Although renal transplantation continues to be associated with increasingly good short- and long-term graft survival, there remains a need for continued chronic immunosuppression and this can lead to significant complications.1–3 This has prompted the evaluation of withdrawal or modification of immunosuppression by several investigators with varying results.4,5 Emerging data proposing dendritic cell migration and repopulation with chimerism following solid organ transplantation as the basis for graft acceptance have further encouraged evaluation of titrated withdrawal of immunosuppression in select groups of patients.6–8 The finding that donor-specific nonreactivity and chimerism occur simultaneously in long-term living related kidney recipients9 led us to evaluate withdrawal of immunosuppression in these patients.
MATERIALS AND METHODS
Eight long-term recipients of continuously functioning living related renal allografts were weaned from immunosuppression. Three patients were noncompliant and had self-weaned their medications many years previously. Patient demographic data are listed in Table 1. An additional five patients were deliberately weaned from their immunosuppression according to the following criteria: the patients were recipients of living related kidneys, more than 5 years posttransplant, with at least 2 years of stable graft function. Baseline renal biopsy without evidence of rejection was required, as was a compliant medical history and complications related to immunosuppression.
Table 1.
Patient Demographic Data
| Patient No. | Age | Sex | Dx | Date of LRRT |
Complications Related to Immunosuppression |
|---|---|---|---|---|---|
| 1. KP | 52 | M | Chronic glomerulonephritis (GN) | 2/9/63 | — |
| 2. SM | 45 | F | Chronic GN | 2/17/64 | — |
| 3. JN | 62 | M | Chronic GN | 1/15/64 | — |
| 4. JW | 49 | M | Chronic GN | 7/10/64 | SCCa/DJD hip |
| 5. DS | 45 | F | Pyelonephritis | 7/19/62 | Verrucous warts |
| 6. LN | 52 | M | Chronic GN | 7/29/66 | Obesity, hypertension, verrucous warts |
| 7. SS | 37 | F | Pyelonephritis | 5/10/78 | Hypertension, verrucous warts |
| 8. JK | 50 | M | Renal agenesis | 2/25/87 | Basal cell carcinoma, verrucous warts |
SCCa/DJD hip, squamous cell cancer/degenerative joint disease of hip.
Immunologic reactivity was assessed when available by in vitro mixed lymphocyte reaction (MLR) and cell-mediated lymphotoxicity (CML). Unidirectional human MLR cultures used 5 × 104 responder and 5 × 104 irradiated stimulator (donor and third party) cells in tissue culture media supplemented with human serum. The degree of labeled thymidine incorporation during the final 20 hours of incubation determined proliferation.
CML was assessed between recipient lymphocytes and donor and third-party targets by using effector lymphocytes incubated with Cr-labeled target cells at various effector:target ratios.
Weaning proceeded in the following manner. After a baseline renal biopsy was obtained, prednisone was weaned to less than 5 mg/d. The ACTH stimulation test was used to assess for intact adrenocortical function, as these patients had been on steroids for many years.10,11 Patients with an appropriate incremental increase in cortisol levels following stimulation with ACTH were weaned off prednisone completely.12 The remaining immunosuppressant (azathioprine in all cases except case no. 8) was sequentially reduced by 50% at monthly intervals. Patient weight, urine output, blood urea nitrogen, and serum creatinine were followed on a monthly basis.
RESULTS
Baseline immunosuppression, donor relationship, serum creatinine before and after withdrawal of immunosuppression, episodes of rejection, and time off immunosuppression are listed in Table 2.
Table 2.
Effect of Weaning Immunosuppression on Renal Function
| Patient No. | Baseline Immunosuppression (mg/d) |
Creat. Pre/Post | Rejection | Time Off Drug (y) |
|---|---|---|---|---|
| 1 | Azathioprine (AZA) | 0.7/0.9 | No | 29 |
| 2 | Pred 15 AZA 25 | 1.0/1.1 | No | 14a |
| 3 | AZA | 0.8/0.9 | No | 28 |
| 4 | AZA 125/pred 10 | 1.6/1. | No | 2 |
| 5 | AZA 75 | 0.8/0.7 | No | 1 |
| 6 | AZA 100/pred 10 | 0.9/0.9 | No | Still weaning; doses decreased by 75% |
| 7 | AZA 75/pred 10 | 1.4/1.0 | No | 3 months |
| 8 | AZA 5 7.5 pred <CyA 50 |
1.0/3.4—1.2 | Yes | 5 months (then restarted) |
Maintenance steroids 5/d prednisone (pred) for adrenal cortical insufficiency.
Renal biopsies obtained on patients 4 to 8 had minimal background infiltrate with no evidence of rejection. One patient developed mild acute cellular rejection diagnosed on biopsy at 5 months postweaning and was treated with pulse steroids and a return to baseline therapy. His creatinine immediately returned to normal. One patient is still in the process of being weaned; to date, her immunosuppression has been decreased by 75% without any untoward effect. Current time off immunosuppression for the remaining six patients ranges from 3 months to 29 years (mean = 12.4 years). One patient is on maintenance steroids (5 mg/d prednisone) because of adrenal insufficiency.
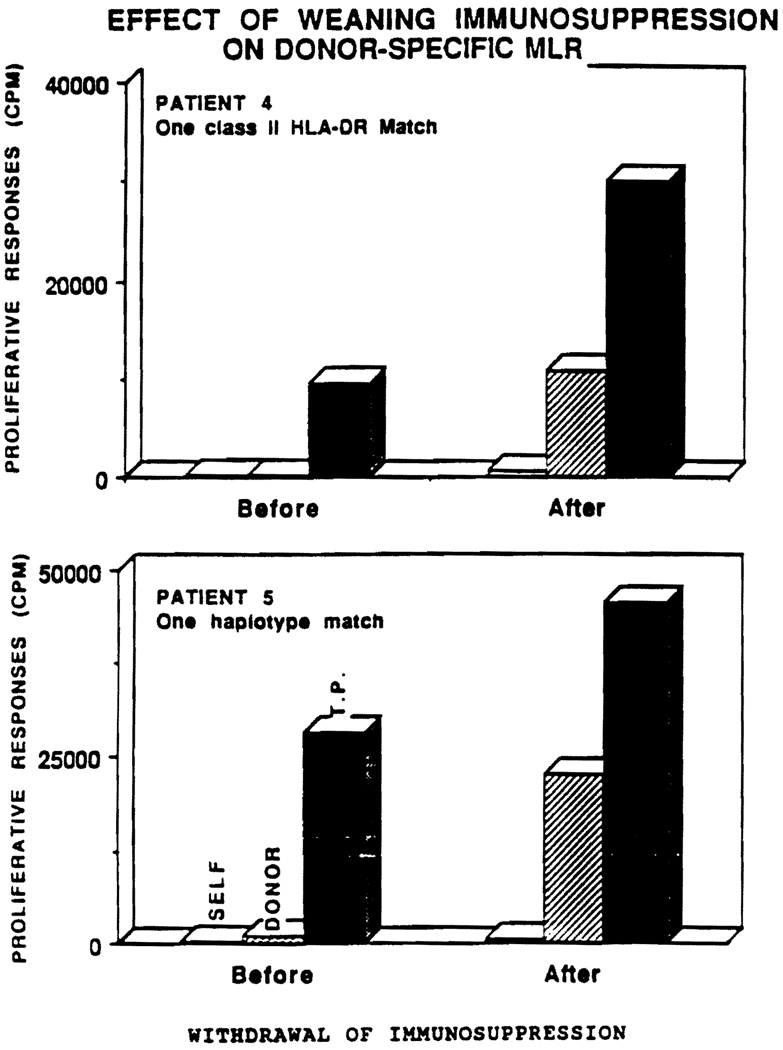
Donor-specific reactivity by MLR in patients 3, 4, and 5 was unresponsive when initially evaluated for weaning of immunosuppression. Repeat MLR in patients 4 and 5 now show a significant response (Fig 1). These patients have remained off immunosuppression for 1 and 2 years, respectively, and have normal serum creatinines. The MLR of patient 3 would be expected to remain unresponsive, as he is a double haplotype match. CML activity remains absent in patients 3, 4, and 5 (Table 3).
Fig 1.
Effect of weaning immunosuppression on donor-specific MLR. T. P. = third party cells.
Table 3.
MLR and CML Data
| Patient No. | Donor | Haplotype Match | MLR | CML |
|---|---|---|---|---|
| 1 | Fraternal twin | Double | Not available (N/A) | N/A |
| 2 | Father | Single | N/A | N/A |
| 3 | Sister | Double | Unresponsive | Nonresponsive |
| 4 | Aunt | No haplotype; one class II match | Unresponsive → responsive | Nonresponsive |
| 5 | Mother | Single | Unresponsive → responsive | Nonresponsive |
| 6 | Brother | Single | N/A | N/A |
| 7 | Brother | Single | N/A | N/A |
| 8 | Sister | Single | N/A | N/A |
Skin lesions were a prominent complication in these long-term recipients. One patient had metastatic squamous cell cancer. Four others had disfiguring verrucous warts. Patients 4 to 7 have had dramatic improvement of their verrucous lesions. Patient 4 had successful surgical therapy for his metastatic squamous cell lesion.
DISCUSSION
Long-term survival following renal transplantation has been previously reported.13 Multiple drug immunosuppressive therapy based on steroids and azathioprine was found to prevent or treat renal allograft rejection in the early clinical experience.14,15 The addition of cyclosporine (CyA) to the immunosuppressive regimen significantly improved graft survival.16
The risks of chronic immunosuppression have been well described,17 and include opportunistic infection and neoplasia.18,19 Less life-threatening but equally disabling sequelae of immunosuppression such as steroid-related weight gain, edema, growth retardation, and osteopenia have also had significant morbidity.20 CyA-related nephrotoxicity and hypertension have also been described extensively.17
The benefits of withdrawal of immunosuppression21,22 must be weighed against the dangers of graft rejection and loss. Our data suggest that weaning of immunosuppression can be carried out in a select group of patients. Only one patient has demonstrated rejection during weaning of immunosuppression. Absolute or relative donor-specific unresponsiveness with MLR prior to weaning was shown in three patients available for study who maintained normal renal function after weaning. Patients 4 and 5 have had return of antidonor reactivity on MLR but not CML; clinically, they have had no evidence of rejection. The recovery of MLR in the absence of cytotoxic effector cells is under investigation.
The study remains preliminary and the enrolled patient number is small. It is known that serum creatinine is not completely accurate as an assessment of renal function. Studies using creatinine clearance or glomerular filtration will be more conclusive. We continue to follow up these patients as well as consider additional suitable patients for withdrawal of immunosuppression.
CONCLUSION
How to safely wean immunosuppression in kidney recipients is still unknown. The kidney is known to be susceptible to rapid rejection from preformed antibodies, whereas the liver, for example, has been said to be tolerogenic.23 We have postulated that the critical difference may be the leukocyte component of the organ, thus explaining why the leukocyte-poor kidney is less likely to be tolerogenic. Thus, weaning is expected to be more dangerous for kidney than for liver recipients and should not be recommended except for disabling or life-threatening indications. Therapies to augment cell migration with infusion of donor bone marrow at the time of solid organ transplantation are a strategy to balance cell traffic in the direction of graft acceptance,24 and these are expected to increase the eventual possibility of achieving a drug-free state. CyA may be safely withdrawn from recipients of HLA identical living related grafts after a year of stable graft function,5 yet others report lack of predictive immunologic parameters in this same “good risk” category of patients.25 Our data suggest that some recipients of living related HLA nonidentical grafts may be able to discontinue immunosuppression. The clinical “tolerance” exhibited here may be explained by the leukocyte microchimerism previously demonstrated. An attempt at complete weaning may be indicated in long-term survivors of living related kidney grafts, especially in those with significant complications, but close monitoring of renal function is required and long-term results are unknown.
REFERENCES
- 1.Starzl TE, Schroter GPJ, Hartmann NJ, et al. Transplant Proc. 1990;22:2361. [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JF, Jr., Pirsh JD, Ramos E, et al. N Engl J Med. 1994;331:358. doi: 10.1056/NEJM199408113310604. [DOI] [PubMed] [Google Scholar]
- 3.Suthanthiran M, Strom TB. N Engl J Med. 1994;331:365. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Heim-Duthoy K, Ma JZ. JAMA. 1993;269:395. [PubMed] [Google Scholar]
- 5.Luke RG. N Engl J Med. 1994;331:393. doi: 10.1056/NEJM199408113310610. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Trucco M, et al. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Inaba K, Aosten JM. Hepatology. 1993;17:1153. [PubMed] [Google Scholar]
- 8.Ramos HC, Reyes J, Abu-Elmagd K, et al. Transplantation. (in press) [Google Scholar]
- 9.Starzl TE, Demetris AJ, Trucco M, et al. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenlet H, Binder C. Br Med J. 1973;2:147. doi: 10.1136/bmj.2.5859.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JB, James VHT, Franckland AW, et al. Lancet. 1965:243. doi: 10.1016/s0140-6736(65)91526-6. [DOI] [PubMed] [Google Scholar]
- 12.Bynny RL. N Engl J Med. 1976;295:30. doi: 10.1056/NEJM197607012950107. [DOI] [PubMed] [Google Scholar]
- 13.Lee HM, Posner MP, King AL, et al. Transplant Proc. 1993;25:1336. [PubMed] [Google Scholar]
- 14.Starzl TE, Marchioro TL, Waddell WR. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders; 1964. [Google Scholar]
- 16.The Canadian Multi-center Transplant Group. N Engl J Med. 1986;314:1219. [Google Scholar]
- 17.Fabrega AJ, Lopez-Boado M, Gonzalez S. Crit Care Clin. 1990;6:979. [PubMed] [Google Scholar]
- 18.Dunn DL. Crit Care Clin. 1990;6:955. [PubMed] [Google Scholar]
- 19.Sheil AGR, Disney APS, Mathew TH, et al. Transplant Proc. 1993;25:1383. [PubMed] [Google Scholar]
- 20.Julian BA, Laskow DA, Dubovsky J, et al. N Engl J Med. 1991;325:544. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 21.Ignulli E, Tejani A, Markell M. Transplantation. 1993;55:1029. doi: 10.1097/00007890-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Ratcliffe PJ, Firth JD, Higgins RM, et al. Transplant Proc. 1993;25:590. [PubMed] [Google Scholar]
- 23.Starzl TE. Transplant Proc. 1993;25:8. [PubMed] [Google Scholar]
- 24.Fontes P, Rao AS, Demetris AJ, et al. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke GW, Allouch M, Cirocco R, et al. Transplantation. 1993;57:750. doi: 10.1097/00007890-199403150-00021. [DOI] [PubMed] [Google Scholar]