Abstract
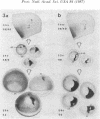
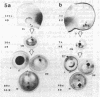
Between 2.5 and 4 days of development, cell proliferation in the Xenopus eye becomes confined to a narrow ring of germinal cells at the front rim of the eye cup. Continued growth of the eye (which lasts until well beyond metamorphosis) is by the continued proliferation of cells in this germinal zone. To determine what factor(s) promotes cell division in this region of the eye long after it ceases at the back of the eye (near the optic nerve), we have transplanted small groups of eye cells from pigmented donor embryos into the eyes of albino hosts, transposing cells from the mitotically quiescent back of the eye to the germinal zone and vice versa. Regardless of their position of origin in the donor eye, only implants into the host germinal zone behaved like germinal cells--as assayed in the living growing eye by the addition of black tissue to the pigment retinal epithelium. Conversely when donor germinal cells were implanted into the back of the host eye, they ceased dividing once they became integrated into the eye and remained as a tiny black spot on the back of the host eye. This suggests that local environmental cues, rather than intrinsic cellular determinants, specify the fates of eye cells ensuring that cells on the eye rim will continue to function as germinal cells while others will withdraw from the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D. H., Jacobson M. Patterns of cell proliferation in the retina of the clawed frog during development. J Comp Neurol. 1979 Feb 1;183(3):603–613. doi: 10.1002/cne.901830308. [DOI] [PubMed] [Google Scholar]
- Bryant P. J., Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q Rev Biol. 1984 Dec;59(4):387–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- Conway K., Feiock K., Hunt R. K. Polyclones and patterns in growing Xenopus eye. Curr Top Dev Biol. 1980;15(Pt 1):217–317. doi: 10.1016/s0070-2153(08)60121-0. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. Embryonic development of identified neurones: differentiation from neuroblast to neurone. Nature. 1979 Jul 19;280(5719):208–214. doi: 10.1038/280208a0. [DOI] [PubMed] [Google Scholar]
- Grant P., Rubin E., Cima C. Ontogeny of the retina and optic nerve in Xenopus laevis. I. Stages in the early development of the retina. J Comp Neurol. 1980 Feb 15;189(4):593–613. doi: 10.1002/cne.901890403. [DOI] [PubMed] [Google Scholar]
- Hollyfield J. G. Differential growth of the neural retina in Xenopus laevis larvae. Dev Biol. 1971 Feb;24(2):264–286. doi: 10.1016/0012-1606(71)90098-4. [DOI] [PubMed] [Google Scholar]
- Hoperskaya O. A. The development of animals homozygous for a mutation causing periodic albinism (ap) in Xenopus laevis. J Embryol Exp Morphol. 1975 Aug;34(1):253–264. [PubMed] [Google Scholar]
- Hunt R. K., Cohen J. S., Mason B. J. Cell patterning in pigment-chimeric eyes in Xenopus: germinal transplants and their contributions to growth of the pigmented retinal epithelium. Proc Natl Acad Sci U S A. 1987 May;84(10):3302–3306. doi: 10.1073/pnas.84.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. Cessation of DNA synthesis in retinal ganglion cells correlated with the time of specification of their central conections. Dev Biol. 1968 Feb;17(2):219–232. doi: 10.1016/0012-1606(68)90062-6. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Histogenesis of retina in the clawed frog with implications for the pattern of development of retinotectal connections. Brain Res. 1976 Feb 27;103(3):541–545. doi: 10.1016/0006-8993(76)90452-2. [DOI] [PubMed] [Google Scholar]
- Shelton P. M., Pfannenstiel H. D., Wachmann E. Regeneration of the eye margin in Periplaneta americana (Insecta, Blattodea). J Embryol Exp Morphol. 1983 Aug;76:9–25. [PubMed] [Google Scholar]
- Straznicky K., Gaze R. M. The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1971 Aug;26(1):67–79. [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977 Mar;56(1):110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Thiébaud C. H. A reliable new cell marker in Xenopus. Dev Biol. 1983 Jul;98(1):245–249. doi: 10.1016/0012-1606(83)90353-6. [DOI] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Weisblat D. A., Stent G. S. Cell lineage analysis by intracellular injection of tracer substances. Curr Top Dev Biol. 1982;17(Pt 3):1–31. doi: 10.1016/s0070-2153(08)60517-7. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and pattern formation. Curr Top Dev Biol. 1971;6(6):183–224. doi: 10.1016/s0070-2153(08)60641-9. [DOI] [PubMed] [Google Scholar]