVA SC. Scale bars are equal to 500 μm.
Figure 3.
Histological images of subcutaneous tissue sections from C57BL/6 mice after 12 weeks of PLGA SC implantation with DX incorporation. Tissue sections were stained with H&E. Panels display both SC materials: A) PLGA DX SC and B) PLGA DX OVA SC. Scale bars are equal to 500 μm.
Humoral response to co-delivered antigen from PLGA or agarose SCs
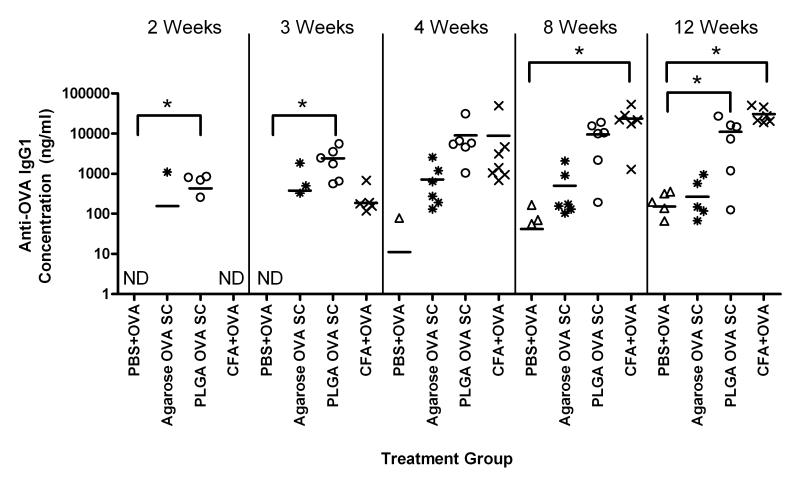
In Figure 4, the serum anti-OVA IgG production is shown for mice receiving similar amounts of OVA delivered with PBS, PLGA SC, agarose SC, or CFA. The mice receiving OVA emulsified in the strong adjuvant, CFA, as the positive control, demonstrated high levels of anti-OVA IgG antibody that were significantly higher than the levels observed for the negative control of OVA in PBS at 8 and 12 weeks. At 2, 3, and 4 weeks, delivery of OVA from PLGA SCs (PLGA OVA SC) was found to induce significantly higher levels of anti-OVA IgG as compared to the levels for the negative controls at the respective times. In contrast, delivery of OVA from agarose SCs (Agarose OVA SC) did not induce significantly different levels of anti-OVA IgG production as compared to negative control at any time point. The implantation of PLGA or agarose SCs without co-delivered OVA did not induce the production of anti-OVA antibodies during the 12-week study.
Figure 4.
Production of anti-OVA IgG antibodies by C57BL/6 mice treated with OVA co-delivered with PBS (PBS+OVA), agarose SC (Agarose OVA SC), PLGA SC (PLGA OVA SC) or CFA (CFA+OVA). Total IgG concentrations were measured by ELISA from sera collected at different times. No detectable anti-OVA antibodies were produced from mice receiving SC without OVA. Data are shown as replicates with means represented as a line (n=5-7 mice). * indicates significant difference between treatment group and negative control. ND indicates none of the collected sera samples contained detectable amount of antibody.
The participation of T helper (TH) cells, particularly TH1 and TH2 cells influence the immune response to co-delivered antigen, leading to the production of specific IgG isotypes, namely IgG2a and IgG1 isotypes, respectively. No detectable serum levels of anti-OVA IgG2a were observed for mice receiving OVA from PLGA SC, agarose SC or with the negative control of PBS. Mice receiving OVA emulsified with CFA as the positive control demonstrated detectable levels of anti-OVA IgG2a at weeks 4, 8, and 12 (data not shown). The production of anti-OVA IgG1 antibodies was detected for mice receiving OVA with either scaffold material or the controls (Figure 5). Initially, at 2 and 3 weeks, delivery of OVA from PLGA SCs induced levels of anti-OVA IgG1 which were statistically higher than the levels for the negative control of OVA in PBS. At later time points (8 and 12 weeks), mice receiving OVA emulsified with CFA as the positive control group demonstrated levels of anti-OVA IgG1 that were significantly higher than the levels for the negative control at the corresponding time points. Furthermore, at 12 weeks, delivery of OVA from PLGA SCs induced significantly higher levels of anti-OVA IgG1 as compared to the level for the negative control. At no time did delivery of OVA from agarose SC induce levels of anti-OVA IgG1 which were significantly greater than the negative control.
Figure 5.
Production of anti-OVA IgG1 antibodies by C57BL/6 mice treated with OVA co-delivered with PBS, CFA, or polymer SC. Antibody concentrations were measured by ELISA from serum collected at different times. No detectable anti-OVA antibodies were produced from mice receiving SC without OVA. Data are shown as replicates with means represented as a line (n=5-7 mice). * indicates significant difference between treatment group and negative control. ND indicates none of the collected sera samples contained detectable amount of antibody.
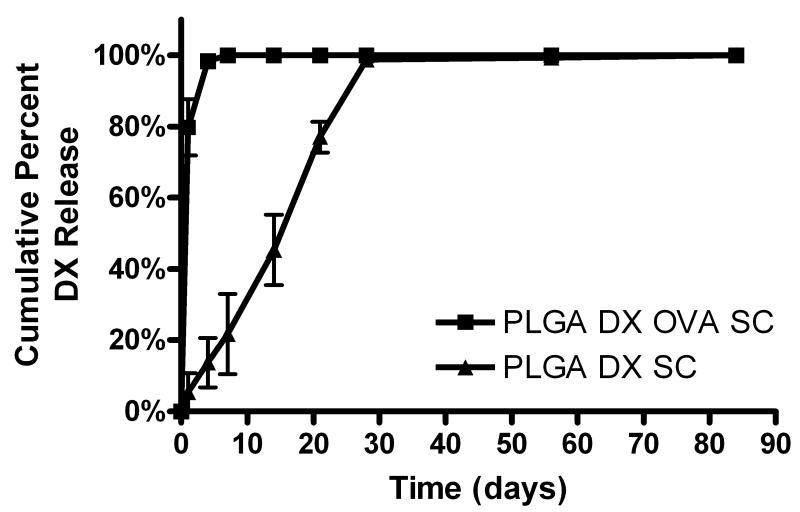
In vitro release of DX from PLGA SC
Given the adjuvant effect observed for PLGA SCs, PLGA SC were modified to incorporate co-release of an anti-inflammatory/immunosuppressive glucocorticoid, DX, to assess if the PLGA adjuvant effect was abrogated. DX release into PBS (sink condition) from PLGA SCs with and without incorporated OVA was assessed and presented as cumulative profiles in Figure 6. PLGA SC prepared with DX and OVA (PLGA DX OVA SC) showed a burst release of DX initially with little to no detectable release of DX after the first 4 days. In contrast, PLGA SC prepared with DX (PLGA DX SC) demonstrated steady release of DX for the first 28 days of study. During these first 4 weeks, the majority of DX was released from PLGA DX SC. No DX was detected released from control PLGA SC prepared without DX for the duration of the in vitro release study, as expected.
Figure 6.
Cumulative DX release from PLGA SC with (PLGA DX OVA SC) and without (PLGA DX SC) incorporated OVA over 84 days (n=3 SCs). Each SC was placed in 4 ml of PBS at pH 7.4 and rotated at 37°C. At each time point, saline samples were collected and replaced with fresh PBS of the same volume. Data are shown as percentage of the total DX release for SC (mean ± SD).
Humoral response to co-delivered antigen with or without anti-inflammatory/immunosuppressive glucocorticoid, DX, from PLGA SCs
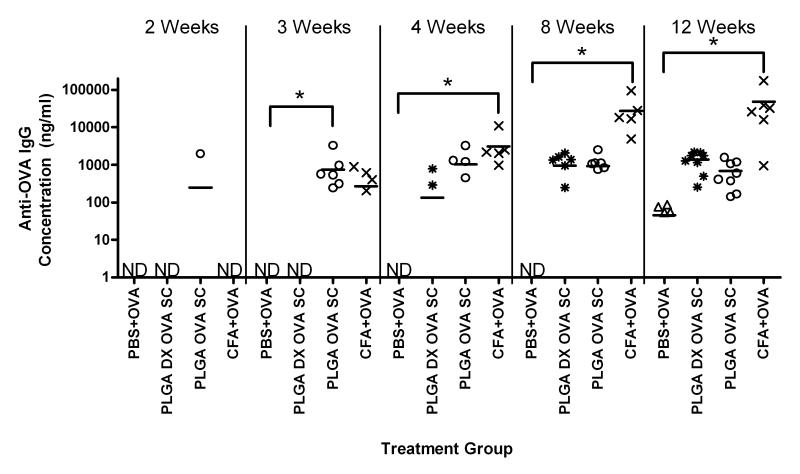
Serum levels of anti-OVA IgG production for mice receiving similar amounts of OVA delivered with PBS, CFA, PLGA SC with or without incorporated DX are shown in Figure 7. Mice receiving OVA emulsified in CFA, as the positive control, demonstrated significantly higher levels of anti-OVA IgG production compared to the levels for mice in the negative control of OVA delivered with PBS, at 4, 8, and 12 weeks. Delivery of OVA from PLGA SCs induced levels of anti-OVA IgG that were significantly different from the level for the negative control, but at the 3 week time point only. However, it was observed that the emergence of detectable levels of anti-OVA IgG for mice receiving PLGA DX OVA SC lagged behind mice receiving PLGA OVA SC. This was apparent because there were not detectable levels of anti-OVA IgG for mice who received PLGA DX OVA SC until 4 weeks after the initial implantation. Despite this delayed response, by 12 weeks delivery of OVA from PLGA SCs, regardless of the co-delivery of DX, demonstrated similar levels of anti-OVA IgG. Implantation of PLGA SCs without co-delivered OVA did not induce detectable levels of anti-OVA antibodies during the 12-week study as expected.
Figure 7.
Production of anti-OVA IgG antibodies by C57BL/6 mice treated with OVA co-delivered with PBS (PBS+OVA), CFA (CFA+OVA), or PLGA SC with (PLGA DX OVA SC) or without (PLGA OVA SC) incorporated DX. Total IgG concentrations were measured by ELISA from sera collected at different times. No detectable anti-OVA antibodies were produced from mice receiving SC without OVA. Data are shown as replicates with means represented as a line (n=6-8 mice). * indicates significant difference between treatment group and negative control. ND indicates none of the collected sera samples contained detectable amount of antibody.
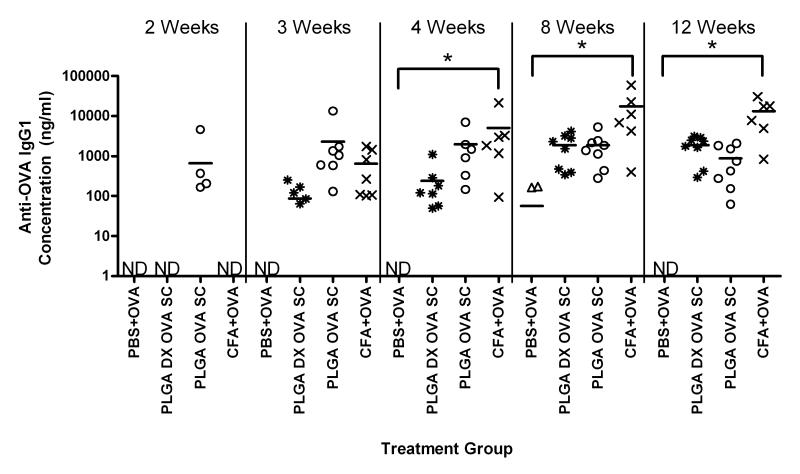
Examining the anti-OVA IgG isotypes induced by OVA delivery from PLGA SC with and without DX co-delivery, IgG1 was the predominant isotype detected (Figure 8). There were no detectable levels of anti-OVA IgG2a isotype in the sera samples from mice receiving OVA in PBS (negative control), or PLGA SCs delivering OVA with and without DX. Only mice that received OVA emulsified in CFA demonstrated detectable levels of anti-OVA IgG2a at weeks 4, 8, and 12 (data not shown). In Figure 8, the sera concentrations of anti-OVA IgG1 antibodies are shown for mice receiving similar amounts of OVA delivered with PBS, CFA, PLGA OVA SC or PLGA DX OVA SC. Mice receiving OVA emulsified in CFA showed significantly higher levels of anti-OVA IgG1 as compared to the level for mice in the negative control group at 4, 8, and 12 weeks. Although anti-OVA IgG1 antibodies were detected early for mice receiving OVA from PLGA SC, the levels were not significantly different from the levels observed for the negative control group at any time point. Likewise, OVA delivered from PLGA SCs with co-delivered DX also did not induce levels of anti-OVA IgG1 that were significantly different from the levels for the negative control group at any time point. Co-delivery of DX from PLGA SCs delivering OVA did delay the production of detectable levels of anti-OVA IgG1 antibodies as compared to the levels for mice receiving PLGA OVA SC (without DX). However, by 12 weeks, mice receiving OVA from PLGA SC with or without DX showed similar concentrations of anti-OVA IgG1 antibodies in their sera.
Figure 8.
Production of anti-OVA IgG1 antibodies by C57BL/6 mice treated with OVA co-delivered with PBS, CFA, or PLGA SC with or without incorporated DX. Total IgG concentrations were measured by ELISA from serum collected at different times. No detectable anti-OVA antibodies were produced from mice receiving SC without OVA. Data are shown as replicates with means represented as a line (n=6-8 mice). * indicates significant difference between treatment group and negative control. ND indicates none of the collected serum samples contained detectable amount of antibody.
Discussion
To correlate the in vitro observations of the differential biomaterial effect on DC maturation with a differential in vivo adjuvant effect, we hypothesized that PLGA scaffolds would stimulate a stronger murine adaptive immune response to co-delivered antigen than agarose scaffolds. The results of this study showed that PLGA SCs acted as an adjuvant in the enhancement of anti-OVA IgG levels that were significantly higher than for OVA delivered with the negative control (Figure 4), consistent with previous studies[22]. Agarose SCs did not show an adjuvant effect in the enhancement of the humoral immune response towards co-delivered OVA (Figure 4). Since IgG1 was the primary IgG isotype detected for all treatment groups, with very little IgG2a isotype detected except for the CFA treatment group, this was primarily a TH2 immune response, consistent with previous studies[22, 24]. The differential affect of PLGA or agarose SCs on the humoral immune response to co-delivered antigen, correlates with the observed in vitro effects of these biomaterials on DC phenotype[19, 20] as a predictor of the adjuvant effect in supporting (or not) a humoral immune response to co-delivered antigen. Furthermore, these in vivo results support the hypothesis that biomaterial selection can be used to modulate the adaptive immune response to co-delivered antigens. In support of this connection between DC contact and biomaterial adjuvant effect, immunohistochemistry has demonstrated DEC-205+ DC infiltration and contact with PLGA SCs with co-delivered OVA[34]. The dependence on DCs for the biomaterial adjuvant effect will be further demonstrated in the near future by conditional DC ablation based on transgenic expression of the diphtheria toxin (DT) receptor under control of the CD11c receptor in B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J mice during the time period of OVA delivery from PLGA SC/antigen constructs[35].
Exposure to OVA to organic solvents, (e.g. DCM), can result in protein denaturation. Uptake of proteins by DCs results in their degraration small peptide fragments are displayed on MHC molecules. The resultant adaptive immune response is based on T-cell recognition of proteins by these peptide fragments or epitopes. While denaturation can hide certain regions, hindering recognition by some T-cells, other peptide sequences will likely be available for recognition, even on any denatured proteins.
The foreign body responses towards PLGA or agarose SCs without incorporated OVA were similar (Figure 1). One difference noted was that porous agarose SCs had more evenly distributed cellular infiltrate than in the porous PLGA SCs (Figure 1 and 2) likely due to the regular porous structure generated by the inverted colloidal crystal templating technique[30, 31, 36]. Although, we did not assess the residual THF in the agarose scaffolds, the scaffolds did not produce significant inflammation. Therefore, we have no evidence that residual THF, if any, produced an undesirable effect with respect to increased inflammation. Incorporation of OVA into the respective scaffolds did not significantly affect the extent of the tissue reaction or extent of cellular infiltration into agarose SCs (Figure 2a), but less cellular infiltration and thicker tissue reactions at the periphery of the scaffolds were observed for PLGA SCs with incorporated OVA (Figure 2b). This may indicate some effect of the more inflammatory PLGA material in being affected by the presence of a foreign antigen on the tissue response. Incorporation of DX in the PLGA SC resulted in a diminished tissue reaction surrounding the scaffold implant (Figure 3a) as expected based on the anti-inflammatory effects of DX. However, incorporation of DX into PLGA SCs also containing the model antigen, OVA, did not demonstrate the benefit of a diminished tissue reaction due to DX delivery (Figure 3b). This extent of tissue reaction after 12 weeks of implantation is hence consistent with the observation that by 12 weeks of delivery of OVA from PLGA SCs, regardless of the co-delivery of DX, similar levels of anti-OVA IgG antibodies were induced (Figure 7). The encapsulation efficiency for DX incorporation was higher for PLGA DX OVA SCs than for PLGA DX SCs resulting in a higher amount of DX incorporated (and delivered) from the former as compared to the latter (Table 2). This difference was presumably due to the inclusion of OVA in the PLGA DX OVA SC group influencing the encapsulation efficiency of DX. However, these formulations were viable to address the aim of this study which was to compare the immune responses to OVA with and without DX release from PLGA SC. The PLGA DX SC group was a control to demonstrate that an immune response would not occur in the absence of OVA.
Although many immunosuppressive drugs have been largely characterized for their effect on T-cell function and proliferation, there are significant effects of these immunosuppressive drugs on DCs. Anti-proliferative drugs (azathioprine and mycophenolate mofetil), calcineurin inhibitors (cyclosporine A and FK506), rapamycin, and glucocorticoids influence DC maturation and T-cell stimulation[37]. Among these drugs, glucocorticoids have potent anti-inflammatory activity as well as broad immunosuppressive activity, affecting DCs, monocytes/macrophages, and T-cells[27, 28, 37, 38]. Glucocorticoids are effective anti-inflammatory agents because they regulate protein synthesis leading to reduced collagen deposition, vasodilation, edema formation, and immune cell recruitment to wound site[39-45]. In vitro DX treatment of immature DC has been shown to reduce the cell surface expression of CD14, CD16, CD32, CD36, and certain C-type lectins (DC-SIGN, mannose receptor, macrophage galatose-type lectin) and of co-stimulatory molecules (CD40 and CD80)[26, 46, 47]. Furthermore, treatment of immature DCs with DX in the presence of maturation stimuli, rendered a cell phenotype (CD14+ CD83−) different from mature DC (CD14− CD83+) and immature DC (CD14− CD83−)[46]. The effect of DX administration on cultured DCs was greatly influenced by when DX was introduced to the cell culture medium (prior to or after addition of maturation stimuli). Expression of Toll-like receptors, TLR2, TLR3, and TLR4, have been shown to be increased in DC that were treated with DX while they were differentiating and in an immature state[25, 48]. As a result, DC differentiated in the presence of DX had an enhanced endocytic capacity, reduced production of pro-inflammatory cytokines (e.g. IL-1β, and IL-12 p70), increased production of anti-inflammatory cytokine, IL-10, and had reduced allo-stimulatory capacity in a mixed lymphocyte assay[25, 26, 49-51]. In contrast, DCs treated with DX after differentiation, either prior or during maturation treatment, have some differing responses to maturation stimuli (i.e. reduced endocytic capacity, no upregulation of CD16 expression, reduced pro-inflammatory cytokine production, and slightly lowered allostimulatory capacity)[25, 26]. The alteration in DC phenotype in the presence of DX may induce the production of tolerogenic DCs, capable of antigen uptake but reduced capacity to stimulate immune responses. In vivo DX administration prior to allergen challenge has been shown to lower Langerhans numbers in lymph nodes[52]. Topical DX treatment reduced Langerhans cell size and density during afferent and efferent phases of contact sensitivity in situ[53]. Graft survival was improved with adoptive transfer of immature DC with systemic DX treatment or adoptive transfer of immature DCs treated with DX prior to transfer[54].
Short-term, local DX delivery was found to temporarily delay the onset of anti-OVA antibody production for PLGA SC delivering OVA (Figures 7 and 8). In vitro release studies demonstrated DX release from PLGA SCs over a short period (Figure 6), with majority of DX was released after 7 days form PLGA DX OVA SCs and after 28 days from PLGA DX SCs. Once the DX release from SC was depleted, antibodies against OVA were detected, no longer suppressed by anti-inflammatory drug administration. More sustained release of DX at an effective concentration for anti-inflammatory/suppression of DC function is expected to prolong the inhibition of an adjuvant effect and will be investigated in the future. Another consideration is the optimization of DX dose and timing for DC treatment as they contact the biomaterial maturation stimulus to render them non-stimulatory for the antigen taken up in this process or toleragenic. In vivo DX treatment of DCs prior to their maturation upon biomaterial treatment would be optimal for inducing toleragenic or maintaining immature DCs. In summary, anti-inflammatory drug delivery is only effective as long as the drug is available at therapeutic levels. Consequently, effective inhibition of a biomaterial adjuvant effect would require determining an effective dosing strategy for DX.
A most significant implication of the results of this study is that delivery of immunogens from agarose SCs is a more attractive approach than delivery of an immunosuppressive drug via controlled release for two reasons: (1) agarose is a material known to not support DC maturation in vitro or to keep DCs in an immature phenotype and (2) the immunosuppressive effect of agarose is longer-lasting (up to 12 weeks the latest time point in this study) and non-pharmacological in its mode of action. The effect of the agarose is also attractive in that it appears to influence host immune response by affecting the DCs and their microenvironment. Through their biomaterial-induced non-inflammatory/immunosuppressive phenotype, the DCs are better able to influence multiple aspects of the cellular and molecular environment (e.g. cytokine profile). In addition, biomaterial-influenced DCs may be better able to tune the host response than through aggressive drug means to achieve immune acceptance of a construct. Future studies include the demonstration of enhanced integration and acceptance of immunogenic cells delivered in a scaffold with less of an adjuvant effect (e.g. agarose) as compared to cell delivery in a scaffold with a known adjuvant effect (e.g. PLGA).
Conclusions
From these studies, we have demonstrated that biomaterial selection, based on in vitro effects on DC maturation, is a powerful tool for controlling the adaptive immune response to co-delivered antigen. The immunomodulatory ability of biomaterials has greater potential than even controlled release of local anti-inflammatory/immunosuppressive due to limited release rate and dosage and the more encompassing and sustained effect of biomaterials on immune microenvironment. Furthermore, we have validated the in vitro assessment of biomaterial effects on DC maturation in predicting the in vivo biomaterial adjuvant effect to co-delivered antigen.
Supplementary Material
Acknowledgements
The authors would like to acknowledge funding from NSF CAREER grant (BES-0239152), NIH RO1EB004633-02, and UNCF-Merck Postdoctoral Fellowship. The authors acknowledge the assistance of Sha’Aqua Asberry for histological services in the Parker H. Petit Institute for Bioengineering and Bioscience (IBB) Sample Analysis Lab and of Todd Rogers in histological analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Padera RF, Colton CK. Time course of membrane microarchitecture-driven neovascularization. Biomaterials. 1996;17:277–284. doi: 10.1016/0142-9612(96)85565-7. [DOI] [PubMed] [Google Scholar]
- [2].Sieminski AL, Gooch KJ. Biomaterial-microvasculature interactions. Biomaterials. 2000;21:2233–2241. doi: 10.1016/s0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- [3].Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- [4].Loudovaris T, Mandel TE, Charlton B. CD4+ T cell mediated destruction of xenografts within cell-impermeable membranes in the absence of CD8(+) T cells and B cells. Transplantation. 1996;61:1678–1684. doi: 10.1097/00007890-199606270-00003. [DOI] [PubMed] [Google Scholar]
- [5].Slutter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider YJ, Van Riet E, Jiskoot W, Preat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release. 2009;138:113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [6].Singh R, Singh S, Lillard JW. Past, present, and future technologies for oral delivery of therapeutic proteins. J. Pharm. Sci. 2008;97:2497–2523. doi: 10.1002/jps.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kang ML, Cho CS, Yoo HS. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol. Adv. 2009;27:857–865. doi: 10.1016/j.biotechadv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- [8].Slutter B, Soema PC, Ding Z, Verheul R, Hennink W, Jiskoot W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J. Control. Release. 2010 doi: 10.1016/j.jconrel.2010.01.007. in press. [DOI] [PubMed] [Google Scholar]
- [9].Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines. 2009;8:937–953. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- [10].Lu JM, Wang XW, Marin-Muller C, Wang H, Lin PH, Yao QZ, Chen CY. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, Torensma R, Figdor CG. Targeted PLGA nano- but no microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control. Release. 2010 doi: 10.1016/j.jconrel.2010.02.013. in press. [DOI] [PubMed] [Google Scholar]
- [12].Thomas C, Gupta V, Ahsan F. Influence of surface charge of PLGA particles of recombinant hepatitis B surface antigen in enhancing systemic and mucosal immune responses. Int. J. Pharm. 2009;379:41–50. doi: 10.1016/j.ijpharm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [13].Kanazawa T, Takashima Y, Murakoshi M, Nakai Y, Okada H. Enhancement of gene transfection into human dendritic cells using cationic PLGA nanospheres with a synthesized nuclear localization signal. Int. J. Pharm. 2009;379:187–195. doi: 10.1016/j.ijpharm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- [14].Munn DH. Tolerogenic antigen-presenting cells. Ann. N. Y. Acad. Sci. 2002;961:343–345. doi: 10.1111/j.1749-6632.2002.tb03119.x. [DOI] [PubMed] [Google Scholar]
- [15].Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- [16].Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- [17].Rock KL, Hearn A, Chen CJ, Shi Y. Natural endogenous adjuvants. Springer Semin. Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- [18].Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- [19].Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J. Biomed. Mater. Res. A. 2005;74A:503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- [20].Yoshida M, Babensee JE. Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation. J. Biomed. Mater. Res. A. 2006;79A:393–408. doi: 10.1002/jbm.a.30798. [DOI] [PubMed] [Google Scholar]
- [21].Yoshida M, Babensee JE. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J. Biomed. Mater. Res. A. 2004;71A:45–54. doi: 10.1002/jbm.a.30131. [DOI] [PubMed] [Google Scholar]
- [22].Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25:295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- [23].Babensee JE, Stein MM, Moore LK. Interconnections between inflammatory and immune responses in tissue engineering. Ann. N.Y. Acad. Sci. 2002;961:360–363. doi: 10.1111/j.1749-6632.2002.tb03124.x. [DOI] [PubMed] [Google Scholar]
- [24].Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26:2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- [25].Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin. Immunol. 2006;120:260–271. doi: 10.1016/j.clim.2006.04.567. [DOI] [PubMed] [Google Scholar]
- [26].Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J. Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- [27].Franchimont D. Overview of the actions of glucocorticoids on the immune response - A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N.Y. Acad. Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- [28].Riccardi C, Bruscoli S, Migliorati G. Molecular mechanisms of immunomodulatory activity of glucocorticoids. Pharmacol. Res. 2002;45:361–368. doi: 10.1006/phrs.2002.0969. [DOI] [PubMed] [Google Scholar]
- [29].Xia CQ, Peng R, Beato F, Clare-Salzler MJ. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand. J. Immunol. 2005;62:45–54. doi: 10.1111/j.1365-3083.2005.01640.x. [DOI] [PubMed] [Google Scholar]
- [30].Liu YF, Wang SP, Lee JW, Kotov NA. A floating self-assembly route to colloidal crystal templates for 3D cell scaffolds. Chem. Mater. 2005;17:4918–4924. [Google Scholar]
- [31].Lee J, Shanbhag S, Kotov NA. Inverted colloidal crystals as three-dimensional microenvironments for cellular co-cultures. J. Mater. Chem. 2006;16:3558–3564. [Google Scholar]
- [32].Jones CA, Vance GHS, Power LL, Pender SLF, MacDonald TT, Warner JO. Costimulatory molecules in the developing human gastrointestinal tract: A pathway for fetal allergen priming. J. Allergy Clin. Immunol. 2001;108:235–241. doi: 10.1067/mai.2001.117178. [DOI] [PubMed] [Google Scholar]
- [33].Norton LW, Tegnell E, Toporek SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26:3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- [34].Babensee JE. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008;20:101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- [35].Jung S, Unutmaz D, Wong P, Sano GI, los Santos K, Sparwasser T, Wu SJ, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv. Mat. 2005;17:399–403. [Google Scholar]
- [37].Abe M, Thomson AW. Influence of immunosuppressive drugs on dendritic cells. Transpl. Immunol. 2003;11:357–65. doi: 10.1016/S0966-3274(03)00050-9. [DOI] [PubMed] [Google Scholar]
- [38].Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell. Mol. Life Sci. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ehrlich HP, Hunt TK. Effects of cortisone and vitamin A on wound healing. Ann. Surg. 1968;167:324–328. doi: 10.1097/00000658-196803000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann. Surg. 1973;177:222–227. doi: 10.1097/00000658-197302000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wicke C, Halliday B, Allen D, Roche NS, Scheuenstuhl H, Spencer MM, Roberts AB, Hunt TK. Effects of steroids and retinoids on wound healing. Arch. Surg. 2000;135:1265–1270. doi: 10.1001/archsurg.135.11.1265. [DOI] [PubMed] [Google Scholar]
- [42].Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T. Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br. J. Dermatol. 2002;147:859–868. doi: 10.1046/j.1365-2133.2002.04949.x. [DOI] [PubMed] [Google Scholar]
- [43].Perretti M, Ahluwalia A. The microcirculation and inflammation: Site of action for glucocorticoids. Microcirculation. 2000;7:147–161. [PubMed] [Google Scholar]
- [44].Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man-impaired suppression of glucose-production and stimulation of glucose-utilization due to a postreceptor defect of insulin action. J. Clin. Endocrinol. Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- [45].Beer HD, Fassler R, Werner S. Glucocorticoid-regulated gene expression during cutaneous wound repair. Vitam. Horm. 2000;59:217–239. doi: 10.1016/s0083-6729(00)59008-6. [DOI] [PubMed] [Google Scholar]
- [46].Matasic R, Dietz AB, Vuk-Pavlovic S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J. Leukoc. Biol. 1999;66:909–914. doi: 10.1002/jlb.66.6.909. [DOI] [PubMed] [Google Scholar]
- [47].van Vliet SJ, van Liempt E, Geijtenbeek TBH, van Kooyk Y. Differential, regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology. 2006;211:577–585. doi: 10.1016/j.imbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- [48].Duperrier K, Velten FW, Bohlender J, Demory A, Metharom P, Goerdt S. Immunosuppressive agents mediate reduced allostimulatory properties of myeloid-derived dendritic cells despite induction of divergent molecular phenotypes. Mol. Immunol. 2005;42:1531–1540. doi: 10.1016/j.molimm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [49].Kitajima T, Ariizumi K, Bergstresser PR, Takashima A. A novel mechanism of glucocorticoid-induced immune suppression: The inhibition of T cell-mediated terminal maturation of a murine dendritic cell line. J. Clin. Invest. 1996;98:142–147. doi: 10.1172/JCI118759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Holt PG, Thomas JA. Steroids inhibit uptake and/or processing but not presentation of antigen by airway dendritic cells. Immunology. 1997;91:145–150. doi: 10.1046/j.1365-2567.1997.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Moser M, Desmedt T, Sornasse T, Tielemans F, Chentoufi AA, Muraille E, Vanmechelen C, Urbain J, Leo O. Glucocorticoids Down-Regulate Dendritic Cell-Function In-Vitro and In-Vivo. Eur. J. Immunol. 1995;25:2818–2824. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- [52].Cumberbatch M, Dearman RJ, Kimber I. Inhibition by dexamethasone of Langerhans cell migration: influence of epidermal cytokine signals. Immunopharmacology. 1999;41:235–243. doi: 10.1016/s0162-3109(99)00037-5. [DOI] [PubMed] [Google Scholar]
- [53].Araki M, Imafuku S, Furue M, Shimada S, Tamaki K. Activation pattern of Langerhans cells in the afferent and efferent phases of contact hypersensitivity. J. Investig. Dermatol. Symp. Proc. 1999;4:164–168. doi: 10.1038/sj.jidsp.5640202. [DOI] [PubMed] [Google Scholar]
- [54].Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation. 2006;81:1451–1459. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.