Summary
Plasma endotoxin was measured in 64 patients undergoing primary liver replacement. Endotoxin concentrations increased during the anhepatic phase of the operations, and remained high for several days. Although the severity of endotoxaemia did not correlate with duration of the anhepatic phase, there was a correlation between endotoxaemia and the need for perioperative platelet transfusions, ventilator dependency postoperatively, and one-month case-fatality.
Introduction
Common complications after orthotopic liver transplantation (OLT) are coagulopathy, cardiovascular instability, respiratory distress syndromes, and multiple organ failure; these abnormalities also occur in animals with endotoxaemia that have not had a transplant.1–4 Using a quantitative blood endotoxin assay,5,6 we have shown a correlation between endotoxaemia and pulmonary failure in man.7 We have now used this assay to see whether there is an association between endotoxaemia after OLT and the development of pulmonary complications or thrombocytopenia.
Patients and Methods
Patients
36 men and 28 women (mean age 46 years, range 18–66) had primary OLT between March 7 and July 31, 1988. None of the patients had had preoperative bacterial infections or massive intraoperative bleeding due to technical mishaps. Samples of systemic venous blood were collected preoperatively, at the end of the anhepatic phase of the operation but before platelet transfusion, and on the 1st, 3rd, and 7th postoperative days. The 64 recipients were divided into two groups: group A consisted of patients whose endotracheal tube could be removed within 5 days (n = 32); group B consisted of those who needed longer ventilatory support (30) or who died within 5 days (2). 6 patients in group B had a retransplantation within 7 days. The two groups did not differ with respect to underlying diseases, medical urgency as judged prospectively,8 or age and sex distribution. 3 patients in each group were on ventilators preoperatively.
Platelet replacement during OLT was guided by thrombelastographic monitoring.9 In most cases, 1 unit of platelets was given with each unit (200 ml) of fresh frozen plasma and 250 ml of crystalloids. Platelets were not given until the end of the anhepatic phase.
Endotoxin Assay
Systemic venous blood was drawn in a pyrogen-free disposable syringe, anticoagulated with heparin (10 units/ml), centrifuged immediately at 1000 g for 10 min to remove platelets, and stored at −80°C. For the colorimetric limulus assay,6 0·32 mol/l perchloric acid, 0·18 mol/l sodium hydroxide, and ‘Toxicolor’ (lyophilised amoebocyte lysate from Tachypleus tridentatus and synthetic chromogenic substrate Boc-Leu-Gly-Arg-p-nitroanilide; Seikagaku Kogyo, Tokyo, Japan) with “tris”-hydrochloric acid buffer (pH 8·0) were used. The standard curve was plotted with Escherichia coli 0111:B4 endotoxin (Westphal type; Difco Laboratories, Detroit, Michigan) in distilled water. The value of plasma endotoxin concentrations from 24 healthy volunteers was always less than 10 pg/ml.
Student’s t test was used to analyse the data.
Results
The mean preoperative endotoxin concentrations of the two groups were similar and were within the normal range. Intraoperative endotoxaemia developed at the end of the anhepatic phase in most of the patients of both groups (fig 1). The severity of endotoxaemia did not correlate with the duration of the anhepatic intervals (fig 1)—range 41–173 min, mean 104·8 (SEM 5) for group A and 108·5 (3·7) for group B. Endotoxaemia was more severe in group B patients who eventually required prolonged ventilatory support (fig 2). Significant differences between groups A and B were maintained for the next 3 days as the endotoxin concentration gradually decreased (fig 2).
Fig 1. Relation of plasma endotoxin to duration of anhepatic phase.
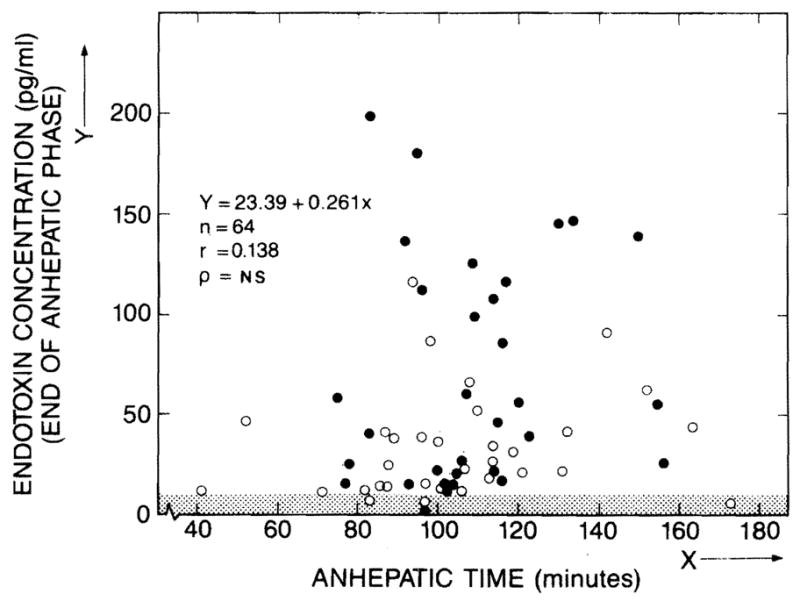
○ = group A; ● = group B; NS = not significant.
Shaded area is normal endotoxin range.
Fig 2. Endotoxin concentrations in group A (----), and group B (——).
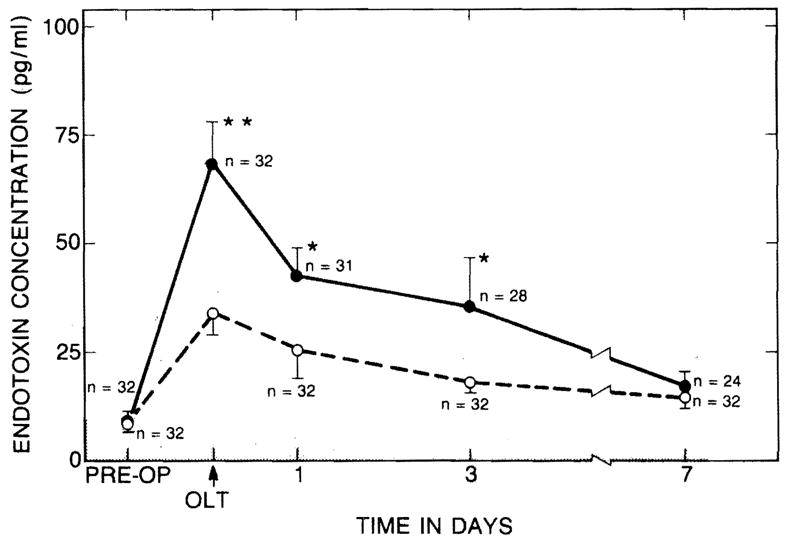
★p < 0·05; ★★p < 0·01.
43 patients received platelet transfusions because of coagulopathy and thrombocytopenia. More transfusions were given (intraoperatively and during the next 24 h) to patients in group B than to those in group A—mean 13·3 units (1·8) vs 4·6 (1·0), p < 0·01. There was a positive correlation between the number of units transfused and endotoxin concentrations at the end of the anhepatic phase (fig 3).
Fig 3. Relation of plasma endotoxin to platelet transfusions.
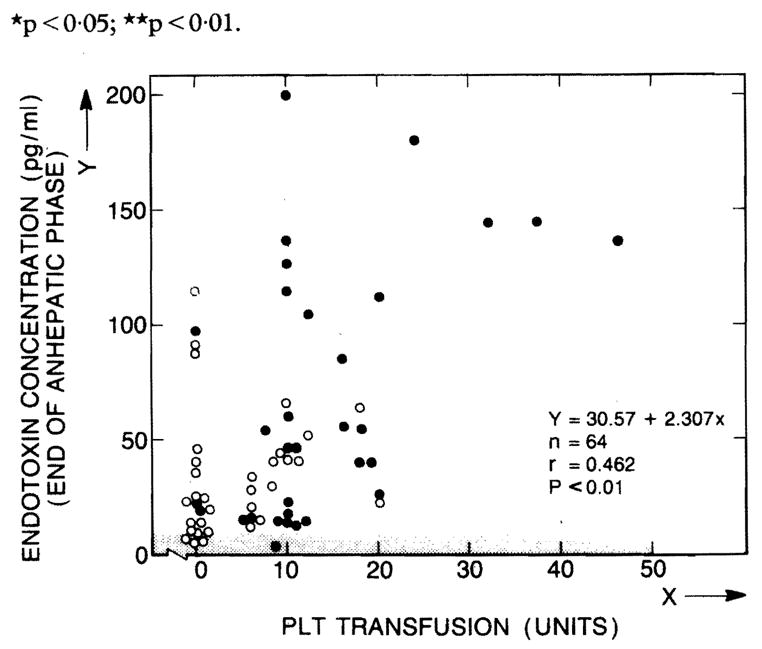
PLT = platelet.
8 of the 64 patients died within a month; 7 could not be extubated until death. The other patient died suddenly on the 11th postoperative day from rupture of a splenic artery aneurysm: convalescence up to this time had been satisfactory. Diffuse pulmonary infiltrates developed in 6 of the group B patients before death.
Discussion
Most of the endotoxin that enters the blood stream is detoxified in the liver.10–12 During the anhepatic phase of liver transplantation endotoxin entering the circulation from intestinal bacterial flora10,13,14 or infectious foci can accumulate in the blood stream. In laboratory animals endotoxaemia leads to a rapid decrease in the number of platelets in peripheral blood.4 Thus, the positive correlation between the volume of platelet transfusion needed perioperatively and the endotoxin concentrations at the end of the anhepatic phase in the present study was not surprising. Similarly, the correlation of high endotoxin with pulmonary complications was as expected from animal experiments1–3 and our previous report in human beings.5
The role of endotoxin in human disease is poorly understood, partly because the lethal dose even in the target organs varies with the animal species.15,16 Additionally, there have been difficulties17,18 with the qualitative assay that was previously available.19 The chromogenic substrate method developed by Iwanaga et al20 in 1978 paved the way to a sensitive quantitative assay of endotoxin.5,6 Study of patients with liver transplants may indicate how endotoxin triggers such diverse processes as pulmonary oedema,1,3 and also shock2 and coagulopathy.4
Acknowledgments
This work was supported by research grants from the Veterans Administration, and by project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Brigham KL, Bowers RE, Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979;45:292–97. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- 2.Balis JU, Rappaport ES, Gerber L, Fareed J, Buddingh F, Messmore HL. A primate model for prolonged endotoxin shock. Lab Invest. 1978;38:511–23. [PubMed] [Google Scholar]
- 3.Hasan FM, Teplitz C, Farrugia R, Huan E, Schwartz S. Lung function and structure after Escherichia coli endotoxin in rabbits: effect of dose and rate of administration. Circ Shock. 1984;13:1–19. [PubMed] [Google Scholar]
- 4.Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. Am J Pathol. 1978;93:527–618. [PMC free article] [PubMed] [Google Scholar]
- 5.Obayashi T, Kawai T, Tamura H, Nakahara C. New limulus amoebocyte lysate test fur endotoxaemia. Lancet. 1982;i:289. doi: 10.1016/s0140-6736(82)91016-9. [DOI] [PubMed] [Google Scholar]
- 6.Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984;104:321–30. [PubMed] [Google Scholar]
- 7.Miyata T, Torisu M. Plasma endotoxin levels and functions of peripheral granulocytes in surgical patients with respiratory distress syndrome. Jpn J Surg. 1986;16:412–17. doi: 10.1007/BF02470608. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Gordon RD, Tzakis A, et al. Equitable allocation of extrarenal organs: with special reference to the liver. Transplant Proc. 1988;20:131–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–96. [PMC free article] [PubMed] [Google Scholar]
- 10.Jabob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268–70. [PubMed] [Google Scholar]
- 11.Zlydassyk JC, Moon RJ. Fate of 51Cr-labeled lipopolysaccharide in tissue culture cells and livers of normal mice. Infect Immun. 1976;14:100–05. doi: 10.1128/iai.14.1.100-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freudenberg MA, Freudenberg N, Galanos C. Time course of cellular distribution of endotoxin in liver, lungs, and kidneys of rats. Br J Exp Pathol. 1982;63:56–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Greene R, Wiznitzer T, Rutenburg S, Frank E, Fine J. Hepatic clearance of endotoxin absorbed from the intestine. Proc Soc Exp Biol Med. 1961;108:261–63. [Google Scholar]
- 14.Gans H, Matsumoto K. The escape of endotoxin from the intestine. Surg Gynecol Obstet. 1974;139:395–402. [PubMed] [Google Scholar]
- 15.Berczi I, Bertok L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Canad J Microbial. 1966;12:1070–71. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 16.Hinshaw LB, Benjamin B, Holmes DD, et al. Responses of the baboon to live Escherichia coli organisms and endotoxin. Surg Gynecol Obstet. 1977;145:1–11. [PubMed] [Google Scholar]
- 17.Stumacher RJ, Kovnat MJ, McCabe WR. Limitations of the usefulness of the limulus assay for endotoxin. N Engl J Med. 1973;288:1261–64. doi: 10.1056/NEJM197306142882402. [DOI] [PubMed] [Google Scholar]
- 18.DuBose DA, Lemaire M, Basamania K, Rowlands J. Comparison of plasma extraction techniques in preparation of samples for endotoxin testing by the limulus amoebocyte lysate test. J Clin Microbiol. 1980;11:68–72. doi: 10.1128/jcm.11.1.68-72.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin J, Bang FB. Clottable protein in Limulus: its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrhag. 1968;19:186–97. [PubMed] [Google Scholar]
- 20.Iwanaga S, Morita T, Harada T, et al. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7:183–88. doi: 10.1159/000214260. [DOI] [PubMed] [Google Scholar]


