Abstract
Central chemoreception is active early in development and likely drives fetal breathing movements, which are influenced by a combination of behavioral state and powerful inhibition. In the premature human infant and newborn rat ventilation increases in response to CO2; in the rat the sensitivity of the response increases steadily after ~P12. The premature human infant is more vulnerable to instability than the newborn rat and exhibits periodic breathing that is augmented by hypoxia and eliminated by breathing oxygen or CO2 or the administration of respiratory stimulants. The sites of central chemoreception active in the fetus are not known, but may involve the parafacial respiratory group which may be a precursor to the adult RTN. The fetal and neonatal rat brainstem spinal-cord preparations promise to provide important information about central chemoreception in the developing rodent and will increase our understanding of important clinical problems, including The Sudden Infant Death Syndrome, Congenital Central Hypoventilation Syndrome, and periodic breathing and apnea of prematurity.
Keywords: central chemoreception, ventilatory response to CO2, fetal breathing, development
1. Introduction
There is common agreement that central respiratory chemoreception is in place early in development. In this review I will focus on the role of CO2 and central chemoreception in the control of breathing in the fetus, preterm human infant and full term newborn rat based on the evidence that brainstem development in the newborn rat from P0 until P8-10 is roughly equivalent to that in the third trimester human fetus. From studies of the rate of growth of the brain (Dobbing et al. 1979), functional studies of sleep and arousal (McNamara et al. 1998; Dauger et al. 2001; Karlsson et al. 2002; Durand et al. 2004; Blumberg et al. 2005; Seelke et al. 2005), control of breathing (Stunden et al. 2001; Putnam et al. 2005; Wong-Riley et al. 2005; Davis et al. 2006), the autonomic control of heart rate (Hofer et al. 1969), and thermoregulation (Lagerspetz 1966; Conklin et al. 1971; Mouroux et al. 1990), three periods during rat development can be recognized that are roughly comparable to stages of human development: 1) The period from P0 to P8-10, roughly equivalent to the third trimester of pregnancy in the human, 2) a period from P10 to P15 roughly equivalent to early infancy in the human in which there is dramatic development of the autonomic control of heart rate, sleep, and thermoregulation, and 3) a period from P15 to P30, roughly equivalent to late infancy through young adulthood.
Rats are relatively immature at birth. They have no fur, their eyes are fused and ear ducts are sealed. In some respects they resemble premature infants at 24–26 weeks gestation. However, rats are born with more mature lungs and may not face the same challenges of the very premature infant including surfactant deficiency and poorly developed mechanisms to maintain lung volume. With respect to respiratory control and lung development, the full term newborn rat may be more like a late preterm infant at 32–36 weeks post menstrual age (gestational age + chronological age) (PMA).
Although it is well recognized that the neuronal and muscular components of the respiratory system, as well as the lungs, continue to develop after birth, even when delivered at term, a certain degree of development must occur in utero to assure adequate ventilation within minutes of birth. Even very immature human infants have the basic tools to breathe spontaneously and relatively continuously, albeit with significant disadvantages, including a highly compliant chest wall with difficulty maintaining functional residual capacity (FRC), a propensity for airway obstruction, difficulties with coordinating sucking, swallowing and breathing, and immature respiratory control mechanisms resulting in irregular and periodic breathing and apnea.
2. Role of CO2 in the Control of Fetal Breathing Movements
It is likely that mechanisms designed to maintain oxygenation and acid base balance in the fetus are largely focused on the cardiovascular system and the placenta, since the lungs play no role in gas exchange. On the other hand, a rapid adaptation must take place at birth to survive in a new and different environment that requires the lungs to become the organ of gas exchange immediately after birth. Thus the discovery of fetal breathing movements in the late 1960s generated interest in learning more about the control of fetal “breathing”. Breathing has no gas-exchange function in the fetus and questions about and the role of breathing movements in the transition to extra-uterine life continue remain largely unanswered. However, understanding the mechanisms involved in the regulation of fetal breathing remains important, particularly in advancing our knowledge about the essential changes that must take place just before and immediately after birth and especially the transition from intermittent to continuous breathing. Because the ability to study the human fetus is limited, much of our knowledge has been gleaned from experiments in the fetal lamb.
2.1 Characterization of fetal breathing movements
Fetal “breathing movements” (FBMs), characterized by rhythmic contractions of the diaphragm, intercostals and laryngeal muscles are present in most mammalian species sometime during the second trimester of pregnancy (Jansen et al. 1983; Jansen et al. 1991; Kobayashi et al. 2001). However, the activity of the genioglossus, the major pharyngeal dilator does not appear to be synchronous with that of the diaphragm, unlike what is observed in infants and adults (Johnston et al. 1988). FBMs characteristically occur during periods of low voltage, high-frequency electrocorticogram (LV-ECoG), rapid eye movements and hypotonia, similar to many features of REM sleep (Dawes et al. 1972). During periods of high voltage low-frequency ECoG (HV-ECoG), the fetus is characteristically apneic. Although controversial, the fetus may spend a very small amount of time in a more active “awake” state characterized by LV-ECoG, fetal breathing, increased tonic and phasic nuchal EMG activity, and increased mean arterial blood pressure. In the fetal lamb FBMs are irregular with an abrupt beginning and ending of diaphragmatic (DIA) EMG electrical activity. There is no evidence for a gradual decrease in DIA EMG activity representing expiratory braking frequently observed in the premature human infant (Harding et al. 1980; Dawes et al. 1982; Rigatto et al. 1986). FBMs generate a negative tracheal pressure change of ~2–5 mmHg and on average have a TI of 0.45 s, TE of 0.74 s and TTOT of about 1.12 s (Dawes et al. 1982; Rigatto et al. 1988). The incidence of FBMs appears to increase after meals and is associated with increased plasma glucose levels. It has been suggested that the discovery of the intermittent nature of FBMs and their relationship to state may be the most important contribution to fetal physiology of the century (Rigatto 1996) and the explanation for the intermittent nature of fetal breathing continues to be incomplete. In the fetal lamb the incidence of FBMs increases and becomes more episodic during development reaching about 30% by term and characteristically decreases during the 5 days prior to the onset of labor (Berger et al. 1986).
2.2 Central control of fetal breathing
Development of the instrumentation in the 1960s that allowed physiologic measurements to be made in the unanesthetized fetal preparation (Meschia et al. 1965) was an important first step in studying mechanisms that control fetal breathing. Fetal breathing responds to chemical stimuli and other agents that have been found to affect postnatal breathing. Increasing PaCO2 increases fetal breathing as evidenced by an increase in tracheal pressure, DIA EMG activity, and frequency (Moss et al. 1979; Dawes et al. 1982; Rigatto et al. 1988), but only during LV-ECoG. However, breathing during HV-ECoG can be stimulated by unphysiologically high levels of CO2 or when pH is low (Molteni et al. 1980). External cooling is also a powerful stimulant of fetal breathing, and unlike CO2, results in breathing during both low and high voltage ECoG states (Gluckman et al. 1983; Moss et al. 1983; Kuipers et al. 1997). Moreover, administering CO2 during cooling enhances breathing during both states (Kuipers et al. 1997). A number of excitatory and inhibitory neuromodulators have also been implicated in controlling fetal breathing, including adenosine, GABA, opioids, prostaglandins, serotonin, progesterone, substance P, TRH, and catecholamines (Moss et al. 1989; Wong-Riley et al. 2005).
Both hypoxia and hypercapnia increase electrical activity in the carotid sinus nerve demonstrating that carotid chemoreceptors are active in the fetus (Blanco et al. 1984). Changes in heart rate (fH) in response to CO2 and hypoxia are also thought to be mediated by peripheral chemoreceptors. Both hypoxia and hypercapnia produce substantial decreases in fH. In the case of hypoxia, the change in fH is inversely related to the resting oxyhemoglobin saturation (Boekkooi et al. 1992). There are several lines of evidence, however, that suggest that central mechanisms drive fetal breathing. Central chemoreceptors appear to be present within days after birth in the rabbit (Wennergren et al. 1983) and piglet (Wolsink et al. 1991) suggesting their previous presence in the fetus. Decreasing PaO2 inhibits rather than stimulates fetal breathing (Koos et al. 1987; Jansen et al. 1991), the inhibition likely originating from a region in the upper lateral pons (Johnston et al. 1989; Johnston et al. 1993). Moreover, denervation of the carotid bodies does not alter fetal breathing, fetal state, or the stimulating effect of CO2 (Koos et al. 1987; Moore et al. 1989). In addition, alteration of bicarbonate concentrations in the CSF can stimulate or depress fetal breathing in the chronically instrumented lamb (Hohimer et al. 1983). However, bilateral surface lesions in on the ventral surface of the medulla corresponding to the classical Area “S” lesions, although reducing the incidence of FBMs for several days after the lesion, do not affect the FBM response to CO2 (Jansen et al. 1993).
2.2.1 Possible sites of central chemoreception in the fetus
The evolving concept of multiple brainstem sites for central chemoreception (Nattie et al. 2009) should be taken into consideration in the interpretation of lesion experiments such as those described above, as there are several candidate sites for central chemoreception in the fetus including the parafacial respiratory group (pfRG), nucleus tractus solitarius (NTS), locus coeruleus (LC), and the medullary raphe (MR). For example, the serotonin (5-HT) system is one of the earliest neurotransmitter systems to develop (Herlenius et al. 2001; Kinney et al. 2007) and 5-HT neurons in the medullary raphe are sensitive to CO2, are located near a rich blood supply, and could be an important modulator of fetal breathing during fetal life (Corcoran et al. 2009). Administration of 5-hydroxytryptamine to the fetus stimulates breathing (Quilligan et al. 1981) and importantly, endogenous serotonin modulates fetal respiratory rhythm in fetal reduced preparations (Di Pasquale et al. 1994).
Although 5-HT can stimulate breathing in the fetus, whether 5-HT neurons are chemosensitive during early development is unknown. There is evidence in the rat that the role of 5-HT neurons in chemoreception may become more important later in development based on experiments showing that the chemosensitivity of 5-HT neurons in slices and culture parallels the increase in the hypercapnic ventilatory response that occurs after about P12 (Wang et al. 1999; Corcoran et al. 2009). In addition, in adult animals, the activity of 5-HT neurons is state dependent, having the highest level of activity during wakefulness, a lower level of activity during NREM sleep and almost no activity during REM. It is unknown whether this state dependence of 5-HT neuronal activity is present during early development. In the fetus, breathing only occurs during the REM-like LV-ECoG state. Furthermore CO2 stimulates breathing only during this state, unless accompanied another stimulus such as cooling. If, as in adults, 5-HT neurons have only limited activity during REM, it is unlikely that they would contribute heavily to central chemoreception, but may provide important state related inputs to other central chemoreceptor sites. This input could be excitatory or possibly inhibitory depending on which receptors were activated. Similarly, the LC might provide a state related drive to breathing. Thus, although central chemosensory activity is present in the fetus, the exact locations or combination of sites involved remains unknown.
2.2.2 Fetal Brainstem-Spinal Cord Preparations
Recent information about the development of neural circuits responsible for fetal breathing has come from studies in in vitro fetal rodent models (Greer et al. 2006). Based on in vitro electrophysiological recordings in the isolated rat fetal brainstem-spinal cord (Di Pasquale et al. 1992; Greer et al. 1992), and ultrasound recordings of the rat fetus in utero in anesthetized dams (Kobayashi et al. 2001), it appears that respiratory rhythmogenesis first emerges around E16.7-17. In ultrasound recordings, respiratory activity begins as single isolated movements progressing to episodes of more clustered events. Unlike breathing in the conscious fetus, the burst patterns from in vitro preparations are continuous, perhaps due to the lack of descending inhibitory influences in these preparations. The episodic nature of fetal breathing movement in conscious intact mammals remains an enigma and the mechanisms remain unknown.
2.3 Role of inhibition in the control of fetal breathing
2.3.1 Inhibitory role of the upper lateral pons
Powerful inhibitory mechanisms active in the fetus, superimposed on state related cycles of excitation and inhibition, may be responsible for the episodic nature of fetal breathing. Based on transection and lesion experiments, it has been postulated that regions located in the upper lateral pons contribute to the inhibition of fetal breathing during hypoxia (Gluckman et al. 1987). In addition, lesions in this region that reverse the breathing depression caused by hypoxia also allow CO2 to stimulate breathing during the HV-ECoG state (Johnston et al. 1989) suggesting that this region may also provide some level of tonic inhibition, especially during periods of HV-ECoG. Further evidence for the presence of a fetal lateral pontine inhibitory area comes from transection and lesion studies in newborn animals showing that the depressive phase of the “biphasic” hypoxic response can be attenuated by mid-collicular but not pre-collicular transections, and red nucleus lesions in rabbits and rats (Martin-Body et al. 1988; Waites et al. 1996).
2.3.2 Inhibitory role of adenosine and other factors
Adenosine has been suggested as an important source of inhibition of FBMs in the fetus. Activation of adenosine A(2A) receptors appears to contribute to hypoxic inhibition of fetal breathing (Koos et al. 1994; Koos et al. 2002). Elevated levels of adenosine, presumably related to the relative hypoxic fetal environment, may also be a source of tonic inhibition by providing inhibition of respiratory drive via A(1) receptors and inhibiting the REM-like behavioral state via A(2A) receptors (Koos et al. 2001). Antagonists of adenosine receptors such as caffeine stimulate fetal breathing movements. Moreover, blocking adenosine A(1) receptors reverses ethanol-induced inhibition of fetal breathing movements (Watson et al. 1999).
In addition to the inhibitory actions of adenosine, both GABAergic and glutamatergic mechanisms appear to be important in suppressing the rarely observed “awake” state characterized by LV-ECoG, increased nuchal tone, increased blood pressure, and fetal breathing. For example, administration of NMDA antagonists increases the incidence of this “awake” state, which is prevented by increasing extracellular GABA by inhibiting reuptake mechanisms (Bissonnette et al. 1995). The role of other inhibitory neuromodulators such as opioids may also contribute to inhibition (Moss et al. 1989; Herlenius et al. 2001). Moreover, since cooling is also a potent stimulator of fetal breathing, the normal warm temperatures of amniotic fluid that bathe peripheral skin thermo-sensors may provide a relative level of tonic inhibition.
2.3.3 Inhibitory role of the placenta
The placenta may also be a source of tonic inhibition of breathing in the fetus, which then disappears when the fetus is separated from the placenta at birth. This mechanism may also be involved in the maintenance of continuous breathing after birth (Adamson et al. 1991; Alvaro et al. 1993) but is likely not the source of the initiation of breathing (Kuipers et al. 1992). The presence of some inhibitory placental factor is also supported by experiments showing that injection of a placental extract into the carotid artery of fetal sheep inhibits breathing induced by previous umbilical cord occlusion (Alvaro et al. 1993). Although the exact nature of such a placental factor remains unknown, prostaglandin E2, the level of which is known to decrease after birth and after cord occlusion, remains a likely candidate (Alvaro et al. 2004). An inhibitory role for prostaglandins is further supported by experiments in fetal lambs showing that the prostaglandin synthetase inhibitor, meclofenamate, stimulates and prostaglandin E2 inhibits fetal breathing movements independent of glucose concentration (Murai et al. 1984).
2.4 Role of sleep or behavioral state in fetal breathing
Fetal breathing is stimulated or inhibited in association with behavioral state. It is possible that increasing levels of state related excitatory influences on breathing are partially achieved by altering the sensitivity of central chemoreception as suggested by Guyenet, et al. (Guyenet et al. 2009). According to this hypothesis putative chemoreceptors receive increasing excitatory inputs, possibly stress related, from neuronal networks with state related activity such as MR 5-HT neurons, LC noradrenergic (NA), or possibly orexinergic neurons, which in turn lower the CO2 threshold and thus contribute to increasing respiratory drive. It is unknown, however, whether MR 5-HT and LC NA neurons have state related activity in the fetus. However, if their activity patterns are similar to adults, both MR 5-HT neurons and LC NA neurons would be expected to be relatively quiescent during REM and active during wakefulness; however both states are associated with breathing in the fetus. An excitatory contribution from the MR and LC may therefore seem reasonable during wakefulness (rarely seen), but less likely during REM. Thus the source of possible excitatory input to breathing during REM remains unknown. Breathing might also be actively inhibited during HV-ECoG since there are many sources of inhibition, but a link between HV-ECoG and any particular source of inhibition has not been identified.
Superimposed on this state related drive is a changing level of tonic inhibition possibly from placental factors including prostaglandins, relatively high concentrations of adenosine related to the hypoxic state of the fetus, and the upper lateral pons. Thus with increasing levels of inhibition, such as during hypoxia or after the administration of narcotics, any state related excitation could be overridden causing all FBMs to disappear. In contrast, decreasing levels of inhibition, as might occur at birth or after cooling, might be the origin of more continuous breathing which could occur both during LV-ECoG and HV-ECoG states. Figure 1 illustrates the possible relationships between state related intermittent breathing activity and varying levels of tonic inhibition.
Figure 1.

Relationship between state related fetal breathing and level of tonic inhibition. The line alternating between HV ECoG and LV ECoG represents alternating combinations of excitation and inhibition that increase the probability of breathing during LV-ECoG and decrease the probability of breathing during HV-EcoG. The shaded box indicates a gradient of tonic inhibition from little or no inhibition (lighter) to heavy inhibition (darker). The boxes in the right side of the diagram indicate known sources of respiratory inhibition and excitation in the fetus. The three dashed lines represent examples of 1) a baseline level of inhibition that results in breathing only during LV-CoG, 2) heavy inhibition as might be encountered during hypoxia where there is no breathing in either state, and 3) little or no inhibition as might occur during cooling or at birth where breathing becomes continuous, occurring during both states.
2.5 Control of breathing in the human fetus
While we have benefited from the study of respiratory control in the fetal lamb and rat, little specific information is known about respiratory control in the human fetus. Human fetuses have episodic rapid irregular breathing movements similar to those observed in other mammals. Also, similar to what is observed in fetal lambs, FBMs occur about 30% of the time when observed with real-time ultrasonic scanners. In late preterm fetuses (34–35 weeks gestation), FBMs are stimulated after meals and are correlated with maternal plasma glucose levels (Patrick et al. 1978). The FBM response to glucose has been observed as early as 24 weeks gestational age (GA) (Nijhuis et al. 1986) and increases with advancing gestation (Harper et al. 1987). In addition, there are sustained increases in FBMs between 0100 and 0700 that are not related to plasma glucose concentrations and may be circadian rhythm related. During labor the incidence of FBMs decreases dramatically and they are no longer stimulated by glucose administration (Boylan et al. 1980). Maternal coffee drinking can cause a doubling in the increase in FBMs and a decrease in baseline fetal fH (Salvador et al. 1989), whereas maternal ETOH and narcotics suppress FBMs. There are limited data about brainstem sites in the human fetus that might be involved in central chemoreception. In an important study, the anatomic relationships of the nucleus tractus solitarius (NTS) to other regions thought to be important for respiration were studied in the human fetus using the bidirectional lipophilic fluorescent tracer, DiI. Labelling was found in several medullary areas involved in autonomic and respiratory control, including stellate cells in the caudal raphe and cells and fibers in the Dorsal Motor Nucleus (DMN) of the vagus, the nucleus ambiguus complex, the rostral and caudal ventrolateral medulla and the medullary reticular formation. This pattern was established by mid-gestation and was consistent with anatomic patterning in animals. An important finding was that of stellate cells at the junction of the nucleus raphe pallidus and the arcuate nucleus at the ventral medullary surface which project to the NTS. These could be homologous to chemosensitive 5-HT neurons found in the midline raphe in rodents (Zec et al. 1997).
3. The premature human infant and the newborn rat
3.1. Breathing irregularity in the premature human infant and newborn rat
3.1.1 The premature human infant
Breathing in premature human infants is characterized by irregularity, periodic breathing, and frequent apnea. Breathing irregularity is largely dependent on sleep/behavioral state and is most common in active or REM sleep (AS), whereas breathing is more regular during quiet or NREM sleep (QS). In the human infant from 30 to 40 weeks PMA, breathing irregularity appears to increase slightly from 30 to 36 weeks and then steadily decreases. Breathing is irregular approximately 60–70% of the time at term and doesn’t decrease below 50% until approximately 3 months of age (Parmelee et al. 1972). The changes in the amount of irregular breathing generally correlates with the changes in the percentage of AS that occurs from 30 weeks to term (Mirmiran et al. 2003). Figure 2 shows the percent of irregular breathing closely matching the amount of active sleep in the premature infant.
Figure 2.

The relationship between the percent of active sleep (AS) (short dashed line) and percent of irregular breathing (heavy dashed line) from 30 to 40 weeks postmenstrual age (PMA). Note that the percentage of quiet sleep (QS) steadily increases and the level of indeterminant sleep (IS) steadily decreases from 30 to 40 weeks PMA. The apparent increase in the percentage of AS that occurs from 30 to 36 weeks may be partially because AS cannot be scored with certainty and any sleep that is clearly not AS or QS is scored as IS. The sleep data is from Mirmiran, et al ((Mirmiran et al. 2003), and the irregular breathing data was taken from Parmelee, et all (Parmelee et al. 1972).
3.1.2 The newborn rat
Full term neonatal rats also exhibit breathing irregularity, often associated with breathing pauses and acute decreases in fH (bradycardia) (Cummings et al. 2009a). This is most pronounced immediately after birth, becoming more regular by P10, and appears to be independent of vagal or supratentorial inputs (Zhou et al. 1996). In the experiments of Zhou, et al, performed in decerebrate and vagotomized newborn rats, adding CO2 to the breathing gases increased phrenic amplitude and minute activity with little effect on the timing variables or the degree of variability. Studies in conscious rat pups studied in their own nests with their siblings have shown that after a regular respiratory pattern is established (P10-P12), bilateral denervation of the carotid sinus nerve causes a reversion to an irregular pattern. These findings suggest that increasing strength of excitatory carotid body reflexes participates in the transition from an irregular to a regular pattern of breathing (Hofer 1986).
Moreover, the degree of irregularity is dependent not only on behavioral state, but also on temperature (Cameron et al. 2000) and is further modulated by the level of hypoxemia (Cameron et al. 2000) and hypercapnia (Cummings et al. 2009b). Interestingly, whether temperature is rising or falling also appears to play a role (Cameron et al. 2000). Measures of respiration, including V̇T, TI, TE, and V̇E, in P4 rat pups are considerably more variable in a warm environment (36 °C) compared a cool environment (24 °C) and the differences are greatest when exposure to a warm environment precedes exposure to cold. Interestingly hypoxia decreases the variability and largely abolishes any effect of temperature. Cummings and Frappell reported that the increasing variability associated with a warm environment was also associated with an increased immediate V̇E response to CO2 (Cummings et al. 2009b). Taken together, these data are consistent with observations in human premature infants that mild cooling regularizes breathing and infants kept in warmer environments exhibit more unstable breathing and apnea (Daily et al. 1969; Perlstein et al. 1970).
3.2 Periodic breathing in the premature human infant
In addition to breath-to-breath variability, human premature infants often exhibit respiratory instability in the form of periodic breathing (PB). Often the terms PB and irregularity are used interchangeably causing some confusion since the mechanisms responsible, although interrelated, are most likely different. In contrast to irregularity where there is characteristic breath-to-breath variability, PB has been defined as alternating periods of breathing and apnea. Indeed, in the human infant, most apnea of prematurity is associated with PB. For study purposes PB has been commonly defined according to Kelly et al, as three or more sequential apneas lasting more than 3 seconds, separated by periods of breathing with a duration of < 20 seconds (Kelly et al. 1979). The incidence of PB and apnea is greatest in the most immature infants and gradually decreases from 30 to 40 weeks PMA (Parmelee et al. 1972) and by 43 weeks PMA, the incidence of PB and apnea approaches that of the full term infant (Ramanathan et al. 2001). The degree of PB can be quantified by measuring the periodic cycle duration (PCD) which is the interval from the initial breath in one cluster of breaths to the initial breath in the next cluster and which takes into account both apnea and breathing cluster duration. In a study of both term and preterm infants, Wilkinson et al reported a decrease in PCD during development. Figure 3 is a plot of the PCD of both preterm and term infants plotted against PMA in days (Wilkinson et al. 2007).
Figure 3.

Periodic cycle duration is inversely related to postconceptional age. Periodic cycle duration is defined during periodic breathing as the interval from the beginning of one cluster of breaths to the beginning of the next cluster. The filled circles are data from full term infants and the open circles are from premature infants. Note that the developmentally younger infants, whether full term or premature have longer cycle durations. From Wilkinson, et al (Wilkinson et al. 2007).
3.2.1 The apneic threshold and periodic breathing
Periodic breathing can be induced by hypoxemia or respiratory depressants and alleviated by respiratory stimulants, including caffeine, and breathing oxygen or low levels (0.5–1%) of CO2. Sleep has long been known to favor the appearance of PB. In premature infants, breathing cycles between PB and non-PB breathing in both QS and AS. It has been suggested that a major factor producing PB during sleep relates to a decrease in ventilation, decrease in the sensitivity to CO2, and a decrease in arterial O2 tension (Rigatto et al. 1972). The apneic threshold has been determined to be much closer to eupnic levels of PaCO2 in premature infants compared to adults (Khan et al. 2005). Thus the combination of greater oscillations in PaCO2 and a decreased CO2 sensitivity may allow PaCO2 to frequently decrease below apneic threshold and contribute to the apnea associated with PB. Figure 4 illustrates the difference between premature infants and adults with respect to the proximity of eupneic PaCO2 and apneic threshold.
Figure 4.
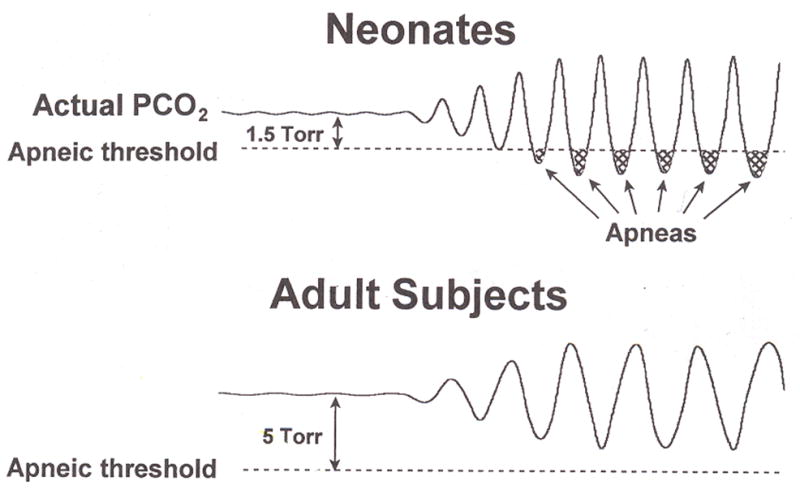
The relationship between the proximity between eupneic PaCO2 and apneic threshold, oscillations in PaCO2, and apnea in neonates and adults. The upper panel represents the neonate where the eupnic PaCO2 is close to apneic threshold in contrast with the adult, shown in the lower panel where there is a greater difference between PaCO2 and apneic threshold. Note that similar levels of oscillation of PaCO2 results in frequent decreases below apneic threshold in the neonate resulting in the apnea associated with periodic breathing. From Rigatto (Rigatto 2003).
3.2.2 Contribution of loop gain to periodic breathing
Periodic breathing can also be examined in terms of the engineering concept of loop gain (Khoo et al. 1982; Khoo 2000). For any negative feedback system, a disturbance (u), which increases alveolar ventilation (V̇A) by ΔV̇A from some steady-state equilibrium level, will lower PaCO2 by ΔPaCO2 and thus evoke a negative corrective action (e) to suppress the disturbance. The magnitude of corrective action comes from a combination of the gain of the controller (for example, the carotid body and/or central chemoreceptors), and the gain of the lungs and respiratory muscles, termed “plant” gain. The ratio of e/u defines the system loop gain. The other essential feature that favors instability of the system is the time delay between the initial change in V̇A caused by the disturbance to the corrective change in V̇A produced by the controller and plant. Thus, for an intermittent disturbance such as a sigh producing an abrupt decrease in PaCO2, when the loop gain is high (the response is high with respect to the initial change) and the response is 180° out of phase with the purturbation, a sustained oscillation can result. In premature infants, the level of arterial oxygen tension is frequently in the 40–70 range, lower than that of adults. After the first few days of life carotid chemoreceptor drive at rest may be a significant component of the overall drive to breathe (Rigatto 2003) and contribute to respiratory instability. This is supported by reports of a positive relationship between apnea frequency and the strength of the carotid body reflex (Nock et al. 2004; Cardot et al. 2007). Moreover, the low FRC in the premature infant may result in decreased “buffering” of changes in ventilation. Thus both increasing carotid body gain and low FRC may be significant contributors to an increased loop gain in the developing infant. Interventions aimed at increasing FRC, including prone positioning (Martin et al. 1979; Wagaman et al. 1979; McEvoy et al. 1997), a negative pressure applied around the chest (Thibeault et al. 1967), and continuous positive airway pressure (Kattwinkel et al. 1975) result in a decrease in periodicity. In this regard the application of CPAP has been shown to reduce loop gain in lambs breathing periodically (Edwards et al. 2009). Since an increase in peripheral chemoreceptor gain is most likely necessary to increase loop gain in the immature system, this might explain why periodic breathing does not occur immediately after birth but begins after several days. This is consistent with carotid body becoming relatively silent immediately after birth and resetting to a higher gain over the first few days of life (Blanco et al. 1984).
3.2.3 Contribution of sleep to periodic breathing
Unlike irregular breathing which is mostly related to stage of development and behavioral state (modulated by temperature and the degree of hypoxia and hypercapnia), PB occurs in both QS and AS. During PB in AS breathing is very irregular and during PB in QS breathing is more regular (Rigatto 2003). Sleep could also promote PB with further reductions in FRC and increases in chest wall compliance, particularly during AS (Knill et al. 1976; Henderson-Smart et al. 1979). In premature infants decreases in FRC have been observed during PB (Thibeault et al. 1967) and are often associated with decreases in oxyhemoglobin saturation.
3.2.4 Sighs, obstructed breaths, and periodic breathing
Augmented breaths or sighs are common in premature infants and are more frequent in AS compared to QS and most likely serve to increase lung volume and maintain FRC. The mechanisms responsible for sighs have not been clearly elucidated but are likely related to changes in irritant and chest wall reflexes caused by a decrease in lung volume, and particularly in the neonate, asphyxia and hypoxemia (Bartlett 1971; Thach et al. 1976; Alvarez et al. 1993). During PB, sighs appear to be distributed roughly equally both before and after respiratory pauses (Alvarez et al. 1993; Weintraub et al. 1994). When occurring after the pause, sighs almost always appear within the first 1–2 breaths, suggesting a decreased lung volume occurring during the pause (Henderson-Smart et al. 1979; Poets et al. 1997). In term infants sighs have also been observed to trigger episodes of PB, presumably associated with a drop in PaCO2 below apneic threshold producing an initial respiratory pause (Rigatto 2003) in combination with high loop gain and response delay.
Obstructed breaths often accompany otherwise central apnea, especially those of longer duration and the combination is often termed “mixed” apnea (Milner et al. 2004). Obstructed breaths can also occur during PB, but the pattern may differ from those occurring with longer apneas. Based on measurements of airway resistance, Miller et al reported complete airway obstruction at the level of the pharynx occurring at the beginning of each respiratory phase and continuing for approximately one-third of the breathing cycle (Miller et al. 1988). Using another approach which involves the presence or absence of a magnified cardiac oscillation signal present in the respiratory flow tracing, Lemke et al determined that during PB, in almost all cases, the magnitude of the cardiac oscillation remained relatively unchanged during the apneic phase of the cycle (Lemke et al. 1996). These data, taken together, indicate that during the relative short apneas associated with PB, the apneic phase is largely unobstructed but airway obstruction frequently occurs during the first third of the respiratory phase.
3.3 Periodic breathing in the newborn rodent
Although common in premature human infants, classic PB is rarely observed in unmanipulated newborn or adult rodents, whereas breath to breath variability (irregularity) is commonly seen. PB can be induced in adult mice of certain strains, however, including the heterozygous Mecp2+/− (Bissonnette et al. 2008), c-ret +/− (Aizenfisz et al. 2002) and inbred B6 (Han et al. 2002) mouse during the recovery phase (air or 100% O2) after exposure to hypoxia or asphyxia. Figure 5 shows the development of a periodic breathing pattern after exposure to hypoxia in the heterozygous Mecp2+/− mouse, a genetic model for Rett Syndrome in humans in which PB often occurs during sleep. The reason for the absence of periodic breathing in the newborn rodent is not known but may be related to the relative insensitivity of peripheral chemoreceptors or perhaps better mechanisms to maintain FRC (low loop gain), relatively short delay between the initial change and resulting response, or a lack of proximity of eupneic PaCO2 to apneic threshold.
Figure 5.
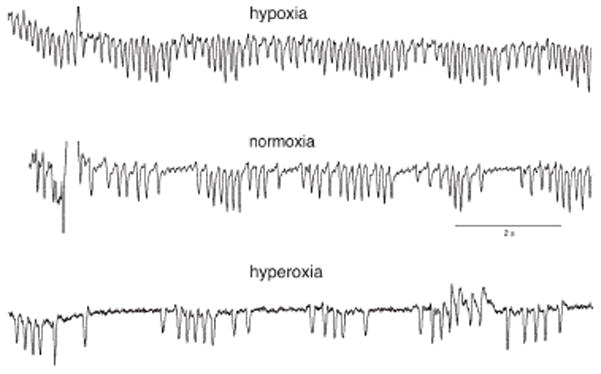
Periodic breathing in the Mecp2+/− Mouse during hypoxia, normoxia and hyperoxia. The experiment was performed by exposing the animal to 30 min of 40% oxygen, 12% oxygen, or room air. The upper panel illustrates a mostly regular breathing pattern during hypoxia. The middle and lower panels show periodicity associated with both normoxia and hyperoxia, respectively. From Bissonnette and Knopp (Bissonnette et al. 2008).
3.4 The ventilatory response to CO2 in the human preterm infant
Inhalation of CO2 results in an increase in V̇E even in the most premature human infants. In humans, the full term infant has a ventilatory response slope (ΔV̇/ΔPACO2) comparable to that of the adult, whether steady state or rebreathing methods are used (Avery et al. 1963), but the position of curve is to the left of that of the adult and shifts to the right with increasing age reflecting the lower eupnic PACO2 in the infant compared to the adult. In the premature infant, the slope of the response to CO2, measured as ΔV̇/ΔPACO2 increases with maturation (Krauss et al. 1975; Rigatto et al. 1975a; Frantz et al. 1976; Krauss et al. 1976). Unfortunately, most studies report on only two gestational age ranges in relatively mature preterm infants over a range of 30–37 weeks; there is little information on more immature infants < 30 weeks. Early studies suggested that the ventilatory response to CO2 in the preterm infant consisted of changes in both respiratory frequency (fR) and V̇T (Cross et al. 1953), thought to be explained by changes in the vagally mediated Hering-Breuer (HB) reflex during development. Bodegard reported a shift in the pattern of the CO2 response to steady state exposure of 4% CO2 from one in which there was an abrupt increase in respiratory frequency fR without much change in V̇T early in prematurity to one in which there was a gradual increase in tidal volume, followed by a fR increase accompanied by a further increase in tidal volume. This was interpreted as a shift from a strong inspiratory inhibitory reflex (the immediate rate increase) during prematurity to a weaker reflex more consistent with that observed in adults (Bodegard 1975).
Later studies using similar steady state techniques showed that once stabilized, the changes in V̇E after CO2 breathing in premature infants are largely secondary to changes in V̇T with little change in fR (Rigatto et al. 1975a) and this continues to be reflected in most reviews. If one considers the entire response, however, there is evidence for a transient increase in fR (1–2 minutes) followed by a gradual decrease to baseline levels. The initial increase in fR is associated with a decrease in TE, not TI as might be expected if the increase in fR were due to activation of the inspiratory inhibitory reflex (Martin et al. 1985; Noble et al. 1987). After the initial increase there is a decrease in fR to baseline values associated with lengthening of TE. It is likely that the initial ventilatory response to CO2 is more related to peripheral chemoreceptor CO2 sensitivity which may be relatively mature at birth.
3.4.1 Presence of expiratory braking in the ventilator response to CO2
The lengthening of TE over the last 5–6 minutes of CO2 exposure is often associated with expiratory braking and in some infants, audible grunting. The first reports of expiratory braking during CO2 exposure in premature infants were later supported by studies of Eichenwald et al (Eichenwald et al. 1993) who measured V̇E and diaphragmatic (DIA) and posterior crycoarytenoid (PCA) muscle EMG activity in 33–34 week gestation infants at 3–4 days of age. They concluded that exposure to steady state CO2 resulted in activation of both the diaphragm and the PCA and that the response to CO2 was associated with an increase in TE characterized by expiratory braking. An examination of the changes in diaphragm and PCA EMG activity and airflow revealed that most of the infants experienced prolonged post-inspiratory DIA activity which was highly correlated with increases in TE, despite increases in PCA activity. In some infants there was also evidence for laryngeal braking. The expiratory braking and prolongation of TE observed in premature infants is in marked contrast to what is observed in older infants and adults where there is a decrease in airway resistance during both inspiration and expiration and an increase in respiratory frequency characterized by a shortening of TE (Avery et al. 1963; Clark et al. 1972; Bartlett 1979).
3.4.2 Etiology of the decreased ventilatory response to CO2 in immature infants
To help answer the question of whether the diminished response in the more immature infants is secondary to a diminished central “sensitivity” to CO2 or whether mechanical or vagally mediated reflexes play a role, Franz et al measured both the V̇E and the peak pressure response to CO2 in two groups of preterm infants (29–32 wks and 33–36 wks) on day 3 and 10 of life. Similar to the findings of other investigators, they found a lower slope of the V̇E response to CO2 in the more immature infants that was correlated with peak pressure responses. Some immature infants had only a minimal ventilatory response to CO2 and were observed to have audible “grunting” and prolongation of TE. These infants, however, had normal peak pressure responses, indicating that their diminished responses were likely due to mechanical factors such as the requirement to maintain lung volume by prolonging expiratory time (Frantz et al. 1976). The mechanisms responsible for the apparent increase in CO2 sensitivity with postnatal maturation independent of changes in the mechanical properties of the lung remain unclear. Possibilities include: 1) developmental enhancement of the integrative function on the central nervous system respiratory controllers, 2) an increase in the sensitivity of the central and/or peripheral chemoreceptors to CO2 and/or [H+], 3) change in the mix of neurotransmitters and receptors, or 4) a maturational change in the effectors of the respiratory system including diaphragm and intercostal muscles (Guthrie et al. 1980). In support of the last possibility, Keens has reported an increase in the percentage of type I fibers (low fatigability) in the diaphragm and intercostals muscles of human infants with advancing gestational and postmenstrual age (Keens et al. 1978).
3.4.3 Interactions between the level of oxygenation and the ventilatory response to CO2
The interactions between the level of oxygenation and the steady state ventilatory response to CO2 also appear to be different from those in adults. In the adult, the sensitivity of the ventilatory response to CO2 increases with decreasing levels of PaO2; the response appears to be the opposite in the preterm infant, with the ventilatory response to CO2 increasing with increasing PaO2 (Rigatto et al. 1975b). However, one must take into consideration that most evaluations of CO2 chemosensitivity in infants are done using a steady state technique where measurements are often taken after 5–10 minutes of exposure. The ventilatory response to hypoxia is biphasic in the preterm infant; thus measurements of the response to CO2 are most often made after there is considerable central ventilatory depression from hypoxia. However, at least one study in preterm infants suggests that CO2 interacts positively with hypoxia (positive CO2/O2 interaction) at the peripheral chemoreceptor level (Albersheim et al. 1976). It is possible that during longer exposures to hypoxia and CO2 that central ventilatory depression associated hypoxia might override the positive CO2/O2 interaction at the peripheral chemoreceptor (Wolsink et al. 1992). There also may be considerable species differences that might affect the balance and timing between peripheral and central chemosensitivity to CO2. The degree of “roll off” during steady state exposure to hypoxia may provide some clues. In the human preterm infant, for example, the roll off is extreme often producing apnea after 4–5 minutes of exposure. This roll off is not as extreme in the newborn rat or piglet and in these species a there is a positive CO2/O2 interaction similar to the adult, except during very severe hypoxia (Saetta et al. 1987; Wolsink et al. 1992).
3.5 Ventilatory response to CO2 in the newborn rat
The steady state ventilatory responses to CO2 in more mature newborn animals such as the piglet or the goat have been reported to be similar to adults although few direct comparisons have been performed (Lowry et al. 1996; Putnam et al. 2005). Ventilatory responses to CO2 in the newborn rat, however, in many respects are similar to the human premature infant. Studies that have examined the response to CO2 in the first few minutes have shown a transient increase in fR (Cummings et al. 2009b). After equilibration, the changes in V̇E are almost entirely secondary to changes in V̇T (Stunden et al. 2001; Davis et al. 2006). In contrast to what is commonly observed in human premature infants, expiratory braking is not as evident in young rats, although in the first week of life, in response to CO2, there is also a decrease in fR and a prolongation of TE. Moreover, the lengthening of TE appears to be influenced by GABAergic mechanisms, since it is abolished by pretreatment with bicuculline, a GABAA receptor antagonist (Abu-Shaweesh et al. 1999). Similar to more mature infants, children and adults, fR commonly increases in response to CO2 in adolescent and adult intact rats (Abu-Shaweesh et al. 1999).
Resting ventilation and the ventilatory response to CO2 in the newborn rat is also affected by body and environmental temperature and this is age dependent. P2-P3 and P5-P6, but not P8-P9 rat pups, have a higher resting ventilation at body temperatures of 32–33°C compared to 36–37°C or 38–39°C. There is some controversy about the effect of temperature on the V̇E response to CO2; some have observed greater overall responses at cooler temperatures, but only in the P2-P3 and P5-P6 rat pups (Malcolm et al. 1996), whereas others have noted greater immediate responses at warmer temperatures (Cummings et al. 2009b) in these same age ranges. The differences in these observations may be partially due to the focus on the immediate (likely peripheral chemoreceptor) responses by Cummings et al and the measurement of a more steady state response by Malcolm et al.
The effect of oxygenation on the steady state ventilatory response to CO2 in the newborn rat is also different from that in the premature human infant and shows a positive correlation between hypoxia and the response to CO2 (Saetta et al. 1987). These differences may be secondary to the combination of the species and age related relative contribution of peripheral and central chemoreceptors to the total ventilatory response to CO2 and to varying degrees hypoxic ventilatory depression.
3.5.1 Changes in the sensitivity of the ventilatory response to CO2 with advancing age in the rodent
In the young rat, the sensitivity of the steady state ventilatory response to CO2 generally increases with age. There is general agreement that the response increases between P12-P15 and P30, but there is considerable debate over the change in the response in the first few days of life. Some have contended that the response is strong immediately after birth but decreases, reaching a nadir by about P8-P12, followed by a steady increase until about P30 (Serra et al. 2001; Stunden et al. 2001; Putnam et al. 2005). Others have suggested that the response is relatively constant over the first few days until P8-12, and then increases steadily until P30 (Davis et al. 2006). A nadir in the ventilatory response to CO2 at P8-12 could represent a “critical period” of development which correlates with an abrupt decrease in glutamate and increase in GABA content in regions of the developing brain stem important for respiratory control (Wong-Riley et al. 2005). This concept is also attractive because it may correlate with the highest incidence of The Sudden Infant Death Syndrome (SIDS) in humans at around 2–3 months of age, the etiology of which is thought to be related to abnormalities in respiratory and autonomic control during a critical period associated with changing control mechanisms.
Some have argued that the ventilatory response to CO2 changes little over the first 10 days of life and have suggested that one contributing factor to the difference in results is the different methods used to express CO2 sensitivity, one normalized to body weight and the other as a percent of eupnic ventilation (Davis et al. 2006). Moreoever, Stunden et al (Stunden et al. 2001) used “head-out” pressure plethysmography and defined sensitivity as the slope of the response (for example, ΔV̇E/Δ%CO2) calculated from exposing each animal to several concentrations of CO2, whereas Davis et al (Davis et al. 2006) used flow through whole body plethysmography and calculated sensitivity from a single measurement of ventilation after 3–5 minutes of breathing 7% CO2. Both of these methods have their limitations that may have contributed to different results. Figure 6 summarizes the changes in the ventilatory response to CO2 with age using different methodologies. Most agree that the ventilatory response to CO2 increases after P12-15, but there remains uncertainty about the course of the response during the first 10 days of life.
Figure 6.

Change in the ventilatory response to CO2 with advancing age in the rat: a comparison of two methods. In one set of experiments (Stunden et al. 2001), the slope of the ΔV̇/Δ%CO2, normalized to body weight, for each animal was calculated using several levels of CO2 at different ages. Each grey symbol represents the slope from a single animal. In another set of experiments (Davis et al. 2006) the CO2 response was calculated as the % of eucapnic V̇E averaged from several breaths between 3–5 minutes of CO2 exposure. The open circles represent averages of measurements on several animals at different ages. Lines have been fitted to the data to demonstrate the trends. Values for the slope data is shown on the left axes and values for the % eucapnia data is shown on the right axis. The ranges have been aligned to approximate the two groups of data. Adapted from Stunden et al (Stunden et al. 2001) and Davis et al (Davis et al. 2006).
The large variation in the individual and daily measurements of the ventilatory response to CO2 may also be influenced by how the experimental methodology takes into account changes in growth and development that occur during the first few days of life. For example, there is a doubling of body weight from P2 to P8 accompanied by changes metabolic rate (Taylor 1960; Mortola 2001), the ideal thermal environment (Mortola et al. 1998), sleep and activity cycling (Blumberg et al. 2005), and interactions with the dam and siblings. Ideally studies should be performed in the nest with the siblings and dam. This is rarely practical but some investigators have made measurements of heart rate and respiratory frequency in this environment (Hofer et al. 1969; Hofer 1986).
3.5.2 The importance of thermoregulation in respiratory measurements in immature rodents
The “head out” plethysmograph is commonly used to make respiratory measurements in small rodents. A commonly used technique is to warm the “body” portion of the chamber to maintain body temperature in some range. Often, however, little attention is made to controlling the temperature that is flowing around the head. It is well known that the trigeminal area of the face is exquisitely sensitive to temperature and cooling the face can increase metabolic rate even if abdominal skin and core temperature are normal (Mestyan et al. 1964). Figure 7 shows increases in metabolic rate associated with facial cooling in a premature human infant in the presence of a stable rectal and abdominal skin temperature. The thermoneutral environment also changes with growth and maturity, so that the environmental temperature designed for the P10 animal may not be warm enough for the younger pup. Similarly, a thermal environment designed for a P2 pup may be “too warm” for the larger P12 pup. The ventilatory response to CO2 is influenced by environmental and body temperature and the effects are age dependent with the greatest effects in the youngest pups (Malcolm et al. 1996; Saiki et al. 1996; Cummings et al. 2009b). Thus the age dependent effects of cooling or warming on the ventilatory response to CO2 itself and/or the changing thermoneutral range with growth could result in apparent trends in the measured response with advancing age.
Figure 7.

The effect of facial cooling on metabolic rate in a premature infant when abdominal temperature is kept constant. Infants were studied on a radiant warmer and facial cooling was accomplished by shading the face from the radiant heat. Note the steady increase in oxygen consumption with decreasing cheek temperature. Darnall, RA, 1978, unpublished observations.
3.5.3 The importance of sleep cycling in respiratory measurements in immature rodents
Sleep cycling also changes over the first few days of development and is entrained to feeding cycles and the circadian rhythm of the dam. In the youngest animals, sleep predominates, and of the sleep time, most is in a state with many features of REM or AS with frequent twitching and hypotonia. This state can easily be confused with wakefulness. Since eye opening does not occur until ~ P12, this cannot be used as a sign of wakefulness in the very young pups. In contrast, quiet immobility is a reasonable sign of QS. However, even with nuchal EMG measurements, this behavioral state cannot be completely differentiated from “non-phasic” REM sleep (Seelke et al. 2008). During development there is a decrease in the amount of AS and an increase in the amount of QS and wakefulness. Thus in the very young animal state may change several times over a single 5–10 minute exposure to hypoxia or hypercapnia. Superimposed on this normal cycling is the varying effect of hypoxia or CO2 on state and arousal. For example in recent experiments, we explored the effect of hypoxia (10% oxygen) on arousal, where the onset of hypoxia was always during quiet immobility. P5 pups switched to REM before arousal nearly 100% of the time, P15 pups 50% of the time, and P25 animals 2% of the time. Moreover, with both hypoxia and hypercapnia, the time to arousal exhibits a kind of “habituation” with progressively increases in latency with repeated exposure. Figure 8 shows the time to arousal (latency) to a brief exposure to either 10% oxygen/90% nitrogen or 8% CO2/21% oxygen in P15 pups at thermoneutrality. Note that hypoxia is a more potent stimulus for arousal and that both hypoxia and hypercapnia result in arousal “habituation”, a progressive blunting of arousal with repeated exposure. Similar to our previous observations during hypoxia, hypercapnia resulted in a switch from QS to REM sleep in 50% of the experiments in P15 pups (Darnall, RA, unpublished observations).
Figure 8.

Arousal habituation in response to repeated exposures either to 10% oxygen or 8% CO2 in the P15 rat pup. Each trial consisted of 3 minutes of either oxygen or CO2 followed by 6 minutes of room air. Each trial was started during a period of quiet immobility, most likely QS. Filled triangles represent data from hypoxia experiments and open triangles represent data from hypercapnia experiments. Note the progressive increase in the time to arousal (latency) for both. All studies were approved by the Dartmouth College Institutional Animal Care and Use Committee. Darnall, RA, unpublished observations.
3.5.4 The importance of the relationship of time after feeding to respiratory measurements
Another factor that is rarely taken into consideration is the time between feeding and exposure to CO2. Increases in metabolic rate and decreases in activity associated with feeding might also variably affect V̇E measurements. The time before or after feeding is routinely taken into consideration in human studies where it has been suggested that the human infant is rarely in a steady state but is either “recovering from the last feed or seeking the next one”. In newborn rat studies, some investigators have eliminated this confounder by feeding the pups by gavage before each experiment (Blumberg et al. 2005).
3.5.1 Sites of central chemoreception in the newborn rat
There is limited information about the sites or developing function of central chemoreception in the developing newborn rat. Single unit recordings in the neonatal brainstem-spinal cord preparation obtained from a region between the caudal end of the vagal roots and 0.5 mm rostral to the caudal end of the hypoglossal roots and from the ventral surface to a depth of 500 μm showed variable responses to hypercapnia; most of the recorded neurons were not active in phase with respiration (Okada et al. 1993). More recently, neuronal recordings from at least four regions thought to be involved in central chemoreception including the MR, the LC, the NTS, and the pfRG/RTN region in slices and reduced neonatal brainstem-spinal cord preparations and have provided some clues (Putnam et al. 2005; Onimaru et al. 2008). LC neuronal activity measured in brain slices using perforated patch techniques increased by ~44% in response to hypercapnic acidosis at all ages from P1-P21 tested (Stunden et al. 2001). Similarly, hypercapnia activated about 40–50% of NTS neurons in slices from several ages between P1 and adult and the firing rate increase with CO2 was constant across ages (Conrad et al. 2009). In contrast to neurons isolated from neonatal NTS or the LC, neurons in slices or culture isolated from the MR appear to be only minimally responsive to CO2 before P12 but thereafter exhibit increasing chemosensitivity (Wang et al. 1999). Thus the increase in neuronal activity in response to CO2 parallels the increase in the ventilatory response to CO2 that occurs starting around P12.
The pfRG contains a group of neurons with respiratory related bursts of activity that were originally identified just ventral to the caudal edge of the facial nucleus in the neonatal brainstem-spinal cord preparation (Onimaru et al. 2006). This group of neurons, which has been identified as early as E19 in the fetal rat and which has intrinsic bursting properties at birth (Onimaru 1995, 2002), has been postulated to be the fetal and early neonatal precursor to the RTN, a major site of central chemoreception in the adult (Guyenet et al. 2009). The most rostral and superficial pfRG neurons located very close to the ventral medullary surface under the facial motor nucleus have the same phenotype as the RTN neurons in the adult. They express Phox2b and possibly VGLUT2 and are not catecholaminergic (Guyenet et al. 2009). Moreover, they are postsynaptically sensitive to CO2 (Onimaru et al. 2008). The intrinsic bursting property present at birth disappears after P7 suggesting that this group of neurons may be particularly important for maintaining breathing at the time of birth. This is supported by convincing evidence showing that severe hypoventilation in neonates occurs in two genetic models of the Congenital Central Hypoventilation Syndrome (CCHS) in which the pfRG is absent but more caudal regions, including the pre-Bötzinger complex, continue to be present (Dubreuil, 2008; Pagliardini, 2008).
4. Conclusions
In conclusion, central chemoreception is active early in development and likely drives fetal breathing movements, which are essential for maintaining fetal lung volume and lung growth and development and in preparation for continuous breathing required after birth. In the fetus breathing is influenced by the combination of behavioral state and powerful inhibition from sources including prostaglandins, adenosine, and neurons located in the upper lateral pons. Inhibition increases during hypoxia and decreases during cooling, cord compression, in response to increasing PaCO2 and after the fetus is separated from the placenta at birth. The ventilation of the premature human infant and the neonatal rat responds to CO2 at birth. In the human the steady state ventilatory response to CO2 increases with development. In the newborn rat, sensitivity of the CO2 ventilatory response is either relatively flat or decreases until about P8-10 and increases thereafter. The premature human infant is perhaps more vulnerable to respiratory instability than the newborn rat and exhibits periodic breathing that is augmented by hypoxia and eliminated by breathing oxygen or CO2 or the administration of respiratory stimulants, including caffeine. Most apnea in premature infants is associated with periodic breathing and pharmacological respiratory stimulants such as caffeine are used clinically. The exact sites of central chemoreception active in the fetus are not known, but may involve the parafacial respiratory group, a likely precursor to the RTN, which contains non-catecholaminergic neurons expressing Phox2b and VGLUT2 that are thought to be a major site of central chemoreception in the adult. Serotonergic neurons located in the midline raphe and noradrenergic neurons in the LC may play important state related roles in the young neonate and adult by enhancing CO2 sensitivity. The brainstem spinal-cord neonatal and fetal rat preparations promise to provide increasing information about respiratory control and central chemoreception in the developing rodent. Information gleaned from rodent experiments both in the fetus and neonate will have an important impact on the clinical care of human unborn fetuses and premature infants and provide insights into the etiology of important clinical problems, including The Sudden Infant Death Syndrome, Congenital Central Hypoventilation Syndrome, and periodic breathing and apnea of prematurity.
Acknowledgments
The author is supported by an NIH grant 5P01 HD036379-12 and thanks Don Bartlett for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Shaweesh JM, Dreshaj IA, Thomas AJ, Haxhiu MA, Strohl KP, Martin RJ. Changes in respiratory timing induced by hypercapnia in maturing rats. J Appl Physiol. 1999;87:484–490. doi: 10.1152/jappl.1999.87.2.484. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Kuipers IM, Olson DM. Umbilical cord occlusion stimulates breathing independent of blood gases and pH. J Appl Physiol. 1991;70:1796–1809. doi: 10.1152/jappl.1991.70.4.1796. [DOI] [PubMed] [Google Scholar]
- Aizenfisz S, Dauger S, Durand E, Vardon G, Levacher B, Simonneau M, Pachnis V, Gaultier C, Gallego J. Ventilatory responses to hypercapnia and hypoxia in heterozygous c-ret newborn mice. Resp Physiol & Neurobiol. 2002;131:213–222. doi: 10.1016/s1569-9048(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Albersheim S, Boychuk R, Seshia MM, Cates D, Rigatto H. Effects of CO2 on immediate ventilatory response to O2 in preterm infants. J Appl Physiol. 1976;41:609–611. doi: 10.1152/jappl.1976.41.5.609. [DOI] [PubMed] [Google Scholar]
- Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol Neon. 1993;63:139–146. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- Alvaro RE, De Almeida V, Al-Alaiyan S, Robertson MA, Nowaczyk B, Cates DB, Rigatto H. A placental extract inhibitis breathing induced by umbilical cord occlusion in fetal sheep. J Dev Physiol. 1993;19:23–28. [PubMed] [Google Scholar]
- Alvaro RE, Hasan SU, Chemtob S, Qurashi M, Al-Saif S, Rigatto H. Prostaglandins are responsible for the inhibition of breathing observed with a placental extract in fetal sheep. Resp Physiol & Neurobiol. 2004;144:35–44. doi: 10.1016/j.resp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Avery ME, Chernick V, Dutton RE, Permutt S. Ventilatory response to inspired carbon dioxide in infants and adults. J Appl Physiol. 1963;18:895–903. doi: 10.1152/jappl.1963.18.5.895. [DOI] [PubMed] [Google Scholar]
- Bartlett D. Effects of hypercapnea and hypoxia on laryngeal resistance to airflow. Resp Physiol. 1979;37:293–302. doi: 10.1016/0034-5687(79)90076-8. [DOI] [PubMed] [Google Scholar]
- Bartlett DJ. Origin and regulation of spontaneous deep breaths. Resp Physiol. 1971;12:230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- Berger PJ, Walker AM, Horne R, Brodecky V, Wilkinson MH, Wilson F, Maloney JE. Phasic respiratory activity in the fetal lamb during late gestation and labour. Resp Physiol. 1986;65:55–68. doi: 10.1016/0034-5687(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Hohimer AR, Knopp SJ. GABAergic and glutamatergic effects on behaviour in fetal sheep. J Physiol. 1995;487.3:677–684. doi: 10.1113/jphysiol.1995.sp020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The responsse to hypoxia of arterial chemoreceptors in fetal shedep and newborn lambs. J Physiol. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegard G. Control of respiration in newborn babies III: Developmental changes of respiratory depth and rate responses to CO2. Acta Paediatr Scand. 1975;64:684–692. doi: 10.1111/j.1651-2227.1975.tb03905.x. [DOI] [PubMed] [Google Scholar]
- Boekkooi PF, Baan J, Jr, Teitel D, Rudolph AM. Chemoreceptor responsiveness in fetal sheep. Am J Physiol Heart Circ Physiol. 1992;263:H162–H167. doi: 10.1152/ajpheart.1992.263.1.H162. [DOI] [PubMed] [Google Scholar]
- Boylan P, Lewis PJ. Fetal breathing in labor. Obstet Gynecol. 1980;58:35–38. [PubMed] [Google Scholar]
- Cameron YL, Merazzi D, Mortola JP. Variability of the breathing pattern in newborn rats: effets of ambient temperature in normoxia and hypoxia. Pediatr Res. 2000;47:813–816. doi: 10.1203/00006450-200006000-00022. [DOI] [PubMed] [Google Scholar]
- Cardot V, Chardon K, Tourneux P, Micallef S, Stephan E, Leke A, Bach V, Libert JP, Telliez F. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res. 2007;62:591–596. doi: 10.1203/PDR.0b013e318155868e. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin P, Heggeness FW. Maturation of temperature homeostasis in the rat. Am J Physiol. 1971;220:333–336. doi: 10.1152/ajplegacy.1971.220.2.333. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Resp Physiol & Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Resp Physiol & Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross KW, Hooper JM, Oppe TE. The effect of inhalation of carbon dioxide in air on the respiration of the full-term and premature infant. J Physiol. 1953;122:264–273. doi: 10.1113/jphysiol.1953.sp004997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R1783–R1796. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol. 2009b;297:R124–R134. doi: 10.1152/ajpregu.91011.2008. [DOI] [PubMed] [Google Scholar]
- Daily WJ, Klaus M, Meyer HB. Apnea in premature infants: Monitoring, incidence, heart rate changes, and an effect of environmental temperature. Pediatrics. 1969;43:510–518. [PubMed] [Google Scholar]
- Dauger S, Aizenfisz S, Renolleau S, Durand E, Vardon G, Gaultier C, Gallego J. Arousal response to hypoxia in newborn mice. Resp Physiol. 2001;128:235–240. doi: 10.1016/s0034-5687(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972;220:119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Gardner WN, Johnston BM, Walker DW. Effects of hypercapnia on tracheal pressure, diaphragm and intercostal electromyograms in unanesthetized fetal lambs. J Physiol. 1982;326:461–474. doi: 10.1113/jphysiol.1982.sp014206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. Endogenous serotonin modulates the fetal respiratory rhythm: an in vitro study in the rat. Brain Res Dev Brain Res. 1994;80:222–232. doi: 10.1016/0165-3806(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Durand E, Lofaso F, Dauger S, Vardon G, Gaultier C, Gallego J. Intermittent hypoxia induces transient arousal delay in newborn mice. J Appl Physiol. 2004;96:1216–1222. doi: 10.1152/japplphysiol.00802.2003. [DOI] [PubMed] [Google Scholar]
- Edwards BA, Sands SA, Feeney C, Skuza EM, Brodecky V, Wilkinson MH, Berger PJ. Continuous positive airway pressure reduces loop gain and resolves periodic central apneas in the lamb. Resp Physiol & Neurobiol. 2009;168:239–249. doi: 10.1016/j.resp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Eichenwald EC, Ungarelli RA, Stark AR. Hypercapnia increases expiratory braking in preterm infants. J Appl Physiol. 1993;75:2665–2670. doi: 10.1152/jappl.1993.75.6.2665. [DOI] [PubMed] [Google Scholar]
- Frantz ID, Adler SM, Thach BT, Taeusch HW. Maturational effects on respiratory responses to carbon dioxide in premature infants. J Appl Physiol. 1976;41:41–45. doi: 10.1152/jappl.1976.41.1.41. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Gunn TR, Johnston BM. The effect of cooling on breathing and shivering in unanaesthetized fetal lambs in utero. J Physiol. 1983;343:495–506. doi: 10.1113/jphysiol.1983.sp014905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Johnston BM. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol. 1987;382:373–383. doi: 10.1113/jphysiol.1987.sp016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath: prenatal maturation of respiratory neural control. J Physiol. 2006;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Generation of respiratory and locomotor patterns by an in vitro brainstem-spinal cord fetal rat preparation. J Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Guthrie RD, Standaert TA, Hodson WA, Woodrum DE. Sleep and maturation of eucapnic ventilation and CO2 sensitivity in the premature primate. J Appl Physiol. 1980;48:347–354. doi: 10.1152/jappl.1980.48.2.347. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SBG, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Resp Physiol & Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol. 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME. Respiratory function of the larynx in developing sheep and the influence of sleep state. Resp Physiol. 1980;40:165–179. doi: 10.1016/0034-5687(80)90090-0. [DOI] [PubMed] [Google Scholar]
- Harper MA, Meis PJ, Rose JC, Swain M, Burns JC, Kardon B. Human fetal breathing response to intravenous glucose is diretly related to gestational age. Am J Obstet Gynecol. 1987;157:1403–1405. doi: 10.1016/s0002-9378(87)80232-6. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Read JC. Reduced lung volume during behavioral active sleep in the newborn. J Appl Physiol. 1979;46:1081–1085. doi: 10.1152/jappl.1979.46.6.1081. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65:21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Role of carotid sinus and aortic nerves in respiratory control of infant rats. Am J Physiol. 1986;251:811–817. doi: 10.1152/ajpregu.1986.251.4.R811. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Reiser MF. The development of cardiac rate regulation in preweanling rats. Psychosom Med. 1969;31:372–388. doi: 10.1097/00006842-196909000-00003. [DOI] [PubMed] [Google Scholar]
- Hohimer AR, Bissonnette JM, Richardson BS, Machida CM. Central chemical regulation of breathing movements in fetal lambs. Resp Physiol. 1983;52:99–111. doi: 10.1016/0034-5687(83)90139-1. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Development of respiratory control. Physiol Rev. 1983;63:437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Fetal breathing and development of control of breathing. J Appl Physiol. 1991;70:1431–1446. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Ioffe S, Chernick V. Effect of medullary lesions, vagotomy and carotid sinus denervation on fetal breathing. Resp Physiol. 1993;94:265–283. doi: 10.1016/0034-5687(93)90023-4. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gluckman PD. Lateral pontine lesions affect central chemosensitivity in unanesthetized fetal lambs. J Appl Physiol. 1989;67:1113–1118. doi: 10.1152/jappl.1989.67.3.1113. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gluckman PD. Peripheral chemoreceptors respond to hypoxia in pontine-lesioned fetal lambs in utero. J Appl Physiol. 1993;75:1027–1034. doi: 10.1152/jappl.1993.75.3.1027. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gunn TR, Gluckman PD. Surface cooling rapidly induces coordinated activity in the upper and lower airway muscles of the fetal lamb in utero. Pediatr Res. 1988;23:257–261. doi: 10.1203/00006450-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Kattwinkel J, Nearman HS, Fanaroff AA, Katona PG, Klaus MH. Apnea of prematurity: Comparative therapeutic effects of cutaneous stimulation and nasal continuous positive airway pressure. J Pediatr. 1975;86:588–592. doi: 10.1016/s0022-3476(75)80158-2. [DOI] [PubMed] [Google Scholar]
- Keens T, Bryan AC, Levison H, Ianuzzo CD. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol. 1978;44:909–913. doi: 10.1152/jappl.1978.44.6.909. [DOI] [PubMed] [Google Scholar]
- Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979;63:355–360. [PubMed] [Google Scholar]
- Khan A, Qurashi M, Kwiatkowski K, Cates D, Rigatto H. Measurement of the CO2 apneic threshold in newborn infants: possible relevance for periodic breathing and apnea. J Appl Physiol. 2005;98:1171–1176. doi: 10.1152/japplphysiol.00574.2003. [DOI] [PubMed] [Google Scholar]
- Khoo MC. Determininants of ventilatory instability and variability. Resp Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Knill R, Bryan AC. An intercostal-phrenic inhibitory reflex in human newborn infants. J Appl Physiol. 1976;40:352–356. doi: 10.1152/jappl.1976.40.3.352. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lemke RP, Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Maeda J, Jan C. Adenosine A(1) and A(2A) receptors modulate sleep state and breathing in fetal sheep. J Appl Physiol. 2001;91:343–350. doi: 10.1152/jappl.2001.91.1.343. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Maeda T, Jan C, Lopex G. Adenosine A(2A) receptors mediate hypoxic inhibition of fetal breathing in sheep. Am J Obstet Gynecol. 2002;186:663–668. doi: 10.1067/mob.2002.122129. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Mason BA, Punla O, Adinolfi AM. Hypoxic inhibition of breathing in fetal sheep: Relationship to brain adenosine concentrations. J Appl Physiol. 1994;77:2734–2739. doi: 10.1152/jappl.1994.77.6.2734. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Sameshima H, Power GG. Fetal breathing, sleep state, and cardiovascular responses to graded hypoxia in sheep. J Appl Physiol. 1987;62:1033–1039. doi: 10.1152/jappl.1987.62.3.1033. [DOI] [PubMed] [Google Scholar]
- Krauss AN, Klain DB, Waldman S, Auld PA. Ventilatory response to carbon dioxide in newborn infants. Pediatr Res. 1975;9:46–50. [Google Scholar]
- Krauss AN, Waldman S, Auld PA. Diminished response to carbon dioxide in premature infants. Biol Neon. 1976;30:216–223. [Google Scholar]
- Kuipers IM, Maertzdorf WJ, De Jong DS, Hanson MA, Blanco CE. The effect of hypercapnia and hypercapnia associated with central cooling on breathing in unanesthetized fetal lambs. Pediatr Res. 1997;41:90–95. doi: 10.1203/00006450-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Kuipers IM, Maertzdorf WJ, Keunen H, De Jong DS, Hanson MA, Blanco CE. Fetal breathing is not initiated after cord occlusion in the unanaesthetized fetal lamb in utero. J Dev Physiol. 1992;17:233–240. [PubMed] [Google Scholar]
- Lagerspetz KY. Temperature relations of oxygen consumption and motor activity in newborn mice. Ann Med Exp Biol Fenn. 1966;44:71–73. [PubMed] [Google Scholar]
- Lemke RP, al-Saedi SA, Alvaro RE, Wiseman NE, Cates DB, Kwiatkowski K, Rigatto H. Use of a magnified cardiac airflow oscillation to classify neonatal apnea. Am J Resp & Crit Care Med. 1996;154:1537–1542. doi: 10.1164/ajrccm.154.5.8912777. [DOI] [PubMed] [Google Scholar]
- Lowry TF, Forster HV, Pan LG, Ohtake PJ, Epshteyn I, Korducki MJ, Franciosi RA. Effect on breathing of ventral medullary surface cooling in neonatal goats. J Appl Physiol. 1996;80:949–1957. doi: 10.1152/jappl.1996.80.6.1949. [DOI] [PubMed] [Google Scholar]
- Malcolm GA, Henderson-Smart DJ. The effect of temperature on the ventilatory response to carbon dioxide in the neonatal rat. Pediatr Res. 1996;40:504–507. doi: 10.1203/00006450-199609000-00022. [DOI] [PubMed] [Google Scholar]
- Martin-Body RI, Johnston BM. Central origin of the hypoxic depression of breathing in the newborn. Resp Physiol. 1988;71:25–32. doi: 10.1016/0034-5687(88)90112-0. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Carlo WA, Robertson SS, Day WR, Bruce EN. Biphasic response of respiratory frequency to hypercapnea in preterm infants. Pediatr Res. 1985;19:791–796. doi: 10.1203/00006450-198508000-00002. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Herrell N, Rubin D, Fanaroff A. Effect of supine and prone positions on arterial oxygen tension in the preterm infant. Pediatrics. 1979;63:528–531. [PubMed] [Google Scholar]
- McEvoy C, Mendoza ME, Bowling S, Hewlett V, Sardesai S, Durand M. Prone positioning decreases episodes of hypoxemia in extremely low birth weight infants (1000 grams or less) with chronic lung disease. J Pediatr. 1997;130:305–309. doi: 10.1016/s0022-3476(97)70360-3. [DOI] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- Meschia D, Cotter JR, Breathnach CS, Barron DH. The hemoglobin, oxygen, carbon dioxide and hydrogen ion concentrations in the umbilical bloods of sheep and goats as sampled via indwelling plastic catheters. Quart J Exp Physiol. 1965;50:185–195. doi: 10.1113/expphysiol.1965.sp001780. [DOI] [PubMed] [Google Scholar]
- Mestyan J, Jarai I, Bata G, Fekete M. The significance of facial skin temperature in the chemical heat regulation of premature infants. Biol Neon. 1964;7:243–254. doi: 10.1159/000239927. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Carlo WA, DiFiore JM, Martin RJ. Airway obstruction during periodic breathing in premature infants. J Appl Physiol. 1988;64:2496–2500. doi: 10.1152/jappl.1988.64.6.2496. [DOI] [PubMed] [Google Scholar]
- Milner AD, Greenough A. The role of the upper airway in neonatal apnoea. Semin Neonatol. 2004;9:213–219. doi: 10.1016/j.siny.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- Molteni RA, Melmed MH, Sheldon RE, Jones MD, Meschia G. Induction of fetal breathing by metabolic acidemia and its effect on blood flow to the respiratory muscles. Am J Obstet Gynecol. 1980;136:609–620. doi: 10.1016/0002-9378(80)91012-1. [DOI] [PubMed] [Google Scholar]
- Moore PJ, Parkes MJ, Nijhuis JG, Hanson MA. The incidence of breathing movements of fetal sheep in normoxia and hypoxia after peripheral chemodenervation and brainstem transection. J Dev Physiol. 1989;11:147–151. [PubMed] [Google Scholar]
- Mortola JP. Metabolic and Ventilatory Requirements. In: Mortola JP, editor. Respiratory Physiology of Newborn Animals: A Comparative Perspective. Johns Hopkins University Press; Baltimore, MD: 2001. pp. 45–79. [Google Scholar]
- Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol. 1998;85:84–90. doi: 10.1152/jappl.1998.85.1.84. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Scarpelli EM. Generation and regulation of breathing in utero: fetal CO2 response test. J Appl Physiol. 1979;47:527–531. doi: 10.1152/jappl.1979.47.3.527. [DOI] [PubMed] [Google Scholar]
- Moss IR, Inman JG. Neurochemicals and respiratory control during development. J Appl Physiol. 1989;67:1–13. doi: 10.1152/jappl.1989.67.1.1. [DOI] [PubMed] [Google Scholar]
- Moss IR, Mautone AJ, Scarpelli EM. Effect of temperature on regulation of breathing and sleep/wake state in fetal lambs. J Appl Physiol. 1983;54:536–543. doi: 10.1152/jappl.1983.54.2.536. [DOI] [PubMed] [Google Scholar]
- Mouroux I, Bertin R, Portet R. Thermogenic capacity of the brown adipose tissue of developing rats; effects of rearing temperature. J Dev Physiol. 1990;14:337–342. [PubMed] [Google Scholar]
- Murai DT, Clyman RI, Mauray FE, Lee CC, Kitterman JA. Meclofenamate and prostaglandin E2 affect breathing movements independently of glucose concentrations in fetal sheep. Am J Obstet Gynecol. 1984;150:758–764. doi: 10.1016/0002-9378(84)90681-1. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhuis JG, Jongsma HW, Crijins IJ, de Valk IM, van der Velden JW. Effects of maternal glucose ingestion on human fetal breathing movements at weeks 24 and 28 weeks of gestation. Early Hum Dev. 1986;13:183–188. doi: 10.1016/0378-3782(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Noble LM, Carlo WA, Miller MJ, Difiore JM, Martin RJ. Transient changes in expiratory time during hypercapnia in premature infants. J Appl Physiol. 1987;62:1010–1013. doi: 10.1152/jappl.1987.62.3.1010. [DOI] [PubMed] [Google Scholar]
- Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr. 2004;144:291–295. doi: 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Okada Y, Mueckenhoff K, Scheid P. Hypercapnia and medullary neurons in the isolated brain stem-spinal cord of the rat. Resp Physiol. 1993;93:327–336. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa H, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Parmelee AH, Stern E, Harris MA. Maturation of respiration in prematures and young infants. Neuropaediatrie. 1972;3:294–304. doi: 10.1055/s-0028-1091768. [DOI] [PubMed] [Google Scholar]
- Patrick J, Natale R, Richardson B. Patterns of human fetal breathing activity at 34 to 35 weeks’ gestational age. Am J Obstet Gynecol. 1978;132:507–513. doi: 10.1016/0002-9378(78)90744-5. [DOI] [PubMed] [Google Scholar]
- Perlstein PH, Edwards NK, Sutherland JM. Apnea in premature infants and incubator-air-temperature changes. NEJM. 1970;282:461–466. doi: 10.1056/NEJM197002262820901. [DOI] [PubMed] [Google Scholar]
- Poets CF, Rau GA, Neuber K, Gappa M, Seidenberg J. Determinants of lung volume in spontaneously breathing preterm infants. Am J of Respir & Crit Care Med. 1997;155:649–653. doi: 10.1164/ajrccm.155.2.9032208. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Resp Physiol & Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilligan EJ, Clewlow F, Johnston BM, Walker DW. Effect of 5-hydroxytryptophan on electrocortical activity and breathing movements of fetal sheep. Am J Obstet Gynecol. 1981;141:271–275. doi: 10.1016/s0002-9378(16)32632-1. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, Silvestri JM, Crowell DH, Hufford D, Martin RJ, Neuman MR, Weese-Mayer DE, Cupples LA, Peucker M, Willinger M, Keens TG The Collaborative Home Infant Monitoring Evaluation Study G. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285:2199–2207. doi: 10.1001/jama.285.17.2199. [DOI] [PubMed] [Google Scholar]
- Rigatto H. Regulation of fetal breathing. Reprod Fertil Dev. 1996;8:23–33. doi: 10.1071/rd9960023. [DOI] [PubMed] [Google Scholar]
- Rigatto H. Periodic Breathing. In: Mathew OP, editor. Respiratory Control and Disorders in the Newborn. Vol. 173. Marcel Dekker, Inc; New York: 2003. pp. 237–272. [Google Scholar]
- Rigatto H, Brady JF, Verduzco RT. Chemoreceptor reflexes in preterm infants: II. the effect of gestational and postnatal age on the ventilatory response to inhaled carbon dioxide. Pediatrics. 1975a;55:614. [PubMed] [Google Scholar]
- Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. I. Evidence for hypoventilation possibly due to cdentral respiratory depression. Pediatrics. 1972;50:202–218. [PubMed] [Google Scholar]
- Rigatto H, Lee D, Davi M, Moore M, Rigatto E, Cates D. Effect of increased CO2 on fetal breathing and behavior in sheep. J Appl Physiol. 1988;64:982–987. doi: 10.1152/jappl.1988.64.3.982. [DOI] [PubMed] [Google Scholar]
- Rigatto H, Moore M, Cates D. Fetal breathing and behavior measured through a double-wall Plexiglas window in sheep. J Appl Physiol. 1986;61:160–164. doi: 10.1152/jappl.1986.61.1.160. [DOI] [PubMed] [Google Scholar]
- Rigatto H, Verduzco RT, Cates DB. Effects of O2 on the ventilatory response to CO2 in preterm infants. Pediatrics. 1975b;39:896–899. doi: 10.1152/jappl.1975.39.6.896. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol. 1987;62:506–512. doi: 10.1152/jappl.1987.62.2.506. [DOI] [PubMed] [Google Scholar]
- Saiki C, Mortola JP. Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol. 1996;491 (Pt 1):261–269. doi: 10.1113/jphysiol.1996.sp021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador HS, Koos BJ. Effects of regular and decaffeinated coffee on fetal breathing and heart rate. Am J Obstet Gynecol. 1989;160:1043–1047. doi: 10.1016/0002-9378(89)90157-9. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AM, Karlsson KA, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol. 2001;91:1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory anbd single dhemosensitive neuron response to hypercapnia in rats. Resp Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Taylor PM. Oxygen consumption in newborn rats. J Physiol. 1960;154:153–169. doi: 10.1113/jphysiol.1960.sp006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT, Tauesch HW. Sighing in human newborn infants: Role of inflation-augmenting reflex. J Appl Physiol. 1976;41:502–507. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- Thibeault DW, Wong MM, Auld PA. Thoracic gas volume changes in premature infants. Pediatrics. 1967;40:403–411. [PubMed] [Google Scholar]
- Wagaman MJ, Shutack JG, Moomjian AS, Schwartz JG, Shaffer TH, Fox WW. Improved oxygenation and lung compliance with prone positioning of neonates. J Pediatr. 1979;94:787–791. doi: 10.1016/s0022-3476(79)80157-2. [DOI] [PubMed] [Google Scholar]
- Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol. 1996;495:217–225. doi: 10.1113/jphysiol.1996.sp021586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neurosci. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Watson CS, White SE, Homan JH, Fraher L, Brien JF, Bocking AD. The adenosine A1-receptor antagonist 8-CPT reverses ethanol-induced inhibition of fetal breathing movements. J Appl Physiol. 1999;87:1333–1338. doi: 10.1152/jappl.1999.87.4.1333. [DOI] [PubMed] [Google Scholar]
- Weintraub Z, Alvaro R, Mills S, Cates D, Rigatto H. Short apneas and their relationship to body movements and sighs in preterm infants. Biol Neon. 1994;66:188–194. doi: 10.1159/000244107. [DOI] [PubMed] [Google Scholar]
- Wennergren G, Wennergren M. Neonatal breathing control mediated via the central chemoreceptors. Acta Physiol Scand. 1983;119:139–146. doi: 10.1111/j.1748-1716.1983.tb07319.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson MH, Skuza EM, Rennie GC, Sands SA, Yiallourou SR, Horne RS, Berger PJ. Postnatal development of periodic breathing cycle duration in term and preterm infants. Pediatr Res. 2007;62:331–336. doi: 10.1203/PDR.0b013e3180db29e5. [DOI] [PubMed] [Google Scholar]
- Wolsink JG, Berkenbosch A, DeGoede J, Olievier CN. Ventilatory sensitivities of peripheral and central chemoreceptors of young piglets to inhalation of CO2 in air. Pediatr Res. 1991;30:491–495. doi: 10.1203/00006450-199111000-00018. [DOI] [PubMed] [Google Scholar]
- Wolsink JG, Berkenbosch A, DeGoede J, Olievier CN. The effects of hypoxia on the ventilatory response to sudden changes in CO2 in newborn piglets. J Physiol (Lond) 1992;456:39–48. doi: 10.1113/jphysiol.1992.sp019325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Resp Physiol & Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Zec N, Filiano JJ, Kinney HC. Anatomic relationships of the human arcuate nucleus of the medulla: a DiI-labeling study [published erratum appears in J Neuropathol. Exp Neurol. 1997;56:509–522. doi: 10.1097/00005072-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Zhou D, Huang Q, Fung ML, Li A, Darnall RA, Nattie EE, St John WM. Phrenic response to hypercapnia in the unanesthetized, decerbrate, newborn rat. Resp Physiol. 1996;104:11–22. doi: 10.1016/0034-5687(95)00098-4. [DOI] [PubMed] [Google Scholar]


