Abstract
Intravenous iron is commonly used in conjunction with erythropoietic agents to treat anemia in patients with chronic kidney disease. Iron has been proposed to promote oxidative stress and endothelial dysfunction in vascular tissues. We studied the acute effects of intravenous iron sucrose on homocysteine-induced endothelial dysfunction in the brachial artery of normal human subjects. In all, 40 healthy subjects received intravenous iron sucrose 100 mg or placebo over 30 min immediately before ingestion of 100 mg/kg of oral methionine in a double-blind, randomized study. Flow- and nitroglycerin-mediated dilation in the brachial artery, serum markers of iron stores, and homocysteine and nitrotyrosine levels were measured before and after study drug administration. Intravenous iron significantly increased transferrin saturation and non-transferrin-bound iron (NTBI) when compared with placebo. Flow-mediated dilation significantly decreased from baseline 1 h after administration of iron sucrose when compared with placebo (from 6.66±0.47 to 1.93±0.35% after iron sucrose vs from 6.00±0.40 to 5.61±0.46% after placebo, P<0.001), but did not differ between groups at 4 h (1.10±0.39 vs 1.33±0.51%). Nitroglycerin-mediated vasodilation, and homocysteine and 3-nitrotyrosine levels did not differ after administration of iron sucrose and placebo. Intravenous administration of iron sucrose in the setting of transient hyperhomocysteinemia induced by methionine ingestion significantly increased transferrin saturation and plasma levels of NTBI and significantly attenuated flow-mediated dilation in the brachial artery when compared with placebo. This potential mechanistic link between intravenous iron and endothelial dysfunction warrants further study of cardiovascular effects of intravenous iron in anemic chronic kidney disease populations.
Keywords: iron, vasodilation, vascular endothelium, oxidative stress
Intravenous iron preparations are commonly administered in conjunction with erythropoietic agents for the treatment of anemia in patients with chronic kidney disease and functional iron deficiency.1,2 Iron is a pro-oxidant cofactor that has been proposed to be linked to increased cardiovascular risk.3-5 Observational studies of the association between various measures of iron stores and coronary risk in general populations and chronic kidney populations have yielded conflicting findings.1,4,6
The vascular endothelial plays an important role in the normal regulation of vasomotor tone, vascular structure, and thrombosis.7 Endothelial dysfunction is associated with increased risk of cardiac events in patients with established atherosclerosis and hypertension.8 Intravenous iron sucrose has been previously reported to transiently increase levels of non-transferrin-bound iron (NTBI) in association with mild attenuation of endothelium-dependent vasodilation in the brachial artery of normal subjects.9 Although this observation is consistent with the overall good safety record of intravenous iron sucrose, physiological response in normal subjects may not be predictive of that in chronic kidney disease populations with multiple comorbidities associated with increased oxidative stress.1 Subjects heterozygous for the hemochromatosis gene with modest increases in serum ferritin levels are at increased risk for cardiovascular events, particularly in the presence of other known risk factors such as hypertension or cigarette smoking.10,11 Accordingly, we hypothesized that iron may increase vascular risk by augmenting oxidative stress in the setting of coexisting cardiac risk factors.
Hyperhomocysteinemia induced by oral methionine ingestion in normal subjects is associated with increased oxidative stress and transient vascular endothelial dysfunction.12,13 Iron chelation with dexrazoxane has been shown to attenuate homocysteine-induced endothelial dysfunction in normal subjects, but the direct interaction between intravenous iron and homocysteine has not been reported previously.14 The current study was undertaken to prospectively test the hypothesis that the pro-oxidant effects of intravenous iron sucrose would augment vascular endothelial dysfunction induced by transient hyperhomocysteinemia in normal subjects. We measured flow-mediated and nitroglycerin-mediated dilation in the brachial artery with high-resolution ultrasound imaging and serum markers of iron stores and oxidative stress before and after administration of intravenous iron sucrose or placebo (normal saline) in 40 healthy subjects with transient hyperhomocysteinemia induced by methionine ingestion in a double-blind, randomized trial.
RESULTS
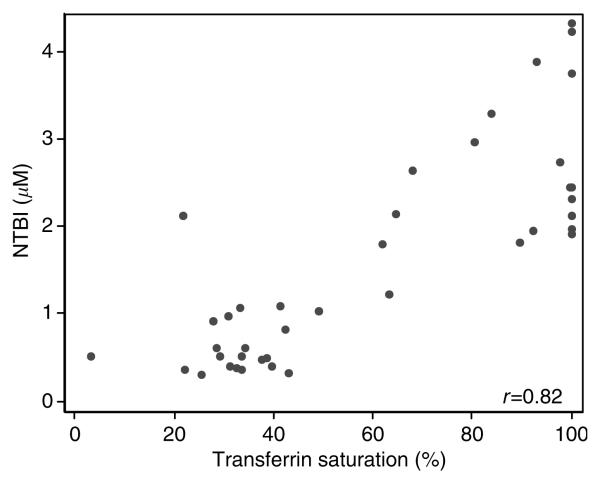
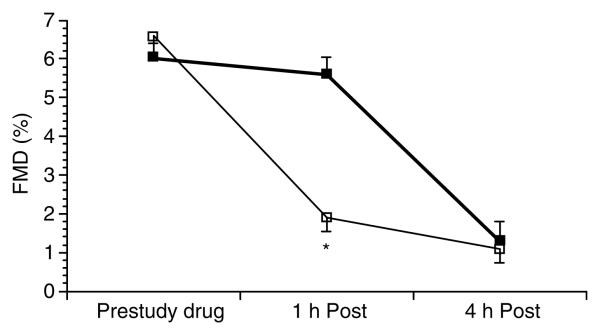
Study drug was well tolerated with no adverse reactions reported. Baseline characteristics of the two treatment groups did not differ (Table 1). Serum iron, transferrin saturation, and NTBI significantly increased after administration of iron sucrose when compared with placebo (Table 2). NTBI levels were highly correlated with percent transferrin saturation 4 h after study drug administration (r = 0.82, P<0.001; Figure 1). Flow-mediated dilation significantly decreased from baseline 1 h after administration of iron sucrose when compared with placebo (from 6.66±0.47 to 1.93±0.35% after iron sucrose vs from 6.00±0.40 to 5.61±0.46% after placebo, P<0.001 for group by time interaction; Figure 2), but did not differ between groups at 4 h (1.10±0.39 vs 1.33±0.51%; Figure 2). Brachial artery flow velocity at rest and during postischemic hyperemia did not differ between subjects receiving iron sucrose or placebo before study drug (rest 8±1 vs 9±1cm/s and hyperemia 59±5 vs 61±5 cm/s, both P=NS) and at 1 and 4 h after study drug (rest 1 h 8±1 vs 871cm/s and hyperemia 1 h 58±5 vs 59±4 cm/s; rest 4 h 10±1 vs 9±1 cm/s and hyperemia 4 h 57±5 vs 56±5 cm/s, all comparisons P=NS). Nitroglycerin-mediated vasodilation, heart rate, mean arterial pressure, homocysteine, and nitrotyrosine levels did not differ before or after administration of iron sucrose and placebo (Table 3).
Table 1.
Clinical characteristics of study population by study drug assignment (means±s.e.m.)
| Iron sucrose (n=20) |
Placebo (n=20) |
P-value | |
|---|---|---|---|
| Age (years) | 27±1 | 27±1 | 0.78 |
| Gender (no. of male/no. of female) | 12/8 | 12/8 | — |
| Body mass index (kg/m2) | 24±1 | 24±1 | 0.99 |
| Total cholesterol (mg/dl) | 152±6 | 163±5 | 0.14 |
| Low-density cholesterol (mg/dl) | 81±6 | 92±5 | 0.17 |
| High-density cholesterol (mg/dl) | 53±4 | 53±3 | 0.99 |
| Triglycerides (mg/dl) | 87±15 | 88±10 | 0.95 |
| Blood glucose (mg/dl) | 92±3 | 88±2 | 0.34 |
| Hemoglobin (gm/dl) | 14.1±0.3 | 14.0±0.4 | 0.77 |
| Brachial artery diameter (mm) | 3.58±0.12 | 3.58±0.15 | 0.32 |
Table 2.
Biomarkers of iron stores (means±s.e.m., or for ferritin median (interquartile range)) before and 4 h after administration of iron sucrose or placebo
| Iron sucrose (n=20) |
Placebo (n=20) |
||||
|---|---|---|---|---|---|
| Before | 4 h post | Before | 4 h post | P-value* | |
| Serum iron (μM) | 14±2 | 53±11 | 12±1 | 16±1 | 0.001 |
| Total iron binding capacity (μM) | 48±3 | 60±11 | 49±3 | 51±3 | 0.36 |
| % transferrin saturation | 31±9 | 87±9 | 24±2 | 32±2 | <0.001 |
| NTBI (μM) | 0.51±0.09 | 2.54±0.21 | 0.64±0.11 | 0.66±0.09 | <0.001 |
| Serum ferritin (ng/ml) | 93 (113) | 92 (213) | 89 (111) | 81 (117) | 0.84 |
P-value for treatment group-by-time interaction term in repeated measures analysis of variance model.
Figure 1.
Scatterplot relating transferrin saturation and plasma levels of NTBI 4 h after study drug administration.
Figure 2.
Flow-mediated dilation in the brachial artery (FMD (%), mean±s.e.m.) before and at 1 and 4 h after administration of intravenous iron sucrose 100 mg (open squares) or placebo (filled squares). *P<0.001 vs placebo.
Table 3.
Brachial artery, systemic hemodynamic, and biochemical measurements (means±s.e.m., or for nitrotyrosine median (interquartile range)) before and 4 h after administration of iron sucrose or placebo
| Iron sucrose (n=20) |
Placebo (n=20) |
||||
|---|---|---|---|---|---|
| Before | 4 h post | Before | 4 h post | P-value* | |
| NTG-mediated dilation (%) | 27±1 | 25±1 | 27±1 | 25±1 | 0.947 |
| Heart rate (min−1) | 60±2 | 65±2 | 65±3 | 61±2 | 0.12 |
| Mean arterial pressure (mmHg) | 82±1 | 79±1 | 80±2 | 79±1 | 0.16 |
| Plasma homocysteine (ng/ml) | 7.2±0.4 | 25.2±1.4 | 7.0±0.5 | 24.7±1.4 | 0.87 |
| Plasma nitrotyrosine (pg/ml) | 5 (62) | 6 (62) | 11 (62) | 11 (49) | 0.90 |
P-value for treatment group-by-time interaction term in repeated measures analysis of variance model.
NTG, nitroglycerin.
Consistent with the gender-stratified randomization code incorporated into our study design, gender distribution in the two treatment groups did not differ (Table 1) and adjustment for gender in multivariate analysis did not alter the findings of the primary unadjusted analysis. Baseline levels of ferritin and homocysteine were lower in women when o compared with men (ferritin 32 (50) vs 126 (93) ng/ml, P<0.001 and homocysteine 6.2±0.5 vs 7.7±0.4 ng/ml, P=0.018). In multivariate analysis, the difference in flow-mediated dilation between the iron sucrose group and the placebo group tended to be greater in women when compared with that in men (P=0.09 for gender-by-treatment interaction; Table 4).
Table 4.
Flow-mediated dilation (%) before and after study drug administration in subjects grouped by gender
| Prestudy drug | 1 h post | 4 h post | |
|---|---|---|---|
| Females (n=16) | |||
| Placebo | 6.5±0.6 | 6.2±0.7 | 2.3±0.8 |
| Iron sucrose | 5.9±0.9 | 2.4±0.7 | 1.0±0.8 |
| Males (n=24) | |||
| Placebo | 5.7±0.5 | 5.2±0.6 | 0.7±0.4 |
| Iron sucrose | 7.2±0.5 | 1.6±0.3 | 1.1±0.4 |
DISCUSSION
The present data demonstrate that administration of intravenous iron sucrose in the setting of transient hyperhomocysteinemia induced by methionine ingestion in healthy subjects significantly increased transferrin saturation and plasma levels of NTBI and significantly attenuated flow-mediated dilation (but not nitroglycerin-mediated dilation) in the brachial artery when compared with placebo.
Iron sucrose is composed of an inner core of ferric hydroxide sequestered by an outer sphere of sucrose molecules with molecular weight of approximately 43 kDa.15 The large majority of the iron contained within iron sucrose is metabolized by reticuloendothelial cells in the liver, spleen, and bone marrow, with rapid incorporation in the red blood cell iron pool.16,17 However, 2–6% of the iron present in iron sucrose and other intravenous iron preparations appears to be more labile and is immediately available for binding to plasma apotransferrin upon injection.18-20 Our finding of increased transferrin saturation and increased NTBI after injection of intravenous iron sucrose is in accord with previous reports in normal subjects and patients with chronic kidney disease.9,21-23 Although there is no consensus on the best analytical approach for the detection of NTBI, this pool of iron appears to be biologically active. Growth of iron-requiring bacteria in culture is supported by the serum of end-stage kidney disease patients treated with intravenous iron and can be blocked by the addition of apotransferrin to the serum.21
The current study is the first study to our knowledge that directly tests the interaction between the effects intravenous iron administration and transient hyperhomocysteinemia on vascular endothelial function in the intact human circulation. Previous studies have demonstrated small decreases in flow-mediated dilation of the brachial artery 1 h after intravenous iron sucrose administration alone or in response to increased homocysteine levels 1 h after methionine ingestion alone.9,12,13,24-26 Our finding of a small decrease in flow-mediated dilation at 1 h after administration of placebo is in accord with these previous reports in normal subjects. In contrast, administration of intravenous sucrose in combination with oral methionine was associated with severe attenuation in flow-mediated dilation to levels comparable to those reported in patients with chronic cardiovascular diseases.8,27 Flow-mediated dilations were severely attenuated in both groups and no longer significantly different at 4 h after administration of iron sucrose or placebo. The observed decrease in flow-mediated dilation at 4 h in the placebo group in response to increased homocysteine levels is generally consistent with prior reports, but was larger than anticipated based on findings from a previous study from our laboratory.14 This large decrease in flow-mediated dilation at 4 h in the placebo group limited our ability to detect an interaction between iron and homocysteine at 4 h due to a ceiling effect and reduced statistical power.
We anticipated that gender might be an important confounding factor and prospectively stratified our random treatment allocation scheme accordingly. As expected, premenopausal women had lower baseline markers of iron stores and homocysteine levels when compared with men. Our finding of a strong trend towards a gender-by-treatment interaction as presented in Table 4 is consistent with our primary hypothesis of interaction between iron and homocysteine.
Tyrosine nitration was initially proposed as a specific marker of increased oxidative stress related to the generation of peroxynitrite from nitric oxide.28,29 Although several non-nitric oxide biosynthetic pathways for 3-nitrotyrosine synthesis have also been proposed, these alternate pathways of tyrosine nitration also involve free radical chemistry reactions.30,31 Increased tyrosine nitration has been reported in patients with heart failure and coronary artery disease.32,33 In the current study of normal subjects, 3-nitrotyrosine did not change in response to intravenous iron or transient hyperhomocysteinemia. This was an unexpected finding, as both homocysteine and iron had been previously reported to be associated with increased oxidative stress in experimental and clinical settings.9,13,34-39 However, clinical studies of serum markers of lipid peroxidation in response to intravenous iron and transient hyperhomocysteinemia have yielded conflicting findings.25,30,40,41 The lack of change in 3-nitrotyrosine in our study may be attributable to the limited utility of serum markers for the detection of acute changes in intracellular redox state. Alternatively, the observed interaction of iron and homocysteine on endothelial function may be related to effects of iron on mitochondrial function, heme-containing enzymes, or iron-responsive intracellular signaling.19,42-44
We studied a disease model of transient homocysteine-induced endothelial dysfunction in normal subjects that may not be predictive of clinical responses to intravenous iron in patients with established vascular disease. The potential clinical sequelae of transient endothelial dysfunction in response to intravenous iron are uncertain. However, since endothelial dysfunction is associated with increased cardiac risk in patients with hypertension and clinical vascular disease,8 our findings raise concern regarding clinical utilization of intravenous iron in patient populations at increased cardiac risk. Current guidelines recommend intravenous iron to correct functional iron deficiency unresponsive to oral iron supplementation in anemic patients with end-stage renal disease.1,2 Chronic kidney disease is known to be associated with vascular endothelial dysfunction, evidence of increased oxidative stress, and increased risk of cardiovascular events.45-49 Intravenous iron preparations (other than iron dextran) are generally well tolerated in chronic kidney disease patients,15,50 but have also been tentatively linked to increased risk of cardiovascular events in some retrospective analyses of clinical trials of erythropoietic therapy in anemic chronic kidney disease populations.6,23,51-53
In conclusion, the current data demonstrate that intravenous iron sucrose augments transient homocysteine-induced endothelial dysfunction in normal subjects. These findings suggest a potential mechanistic link between intravenous iron and cardiac risk. Additional work is needed to determine the effects of intravenous iron on endothelial function and cardiovascular risk in anemic hemodialysis patients with functional iron deficiency.
MATERIALS AND METHODS
Study sample
All subjects were normotensive nonsmokers with no history of chronic illness or chronic medication use. The criteria for exclusion were low-density lipoprotein (LDL) >160 mg/dl, fasting blood sugar >110 mg/dl, plasma homocysteine >10 μmol/l, and pregnancy. The study protocol was approved by the Institutional Ethical Review Committee. All subjects provided informed written consent before participation.
Ultrasound imaging
Flow-mediated endothelium-dependent vasodilation in the brachial artery was determined with an ATL Apogee 800 duplex ultrasound imaging system connected to an 11 MHz high-resolution transducer by a single blinded investigator, adapted from published guidelines as described previously.54,27 The brachial artery was imaged longitudinally approximately 5 cm above the antecubital fossa. Arterial diameter (mm) was derived from the average of five on-screen electronic caliper measurements. External landmarks and internal landmarks (veins, arterial branches, or distinctive soft-tissue markings) were utilized to ensure that all measurements were derived from the same arterial segment. Brachial artery diameter and Doppler blood flow velocity were measured at rest and after transient arterial occlusion induced by 5-min inflation of a forearm pneumatic cuff to 200 mmHg. Flow-mediated dilatation was calculated as the percent increase in brachial artery diameter 60 s after the release of the occluding cuff. Endothelium-independent vasodilation was determined as the percentage increase in brachial diameter 5 min after administration of 0.4 mg of sublingual nitroglycerin.
Analytical methods
Techniques for determining serum iron (SI) and deriving the total iron binding capacity and transferrin saturation (%) are outlined as follows:55,56 at acidic pH (acetate buffer, pH 4.5) and in the presence of hydroxylamine (a reducing agent), transferrin-bound iron or SI dissociates to release ferrous ions. These react with ferrozine to form a stable magenta-colored complex (Fe2+-ferrozine) with a maximum absorption at 560 nm. The difference in absorbance at 560 nm before and after ferrozine addition in serum sample is proportional to SI concentration. In contrast to SI, serum-unbound iron binding capacity was measured at alkaline pH (TRIZMA®, pH 8.1). Ferrous ions added to the serum bind specifically with transferrin at unsaturated iron-binding sites and then remaining unbound ferrous ions are measured with the ferrozine reaction. The difference between the amount of unbound iron and the total amount added to serum is equivalent to the quantity bound to transferrin, which is the unbound iron binding capacity. The serum total iron binding capacity equals the SI plus the unbound iron binding capacity. Serum transferrin saturation (%) was calculated using SI divided by total iron binding capacity × 100.
Ferritin in sera was determined according to a previously published protocol.55,57 In brief, an antibody to a mixture of human spleen and liver ferritin was used as the capture antibody to coat an enyme-linked immunosorbent assay plate. Human liver ferritin was used as the standard (Roche Molecular Bio-Chemicals, Indianapolis, IN, USA). The conjugate of peroxidase and antibody to human spleen and liver ferritin was then added to serve as the detector to determine the amount of ferritin bound to the capture antibody. Tetramethylbenzidine was then added as the peroxidase substrate, and the absorbance of the peroxidase-mediated tetramethylbenzidine oxidation product was determined at 450 nm using the UV–visible microplate reader (SpectraMax Plus, Molecular Devices, Sunnyvale, CA, USA).
Levels of NTBI were determined using nitrilotriacetic acid to capture iron that is nonspecifically bound to serum proteins and low-molecular-weight ligands, without chelating transferrin- or ferritin-bound iron.58 Sodium tris-carbonatocobaltate(III) trihydrate was preincubated with serum prior to nitrilotriacetic acid-iron complexation to block the free iron binding sites of transferrin. This procedure is to ensure that unsaturated transferrin will not chelate NTBI during sample preparation. After the addition of nitrilotriacetic acid, all the NTBI is quantitatively converted to the Fe-nitrilotriacetic acid complex, which can be monitored spectrophotometrically after ultracentrifugation. In brief, 350 μl serum was incubated with 100 μml 4.8 mm sodium tris-carbonatocobaltate(III) trihydrate at 37°C for 1 h. Then, nitrilotriacetic acid was added to the serum mixture to obtain a final concentration of 80 mm. After standing at room temperature for 30 min, the samples were filtered through a Centricon membrane with a nominal molecular weight limit of 30 kDa (Centricon-30) at 3000 g for 1 h. The ultrafiltrate was then diluted at 1:1 ratio with 5 mm 3-[N-morpholino]propane sulfonic acid buffer (pH 7.4). To form the chromogenic complex, of total reaction volume of 120 mm sodium thioglycolic acid and 60 mm sodium bathophenanthroline disulfonic acid (both dissolved in deionized water) was added to the solution and allowed to stand for 30 min at room temperature. The resulting complex was measured by spectrophotometry at 537 nm.
Total plasma homocysteine were determined in μmol/l by high-pressure liquid chromatography.59 Nitrotyrosine was measured with a commercially available enzyme-linked immunoabsorbent assay.
Study protocol
The study followed a randomized, double-blind, placebo-controlled parallel group design. Study drug assignment was determined by a blocked randomization scheme stratified by gender and held by the research pharmacy. All subjects were studied in the fasting state. Intravenous iron sucrose 100 mg in 250 cm3 of normal saline (iron(III) hydroxide sucrose, Venofer®, American Regent) or 250 cm3 of plain normal saline was administered over 30 min by a member of the Yale General Clinical Research Center nursing staff. To maintain the double-blind protocol, investigators were not allowed inside the room at the time of the study drug infusion and the infusion lines were covered with an opaque material. Immediately after completion of the study drug infusion, oral methionine 100 mg/kg mixed in fruit juice was administered. Flow-mediated dilation in the brachial artery was determined before and at 1 and 4 h after study drug administration. Nitroglycerin-mediated dilation in the brachial artery, and venous blood for serum markers of iron stores, homocysteine and nitrotyrosine levels were obtained before and 4 h after study drug administration. Plasma was separated from venous blood by cold centrifugation and stored at −80°C until analysis.
Statistical analysis
Descriptive statistics were used to determine means and distributions of clinical characteristics and physiological and laboratory measurements in the study sample. Continuous variables with normal distributions are presented as means±s.e.m. in the text and tables. Serum ferritin and nitrotyrosine levels were noted to significantly deviate from the normal distribution (by Shapiro–Wilk test), so the natural logarithm of these variables was used in all analyses; median values and interquartile range for these variables are presented in the text and tables. Unpaired Student's t-tests and χ2 tests were used to compare means and proportions of baseline clinical and laboratory characteristics, respectively, in the two treatment groups. Differences in physiological and laboratory variables in response to study drug were analyzed with a repeated measures analysis of variance model with factors of treatment group, time, and the interaction of treatment group-by-time (Stata statistical software version 8.0, College Park, Austin, TX, USA). From previous data derived from our laboratory, the anticipated mean and standard deviation of the change in flow-mediated dilation after administration of methionine and placebo were 2.3±0.8%. In all, 20 subjects per treatment arm provided >80% power to detect a clinically relevant 30% further decrease in flow-mediated dilation in response to methionine after iron sucrose administration. For all analyses, a two-tailed P-value <0.05 was used to infer statistical significance.
ACKNOWLEDGMENTS
This work is supported in part by Division of Research Resources, General Clinical Research Centers Program, NIH, 5 MO1 RR00645, and an unrestricted grant from Amgen Inc.
REFERENCES
- 1.Besarab A, Frinak S, Yee J. An indistinct balance: the safety and efficacy of parenteral iron therapy. J Am Soc Nephrol. 1999;10:2029–2043. doi: 10.1681/ASN.V1092029. [DOI] [PubMed] [Google Scholar]
- 2.NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2000. Am J Kidney Dis. 2001;37:S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 4.Meyers DG. The iron hypothesis: does iron play a role in atherosclerosis? Transfusion. 2000;40:1023–1029. doi: 10.1046/j.1537-2995.2000.40081023.x. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan JL. Is stored iron safe? J Lab Clin Med. 2004;144:280–284. doi: 10.1016/j.lab.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Kletzmayr J, Horl WH. Iron overload and cardiovascular complications in dialysis patients. Nephrol Dial Transplant. 2002;17(Suppl 2):25–29. doi: 10.1093/ndt/17.suppl_2.25. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 8.Mancini GB. Vascular structure versus function: is endothelial dysfunction of independent prognostic importance or not? J Am Coll Cardiol. 2004;43:624–628. doi: 10.1016/j.jacc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Rooyakkers TM, Stroes ES, Kooistra MP, et al. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest. 2002;32(Suppl 1):9–16. doi: 10.1046/j.1365-2362.2002.0320s1009.x. [DOI] [PubMed] [Google Scholar]
- 10.Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100:1268–1273. doi: 10.1161/01.cir.100.12.1268. [DOI] [PubMed] [Google Scholar]
- 11.Tuomainen TP, Kontula K, Nyyssonen K, et al. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100:1274–1279. doi: 10.1161/01.cir.100.12.1274. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy MF, McDowell IF, Ramsey MW, et al. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–1852. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- 13.Chambers JC, McGregor A, Jean-Marie J, et al. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation. 1999;99:1156–1160. doi: 10.1161/01.cir.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 14.Zheng H, Dimayuga C, Hudaihed A, Katz SD. Effect of dexrazoxane on homocysteine-induced endothelial dysfunction in normal subjects. Arterioscler Thromb Vasc Biol. 2002;22:E15–E18. doi: 10.1161/01.atv.0000023187.25914.5b. [DOI] [PubMed] [Google Scholar]
- 15.Yee J, Besarab A. Iron sucrose: the oldest iron therapy becomes new. Am J Kidney Dis. 2002;40:1111–1121. doi: 10.1053/ajkd.2002.36853. [DOI] [PubMed] [Google Scholar]
- 16.Beshara S, Lundqvist H, Sundin J, et al. Pharmacokinetics and red cell utilization of iron(III) hydroxide-sucrose complex in anaemic patients: a study using positron emission tomography. Br J Haematol. 1999;104:296–302. doi: 10.1046/j.1365-2141.1999.01179.x. [DOI] [PubMed] [Google Scholar]
- 17.Beshara S, Lundqvist H, Sundin J, et al. Kinetic analysis of 52Fe-labelled iron(III) hydroxide–sucrose complex following bolus administration using positron emission tomography. Br J Haematol. 1999;104:288–295. doi: 10.1046/j.1365-2141.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Wyck D, Anderson J, Johnson K. Labile iron in parenteral iron formulations: a quantitative and comparative study. Nephrol Dial Transplant. 2004;19:561–565. doi: 10.1093/ndt/gfg579. [DOI] [PubMed] [Google Scholar]
- 19.Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 20.Esposito BP, Breuer W, Slotki I, Cabantchik ZI. Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur J Clin Invest. 2002;32(Suppl 1):42–49. doi: 10.1046/j.1365-2362.2002.0320s1042.x. [DOI] [PubMed] [Google Scholar]
- 21.Parkkinen J, von Bonsdorff L, Peltonen S, et al. Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant. 2000;15:1827–1834. doi: 10.1093/ndt/15.11.1827. [DOI] [PubMed] [Google Scholar]
- 22.Kooistra MP, Kersting S, Gosriwatana I, et al. Nontransferrin-bound iron in the plasma of haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Invest. 2002;32(Suppl 1):36–41. doi: 10.1046/j.1365-2362.2002.0320s1036.x. [DOI] [PubMed] [Google Scholar]
- 23.Roob JM, Khoschsorur G, Tiran A, et al. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539–549. doi: 10.1681/ASN.V113539. [DOI] [PubMed] [Google Scholar]
- 24.Chambers JC, Ueland PM, Wright M, et al. Investigation of relationship between reduced, oxidized, and protein-bound homocysteine and vascular endothelial function in healthy human subjects. Circ Res. 2001;89:187–192. doi: 10.1161/hh1401.093459. [DOI] [PubMed] [Google Scholar]
- 25.Chao CL, Kuo TL, Lee YT. Effects of methionine-induced hyperhomocysteinemia on endothelium-dependent vasodilation and oxidative status in healthy adults. Circulation. 2000;101:485–490. doi: 10.1161/01.cir.101.5.485. [DOI] [PubMed] [Google Scholar]
- 26.Kanani PM, Sinkey CA, Browning RL, et al. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999;100:1161–1168. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 27.Katz SD, Balidemaj K, Homma S, et al. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–851. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt RT, Forgione MA, Cap A, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 31.Hazen SL, Zhang R, Shen Z, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 32.Colombo PC, Ashton AW, Celaj S, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 33.Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 34.Durand P, Prost M, Loreau N, et al. Impaired homocysteine metabolism and atherothrombotic disease. Lab Invest. 2001;81:645–672. doi: 10.1038/labinvest.3780275. [DOI] [PubMed] [Google Scholar]
- 35.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upchurch GR, Jr, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 37.Nappo F, De Rosa N, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999;281:2113–2118. doi: 10.1001/jama.281.22.2113. [DOI] [PubMed] [Google Scholar]
- 38.Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 39.Lim CS, Vaziri ND. The effects of iron dextran on the oxidative stress in cardiovascular tissues of rats with chronic renal failure. Kidney Int. 2004;65:1802–1809. doi: 10.1111/j.1523-1755.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 40.Lim PS, Wei YH, Yu YL, Kho B. Enhanced oxidative stress in haemodialysis patients receiving intravenous iron therapy. Nephrol Dial Transplant. 1999;14:2680–2687. doi: 10.1093/ndt/14.11.2680. [DOI] [PubMed] [Google Scholar]
- 41.Cavdar C, Temiz A, Yenicerioglu Y, et al. The effects of intravenous iron treatment on oxidant stress and erythrocyte deformability in hemodialysis patients. Scand J Urol Nephrol. 2003;37:77–82. doi: 10.1080/00365590310008758. [DOI] [PubMed] [Google Scholar]
- 42.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 43.Weiss G, Werner-Felmayer G, Werner ER, et al. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponka P. Cellular iron metabolism. Kidney Int Suppl. 1999;69:S2–S11. doi: 10.1046/j.1523-1755.1999.055suppl.69002.x. [DOI] [PubMed] [Google Scholar]
- 45.Janssen MJ, Lambert J, et al. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol Dial Transplant. 1998;13:1782–1786. doi: 10.1093/ndt/13.7.1782. [DOI] [PubMed] [Google Scholar]
- 46.Thambyrajah J, Landray MJ, McGlynn FJ, et al. Abnormalities of endothelial function in patients with predialysis renal failure. Heart. 2000;83:205–209. doi: 10.1136/heart.83.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni Z, Oveisi F, et al. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39:135–141. doi: 10.1161/hy0102.100540. [DOI] [PubMed] [Google Scholar]
- 48.Massy ZA, Nguyen-Khoa T. Oxidative stress and chronic renal failure: markers and management. J Nephrol. 2002;15:336–341. [PubMed] [Google Scholar]
- 49.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Chandler G, Harchowal J, Macdougall IC. Intravenous iron sucrose: establishing a safe dose. Am J Kidney Dis. 2001;38:988–991. doi: 10.1053/ajkd.2001.28587. [DOI] [PubMed] [Google Scholar]
- 51.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 52.Furuland H, Linde T, Ahlmen J, et al. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003;18:353–361. doi: 10.1093/ndt/18.2.353. [DOI] [PubMed] [Google Scholar]
- 53.Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol. 2004;15:1623–1632. doi: 10.1097/01.asn.0000128009.69594.be. [DOI] [PubMed] [Google Scholar]
- 54.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 55.Ali MA, Akhmedkhanov A, Zeleniuch-Jaquotte A, et al. Reliability of serum iron, ferritin, nitrite, and association with risk of renal cancer in women. Cancer Detect Prev. 2003;27:116–121. doi: 10.1016/s0361-090x(03)00027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali A, Zhang Q, Dai J, Huang X. Calcein as a fluorescent iron chemosensor for the determination of low molecular weight iron in biological fluids. Biometals. 2003;16:285–293. doi: 10.1023/a:1020642808437. [DOI] [PubMed] [Google Scholar]
- 57.Fang R, Aust AE. Induction of ferritin synthesis in human lung epithelial cells treated with crocidolite asbestos. Arch Biochem Biophys. 1997;340:369–375. doi: 10.1006/abbi.1997.9892. [DOI] [PubMed] [Google Scholar]
- 58.Gosriwatana I, Loreal O, Lu S, et al. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem. 1999;273:212–220. doi: 10.1006/abio.1999.4216. [DOI] [PubMed] [Google Scholar]
- 59.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991;565:441–446. doi: 10.1016/0378-4347(91)80407-4. [DOI] [PubMed] [Google Scholar]